Immunopathology of Renal Tissue in Fatal Cases of Dengue in Children
Abstract
:1. Introduction
2. Results
2.1. Levels of Serum Creatinine and Urea in the Cases Studied during Hospitalization Days
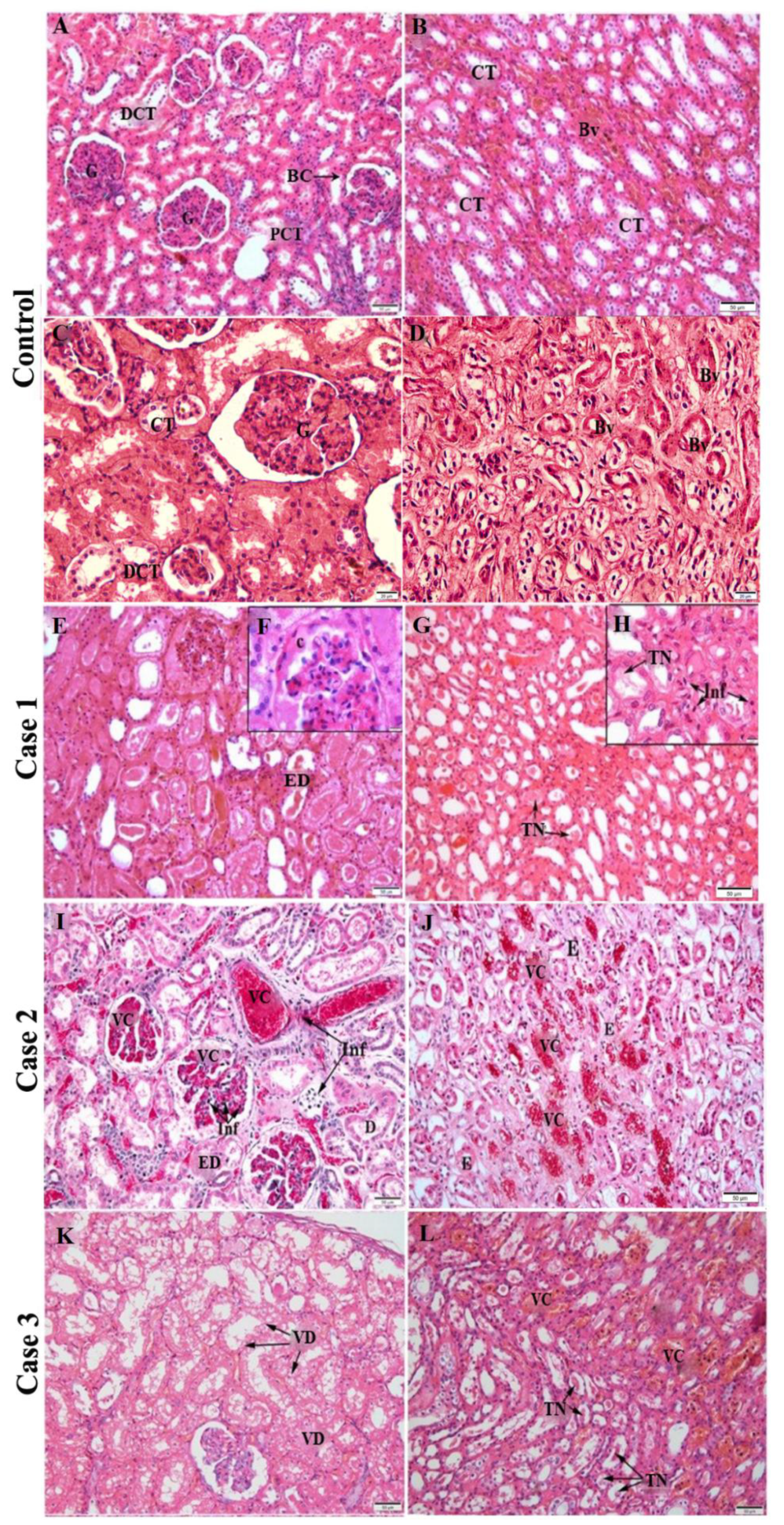
2.2. Histopathological Analysis
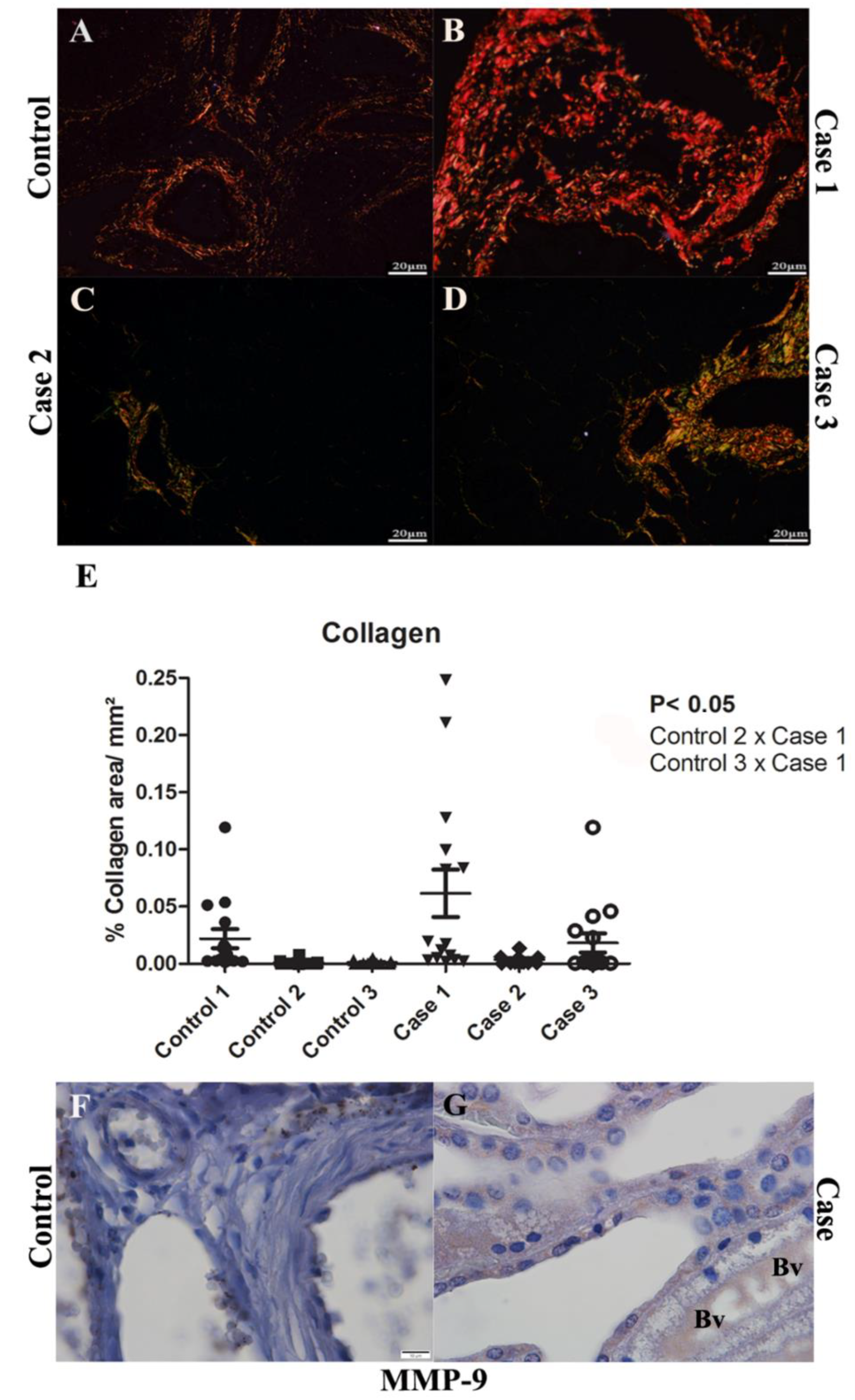
2.3. Evaluation of the Fibrosis by Collagen Quantification and MMP-9 Detection in Renal Tissues
2.4. Detection of Viral Antigen in Infected Renal Tissue
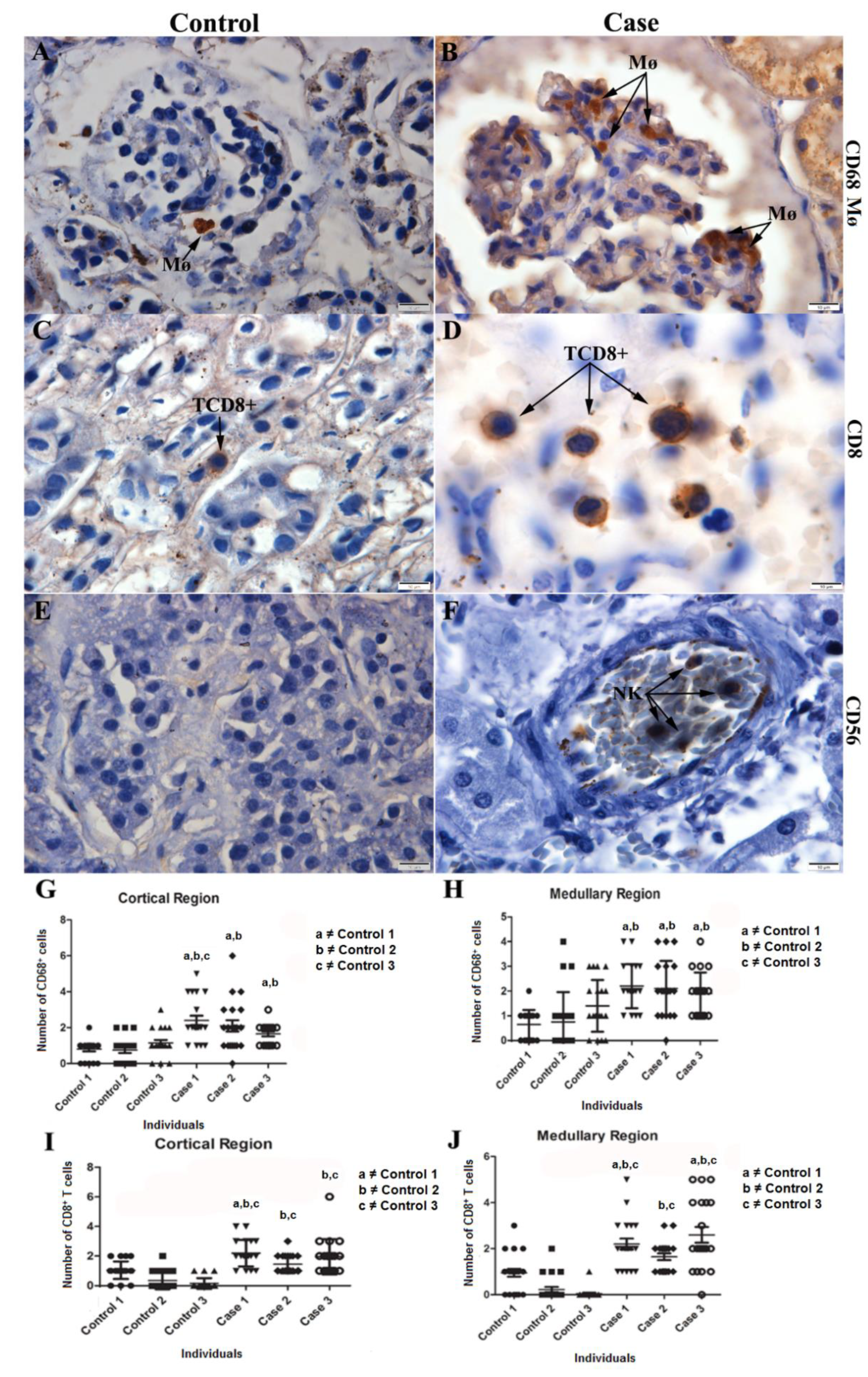
2.5. Characterization of the Cell Expression Pattern in Infected Renal Tissue
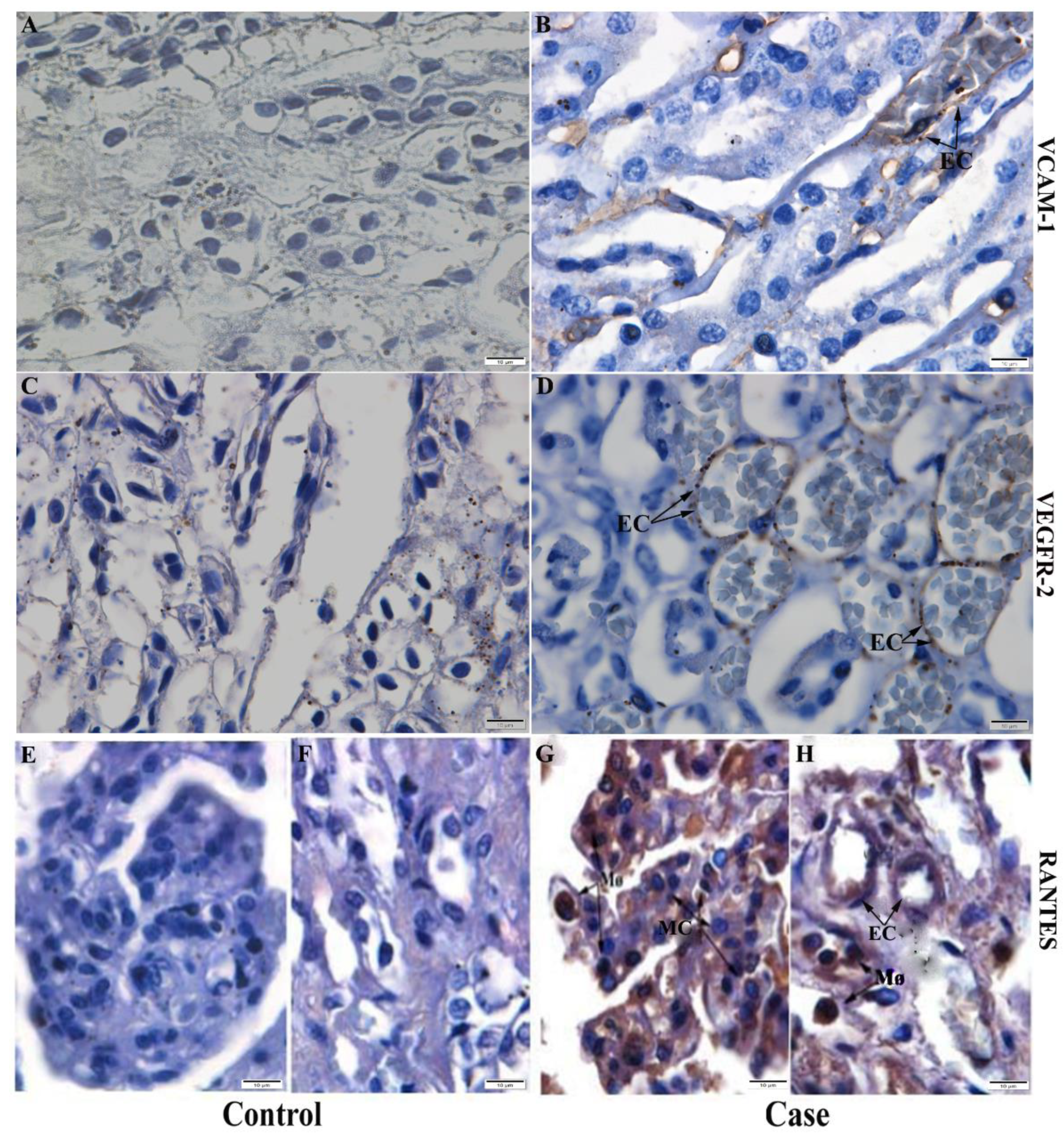
2.6. Detection of Cytokines, Chemokines and Inflammatory Mediators in Kidney Tissue
3. Discussion
4. Materials and Methods
4.1. Ethical Procedures
4.2. Children Fatal Cases
4.3. Case Presentation
- -
- Controls: Three children who were approximately the same age of the cases (8 to 13 years old) and died of natural causes or trauma, without any history of dengue and other infectious diseases, and without any pathology directly or indirectly related to the kidney.
- -
- Case 1: Male, melanodermic, 7 years and 8 months old, born in Rio de Janeiro, Brazil. Admitted to HMJ (Hospital Municipal Jesus) on 30 January 2008. On 23 January 2008, after five days of high fever, nausea and vomiting, the mother sought medical attention at a health center, being medicated with amoxicillin for diagnosis of “infection”. Not improving, he returned to the health care in serious condition, where the presumptive diagnosis of dengue was made by serology (antibody anti-IgM) and hospitalization was provided. Blood culture was positive for bacterial comorbidity (Pseudomonas aeruginosa) after two days of hospitalization, probably acquired in the hospital setting. After 6 days, acute renal failure was diagnosed and requested hemodialysis. After 12 days in hospital, he died on 2 November 2008 and submitted to necropsy. The last blood count performed showed leukocytosis, neutrophilia, lymphopenia, hyperchromic microcytic anemia, and thrombocytopenia. The main diagnosis was hemorrhagic dengue, and the cause of death was extensive bilateral pulmonary hemorrhage. In the analysis of the kidneys proximal tubular was altered due to hydro-electrolyte disturbance, and congestion was present.
- -
- Case 2: Female, 9 years, 11 months, 28 days, born in Itaboraí, Rio de Janeiro, Brazil. On 23 April 2011 started to present fever and general malaise, being taken to the Health Center on 24 April 2011. During physical examination, she presented drowsiness and prostration, but cooperated with the examination. She was also dehydrated, pale, eupneic (respiratory rate of 22 irpm, 20 irpm, 24 irpm), cyanotic, tachycardic (heart rate of 130bpm), with fever and had blood pressure of 90 × 50 mmHg. Petechiae were observed on the face and lower limbs. She required replacement and maintenance hydration with saline and glucose solutions, with the addition of chloride of sodium and potassium chloride. She was transferred to the intensive care unit at a hospital on 25 April 2011 for 10 h and 40 min. In the blood count was noted leukopenia, neutropenia, lymphopenia, anemia, thrombocytopenia. Heart rate reached 160bpm and unmeasurable blood pressure (cold shock). Despite the efforts to maintain cardiovascular and respiratory functions, she did not resist. There was a drop in heart rate to irreversible cardiac arrest. The death occurred on the same day of her hospitalization. The main diagnosis was dengue; and the cause of death was hemorrhagic shock, with multiple organ failure syndrome. She underwent a complete necropsy the following day, with kidneys exhibiting glomerular and interstitial vascular congestion, erythrophakemia, tubular alterations resulting from hydro-electrolytic disturbance and focal acute tubular necrosis. Dengue NS1 Kit (immunochromatographic test for the detection of the NS1 antigen of dengue virus was used, with a positive result.
- -
- Case 3: Male, aged 10 years, 8 months and 20 days, born in Rio de Janeiro, Brazil, presented fever (38.5 °C) and frontal headache on 14 April 2012 before seeking medical attention in the Emergency Care Unit, since the previous day, he started presenting prostration, non-food vomiting, and with persistent fever. He received initial medical treatment at the emergency care unit. After blood count results on 18 April 2012 (leukopenia, thrombocytopenia), dengue was suspected; then he was referred to the Dengue Center and later for hospitalization. Received venous hydration, fast and maintenance, with hydro-electrolytic control. On 19 April 201, he was acyanotic, with dyspnea, and orotracheal intubation was performed. Severe, shocked, pulseless, required mechanical ventilation. In the same day, serology was performed by using Dengue IgM kit—Elisa Capture (PanBio), with positive result. There was no improvement and having his death recorded on 20 April 2012. The main diagnosis was hemorrhagic dengue; the cause of death was brain edema. At necropsy, the kidneys showed diffuse tubular alterations resulting from hydro-electrolytic disturbance.
4.4. Histopathological Analysis
4.5. Immunohistochemistry Procedure
4.6. Kidney Tissue Quantifications/Morphometry
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control, 1st ed.; World Health Organization: Geneva, Switzerland, 2009; pp. 3–17. ISBN 9789241547871. [Google Scholar]
- Gubler, D.J. Dengue and Dengue Hemorrhagic Fever. Clin. Microbiol. Rev. 1998, 11, 480–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guzman, M.G.; Halstead, S.B.; Artsob, H.; Buchy, P.; Farrar, J.; Gubler, D.J.; Hunsperger, E.; Kroeger, A.; Margolis, H.S.; Martínez, E.; et al. Dengue: A Continuing Global Threat. Nat. Rev. Microbiol. 2010, 8, S7–S16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, S.; Jamdar, S.F.; Alalowi, M.; Al Beaiji, S.M.A.A. Dengue Virus: A Global Human Threat: Review of Literature. J. Int. Soc. Prev. Community Dent. 2016, 6, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ajlan, B.A.; Alafif, M.M.; Alawi, M.M.; Akbar, N.A.; Aldigs, E.K.; Madani, T.A. Assessment of the New World Health Organization’s Dengue Classification for Predicting Severity of Illness and Level of Healthcare Required. PLoS Negl. Trop. Dis. 2019, 13, e0007144. [Google Scholar] [CrossRef] [Green Version]
- Halstead, S.B. Dengue. Lancet 2007, 370, 1644–1652. [Google Scholar] [CrossRef]
- Nunes, P.; Nogueira, R.; Coelho, J.; Rodrigues, F.; Salomão, N.; José, C.; de Carvalho, J.; Rabelo, K.; de Azeredo, E.; Basílio-de-Oliveira, R.; et al. A Stillborn Multiple Organs’ Investigation from a Maternal DENV-4 Infection: Histopathological and Inflammatory Mediators Characterization. Viruses 2019, 11, 319. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, J.F.P.; Burdmann, E.A. Dengue-Associated Acute Kidney Injury. Clin. Kidney J. 2015, 8, 681–685. [Google Scholar] [CrossRef]
- Vachvanichsanong, P.; McNeil, E. Electrolyte Disturbance and Kidney Dysfunction in Dengue Viral Infection. Southeast Asian J. Trop. Med. Public Health 2015, 46 (Suppl. S1), 108–117. [Google Scholar] [CrossRef]
- Nunes, P.C.G.; Rioja, L.D.S.; Coelho, J.M.C.D.O.; Salomão, N.G.; Rabelo, K.; José, C.C.; Rodrigues, F.D.C.D.C.; de Azeredo, E.L.; Basílio-de-Oliveira, C.A.; Basílio-de-Oliveira, R.; et al. Renal Injury in DENV-4 Fatal Cases: Viremia, Immune Response and Cytokine Profile. Pathogens 2019, 8, 223. [Google Scholar] [CrossRef] [Green Version]
- Ismail, J.; Sankar, J. Acute Kidney Injury in Dengue—Not Unprecedented. Indian J. Pediatr. 2020, 87, 993–994. [Google Scholar] [CrossRef]
- Poddar, S.; Sharma, S.; Kaur, C.; Chellani, H. Acute Kidney Injury in Dengue among Hospitalized Children: A Prospective View. Saudi J. Kidney Dis. Transplant. 2020, 31, 407. [Google Scholar] [CrossRef]
- Lizarraga, K.J.; Nayer, A. Dengue-Associated Kidney Disease. J. Nephropathol. 2014, 3, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Dalton, R.N. Creatinina Sérica e Taxa de Filtração Glomerular: Percepção e Realidade. J. Bras. Patol. Med. Lab. 2011, 47, 8–11. [Google Scholar] [CrossRef]
- Chang, H.-L.; Wu, C.-C.; Lee, S.-P.; Chen, Y.-K.; Su, W.; Su, S.-L. A Predictive Model for Progression of CKD. Medicine 2019, 98, e16186. [Google Scholar] [CrossRef] [PubMed]
- Póvoa, T.F.; Oliveira, E.R.; Basilio-de-Oliveira, C.A.; Nuovo, G.J.; Chagas, V.L.; Salomão, N.G.; Mota, E.M.; Paes, M.V. Peripheral Organs of Dengue Fatal Cases Present Strong Pro-Inflammatory Response with Participation of IFN-Gamma-, TNF-Alpha- and RANTES-Producing Cells. PLoS ONE 2016, 11, e0168973. [Google Scholar] [CrossRef] [Green Version]
- Alves, F.D.A.V.; Oliveira, L.D.L.S.; Salomão, N.G.; Provance, D.W., Jr.; Basilio-de-Oliveira, C.A.; Basílio-de-Oliveira, R.; Moragas, L.J.; de Carvalho, J.J.; Mohana-Borges, R.; Rabelo, K.; et al. Cytokines and Inflammatory Mediators: Markers Involved in Interstitial Damage to the Pancreas in Two Dengue Fever Cases Associated with Acute Pancreatitis. PLoS ONE 2022, 17, e0262785. [Google Scholar] [CrossRef]
- Prestwood, T.R.; May, M.M.; Plummer, E.M.; Morar, M.M.; Yauch, L.E.; Shresta, S. Trafficking and Replication Patterns Reveal Splenic Macrophages as Major Targets of Dengue Virus in Mice. J. Virol. 2012, 86, 12138–12147. [Google Scholar] [CrossRef] [Green Version]
- Beltrán, D.; López-Vergès, S. NK Cells during Dengue Disease and Their Recognition of Dengue Virus-Infected Cells. Front. Immunol. 2014, 5, 192. [Google Scholar] [CrossRef] [Green Version]
- Ceriotti, F.; Boyd, J.C.; Klein, G.; Henny, J.; Queraltó, J.; Kairisto, V.; Panteghini, M. Reference Intervals for Serum Creatinine Concentrations: Assessment of Available Data for Global Application. Clin. Chem. 2008, 54, 559–566. [Google Scholar] [CrossRef] [Green Version]
- Burritt, M.F.; Slockbower, J.M.; Forsman, R.W.; Offord, K.P.; Bergstralh, E.J.; Smithson, W.A. Pediatric Reference Intervals for 19 Biologic Variables in Healthy Children. Mayo Clin. Proc. 1990, 65, 329–336. [Google Scholar] [CrossRef]
- Junqueira, L.C.; Carneiro, J. Histologia Básica, 13th ed.; Guanabara Koogan: Rio de Janeiro, Brazil, 2017. [Google Scholar]
- Hosten, A.O. BUN and Creatinine. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Butterworths: Boston, MA, USA, 1990; Chapter 193; ISBN 040990077X. [Google Scholar]
- Levey, A.S.; Perrone, R.D.; Madias, N.E. Serum Creatinine and Renal Function. Annu. Rev. Med. 1988, 39, 465–490. [Google Scholar] [CrossRef] [PubMed]
- Schutz, Y. Protein Turnover, Ureagenesis and Gluconeogenesis. International journal for vitamin and nutrition research. Internationale Zeitschrift fur Vitamin- und Ernahrungsforschung. J. Int. Vitaminol. Nutr. 2011, 81, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Sodré, F.L.; Costa, J.C.B.; Lima, J.C.C. Avaliação Da Função e Da Lesão Renal: Um Desafio Laboratorial. J. Bras. Patol. Med. Lab. 2007, 43, 329–337. [Google Scholar] [CrossRef]
- Diptyanusa, A.; Phumratanaprapin, W.; Phonrat, B.; Poovorawan, K.; Hanboonkunupakarn, B.; Sriboonvorakul, N.; Thisyakorn, U. Characteristics and Associated Factors of Acute Kidney Injury among Adult Dengue Patients: A Retrospective Single-Center Study. PLoS ONE 2019, 14, e0210360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallhi, T.H.; Khan, A.H.; Sarriff, A.; Adnan, A.S.; Khan, Y.H.; Jummaat, F. Defining Acute Kidney Injury in Dengue Viral Infection by Conventional and Novel Classification Systems (AKIN and RIFLE): A Comparative Analysis. Postgrad. Med. J. 2016, 92, 78–86. [Google Scholar] [CrossRef] [PubMed]
- Premaratna, R.; Dissanayake, D.; Silva, F.; Dassanayake, M.; de Silva, H. Secondary Bacteraemia in Adult Patients with Prolonged Dengue Fever. Ceylon Med. J. 2015, 60, 10. [Google Scholar] [CrossRef] [Green Version]
- Samarasekara, K.; Munasinghe, J. Dengue Shock Syndrome Complicated with Acute Liver Failure and Kidney Injury, Infective Endocarditis, and Deep Vein Thrombosis: A Case Report. J. Med. Case Rep. 2018, 12, 321. [Google Scholar] [CrossRef]
- Berube, B.J.; Rangel, S.M.; Hauser, A.R. Pseudomonas Aeruginosa: Breaking down Barriers. Curr. Genet. 2016, 62, 109–113. [Google Scholar] [CrossRef] [Green Version]
- Gürtler, N.; Osthoff, M.; Rueter, F.; Wüthrich, D.; Zimmerli, L.; Egli, A.; Bassetti, S. Prosthetic Valve Endocarditis Caused by Pseudomonas Aeruginosa with Variable Antibacterial Resistance Profiles: A Diagnostic Challenge. BMC Infect. Dis. 2019, 19, 530. [Google Scholar] [CrossRef]
- Macedo, R.N.; Rocha, F.A.; Rolim, D.B.; Vilar, D.C.L.F.; Araújo, F.M.D.C.; Vieira, N.N.; Teixeira, J.R.; Carvalho, M.C.; Oliveira, F.G.M.; Cavalcanti, L.P.D.G. Severe Coinfection of Melioidosis and Dengue Fever in Northeastern Brazil: First Case Report. Rev. Soc. Bras. Med. Trop. 2012, 45, 132–133. [Google Scholar] [CrossRef]
- Póvoa, T.F.; Alves, A.M.B.; Oliveira, C.A.B.; Nuovo, G.J.; Chagas, V.L.A.; Paes, M.V. The Pathology of Severe Dengue in Multiple Organs of Human Fatal Cases: Histopathology, Ultrastructure and Virus Replication. PLoS ONE 2014, 9, e83386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, T.; Tsuboi, N.; Haruhara, K.; Okabayashi, Y.; Kanzaki, G.; Koike, K.; Kobayashi, A.; Yamamoto, I.; Ogura, M.; Yokoo, T. Bowman Capsule Volume and Related Factors in Adults With Normal Renal Function. Kidney Int. Rep. 2018, 3, 314–320. [Google Scholar] [CrossRef] [Green Version]
- Mason, J.; Joeris, B.; Welsch, J.; Kriz, W. Vascular Congestion in Ischemic Renal Failure: The Role of Cell Swelling. Miner. Electrolyte Metab. 1989, 15, 114–124. [Google Scholar] [PubMed]
- Walsh, S.B.; Unwin, R.J. Renal Tubular Disorders. Clin. Med. 2012, 12, 476–479. [Google Scholar] [CrossRef]
- Kumar, V.; Abbas, A.K.; Aster, J.C.; Perkins, J.A. Robbins and Cotran Pathologic Basis of Disease, 9th ed.; Elsevier/Saunders: Philadelphia, PA, USA, 2015; pp. 31–68. [Google Scholar]
- Su, H.; Yang, M.; Wan, C.; Yi, L.-X.; Tang, F.; Zhu, H.-Y.; Yi, F.; Yang, H.-C.; Fogo, A.B.; Nie, X.; et al. Renal Histopathological Analysis of 26 Postmortem Findings of Patients with COVID-19 in China. Kidney Int. 2020, 98, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Li, X.; Zhu, X.; Chen, Q.; Jiang, L.; Zhu, Z. Renal Tubular Complement 3 Deposition in Children with Primary Nephrotic Syndrome. BioMed Res. Int. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zuo, X.; Pan, W.; Feng, T.; Shi, X.; Dai, J. Matrix Metalloproteinase 3 Promotes Cellular Anti-Dengue Virus Response via Interaction with Transcription Factor NFκB in Cell Nucleus. PLoS ONE 2014, 9, e84748. [Google Scholar] [CrossRef]
- Afroz, S.; Giddaluru, J.; Abbas, M.; Khan, N. Transcriptome Meta-Analysis Reveals a Dysregulation in Extra Cellular Matrix and Cell Junction Associated Gene Signatures during Dengue Virus Infection. Sci. Rep. 2016, 6, 33752. [Google Scholar] [CrossRef]
- Sharma, A.K.; Mauer, S.M.; Kim, Y.; Michael, A.F. Interstitial Fibrosis in Obstructive Nephropathy. Kidney Int. 1993, 44, 774–788. [Google Scholar] [CrossRef] [Green Version]
- Behrendtsen, O.; Alexander, C.M.; Werb, Z. Metalloproteinases Mediate Extracellular Matrix Degradation by Cells from Mouse Blastocyst Outgrowths. Development 1992, 114, 447–456. [Google Scholar] [CrossRef]
- Leaungwutiwong, P.; Kelley, J.F.; Sachair, A.; Jittmittraphap, A.; Luplertlop, N. Relationship between MMP Expression and Virulence of Dengue Virus Type-2 in Infected Mosquito and Mammalian Cells. Jpn. J. Infect. Dis. 2016, 69, 45–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, P.; Li, G.; Shen, M.; Yu, Z.; Ge, W.; Lao, Z.; Fan, Y.; Chen, K.; Ding, Z.; Wang, W.; et al. DENV NS1 and MMP-9 Cooperate to Induce Vascular Leakage by Altering Endothelial Cell Adhesion and Tight Junction. PLoS Pathog. 2021, 17, e1008603. [Google Scholar] [CrossRef] [PubMed]
- Swarbrick, C.M.D.; Basavannacharya, C.; Chan, K.W.K.; Chan, S.-A.; Singh, D.; Wei, N.; Phoo, W.W.; Luo, D.; Lescar, J.; Vasudevan, S.G. NS3 Helicase from Dengue Virus Specifically Recognizes Viral RNA Sequence to Ensure Optimal Replication. Nucleic Acids Res. 2017, 45, 12904–12920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Assenberg, R.; Mastrangelo, E.; Walter, T.S.; Verma, A.; Milani, M.; Owens, R.J.; Stuart, D.I.; Grimes, J.M.; Mancini, E.J. Crystal Structure of a Novel Conformational State of the Flavivirus NS3 Protein: Implications for Polyprotein Processing and Viral Replication. J. Virol. 2009, 83, 12895–12906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basílio-de-Oliveira, C.A.; Aguiar, G.R.; Baldanza, M.S.; Barth, O.M.; Eyer-Silva, W.A.; Paes, M.V. Pathologic Study of a Fatal Case of Dengue-3 Virus Infection in Rio de Janeiro, Brazil. Braz. J. Infect. Dis. 2005, 9, 341–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talarico, L.B.; Batalle, J.P.; Byrne, A.B.; Brahamian, J.M.; Ferretti, A.; García, A.G.; Mauri, A.; Simonetto, C.; Hijano, D.R.; Lawrence, A.; et al. The Role of Heterotypic DENV-Specific CD8+T Lymphocytes in an Immunocompetent Mouse Model of Secondary Dengue Virus Infection. EBioMedicine 2017, 20, 202–216. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Grifoni, A.; Sette, A.; Weiskopf, D. Human T Cell Response to Dengue Virus Infection. Front. Immunol. 2019, 10, 2125. [Google Scholar] [CrossRef] [Green Version]
- Rivino, L.; Kumaran, E.A.; Thein, T.L.; Too, C.T.; Hao Gan, V.C.; Hanson, B.J.; Wilder-Smith, A.; Bertoletti, A.J.; Gascoigne, N.R.; Lye, D.C.; et al. Virus-Specific T Lymphocytes Home to the Skin during Natural Dengue Infection. Sci. Transl. Med. 2015, 7, 278ra35. [Google Scholar] [CrossRef]
- Chen, A.; Lee, K.; Guan, T.; He, J.C.; Schlondorff, D. Role of CD8+ T Cells in Crescentic Glomerulonephritis. Nephrol. Dial. Transplant. 2020, 35, 564–572. [Google Scholar] [CrossRef]
- Sun, P.; Kochel, T.J. The Battle between Infection and Host Immune Responses of Dengue Virus and Its Implication in Dengue Disease Pathogenesis. Sci. World J. 2013, 2013, 1–11. [Google Scholar] [CrossRef]
- Srikiatkhachorn, A.; Mathew, A.; Rothman, A.L. Immune-Mediated Cytokine Storm and Its Role in Severe Dengue. Semin. Immunopathol. 2017, 39, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, N.; Mohamed, E.; Gaber, M.; Obaidani, I.; Budruddin, M.; al Busaidy, S. Acute Tubular Necrosis Associated with Non-Hemorrhagic Dengue Fever: A Case Report. Ren. Fail. 2009, 31, 736–739. [Google Scholar] [CrossRef] [PubMed]
- Begum, F.; Das, S.; Mukherjee, D.; Mal, S.; Ray, U. Insight into the Tropism of Dengue Virus in Humans. Viruses 2019, 11, 1136. [Google Scholar] [CrossRef] [Green Version]
- Takeda, K.; Tsutsui, H.; Yoshimoto, T.; Adachi, O.; Yoshida, N.; Kishimoto, T.; Okamura, H.; Nakanishi, K.; Akira, S. Defective NK Cell Activity and Th1 Response in IL-18–Deficient Mice. Immunity 1998, 8, 383–390. [Google Scholar] [CrossRef] [Green Version]
- Yamaguchi, S.; Tashiro-Yamaji, J.; Lee, K.; Takahashi, T.; Sano, K.; Endo, Y.; Nakanishi, M.; Eguchi, A.; Okada, M.; Nomi, H.; et al. IFN-γ: A Cytokine Essential for Rejection of CTL-Resistant, Virus-Infected Cells. J. Interferon Cytokine Res. 2005, 25, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, R.A.X.; de Oliveira, S.A.; Gandini, M.; da Cunha Ferreira, L.; Correa, G.; Abiraude, F.M.; Reid, M.M.; Cruz, O.G.; Kubelka, C.F. Circulating Cytokines and Chemokines Associated with Plasma Leakage and Hepatic Dysfunction in Brazilian Children with Dengue Fever. Acta Trop. 2015, 149, 138–147. [Google Scholar] [CrossRef]
- Singh, A.; Bisht, P.; Bhattacharya, S.; Guchhait, P. Role of Platelet Cytokines in Dengue Virus Infection. Front. Cell Infect. Microbiol. 2020, 10, 561366. [Google Scholar] [CrossRef]
- Kong, D.-H.; Kim, Y.; Kim, M.; Jang, J.; Lee, S. Emerging Roles of Vascular Cell Adhesion Molecule-1 (VCAM-1) in Immunological Disorders and Cancer. Int. J. Mol. Sci. 2018, 19, 1057. [Google Scholar] [CrossRef] [Green Version]
- Cerutti, C.; Ridley, A.J. Endothelial Cell-Cell Adhesion and Signaling. Exp. Cell Res. 2017, 358, 31–38. [Google Scholar] [CrossRef]
- Soe, H.J.; Khan, A.M.; Manikam, R.; Samudi Raju, C.; Vanhoutte, P.; Sekaran, S.D. High Dengue Virus Load Differentially Modulates Human Microvascular Endothelial Barrier Function during Early Infection. J. Gen. Virol. 2017, 98, 2993–3007. [Google Scholar] [CrossRef]
- Seet, R.C.S.; Chow, A.W.L.; Quek, A.M.L.; Chan, Y.-H.; Lim, E.C.H. Relationship between Circulating Vascular Endothelial Growth Factor and Its Soluble Receptors in Adults with Dengue Virus Infection: A Case–Control Study. Int. J. Infect. Dis. 2009, 13, e248–e253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lorier, G.; Touriño, C.; Kalil, R.A.K. Angiogênese Coronariana Como Resposta Endógena Da Isquemia Miocárdica No Adulto. Arq. Bras. De Cardiol. 2011, 97, e140–e148. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-W.; Liu, S.-C.; Sun, H.-L.; Huang, T.-Y.; Chan, C.-H.; Yang, C.-Y.; Yeh, H.-I.; Huang, Y.-L.; Chou, W.-Y.; Lin, Y.-M.; et al. CCL5/CCR5 Axis Induces Vascular Endothelial Growth Factor-Mediated Tumor Angiogenesis in Human Osteosarcoma Microenvironment. Carcinogenesis 2015, 36, 104–114. [Google Scholar] [CrossRef] [PubMed]
- Taal, M.W.; Zandi-Nejad, K.; Weening, B.; Shahsafaei, A.; Kato, S.; Lee, K.-W.; Ziai, F.; Jiang, T.; Brenner, B.M.; Mackenzie, H.S. Proinflammatory Gene Expression and Macrophage Recruitment in the Rat Remnant Kidney. Kidney Int. 2000, 58, 1664–1676. [Google Scholar] [CrossRef]
- Isner, J.M. Angiogenesis. In Textbook of Cardiovascular Medicine; Topol, E.J., Ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1998; pp. 2491–2518. [Google Scholar]



| Case 1 | Case 2 | Case 3 | Reference Values | |
|---|---|---|---|---|
| Creatinine (mg/dL) | 0.9236 | 0.6 | 0.5 | 0.300–0.800 mg/dL |
| Urea (mg/dL) | 54.54 | 18.0 | 19.0 | 16–42 mg/dL |
| Case 1 | Case 2 | Case 3 | |
|---|---|---|---|
| Age | 7 years and 8 months | 9 years, 11 months, 28 days | 10 years, 8 months and 20 days |
| Symptoms | high fever, nausea and vomiting | fever and general malaise; drowsiness and prostration; dehydrated, pale, eupneic, cyanotic, tachycardic, blood pressure of 90 × 50 mmHg, petechiae (face and lower limbs) | frontal headache; presenting prostration, non-food vomiting, and with persistent fever; leukopenia, thrombocytopenia |
| Admission to Hospital | 30 January 2008 | 24 April 2011 | 14 April 2012 |
| Laboratory tests | Dengue serology positive Blood culture positive for Pseudomonas aeruginosa | Immunochromatographic test for the detection of Dengue virus NS1 antigen: reagent | Dengue serology positive |
| Death | 12 February 2008 | 25 April 2011 | 20 April 2012 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Lima Siqueira Oliveira, L.; de Andrade Vieira Alves, F.; Rabelo, K.; Moragas, L.J.; Mohana-Borges, R.; de Carvalho, J.J.; Basílio-de-Oliveira, C.; Basílio-de-Oliveira, R.; Rosman, F.C.; Salomão, N.G.; et al. Immunopathology of Renal Tissue in Fatal Cases of Dengue in Children. Pathogens 2022, 11, 1543. https://doi.org/10.3390/pathogens11121543
de Lima Siqueira Oliveira L, de Andrade Vieira Alves F, Rabelo K, Moragas LJ, Mohana-Borges R, de Carvalho JJ, Basílio-de-Oliveira C, Basílio-de-Oliveira R, Rosman FC, Salomão NG, et al. Immunopathology of Renal Tissue in Fatal Cases of Dengue in Children. Pathogens. 2022; 11(12):1543. https://doi.org/10.3390/pathogens11121543
Chicago/Turabian Stylede Lima Siqueira Oliveira, Lucca, Felipe de Andrade Vieira Alves, Kíssila Rabelo, Leandro Junqueira Moragas, Ronaldo Mohana-Borges, Jorge José de Carvalho, Carlos Basílio-de-Oliveira, Rodrigo Basílio-de-Oliveira, Fernando Colonna Rosman, Natália Gedeão Salomão, and et al. 2022. "Immunopathology of Renal Tissue in Fatal Cases of Dengue in Children" Pathogens 11, no. 12: 1543. https://doi.org/10.3390/pathogens11121543






