New Vistas in the Biology of the Flagellum—Leishmania Parasites
Abstract
:1. Introduction
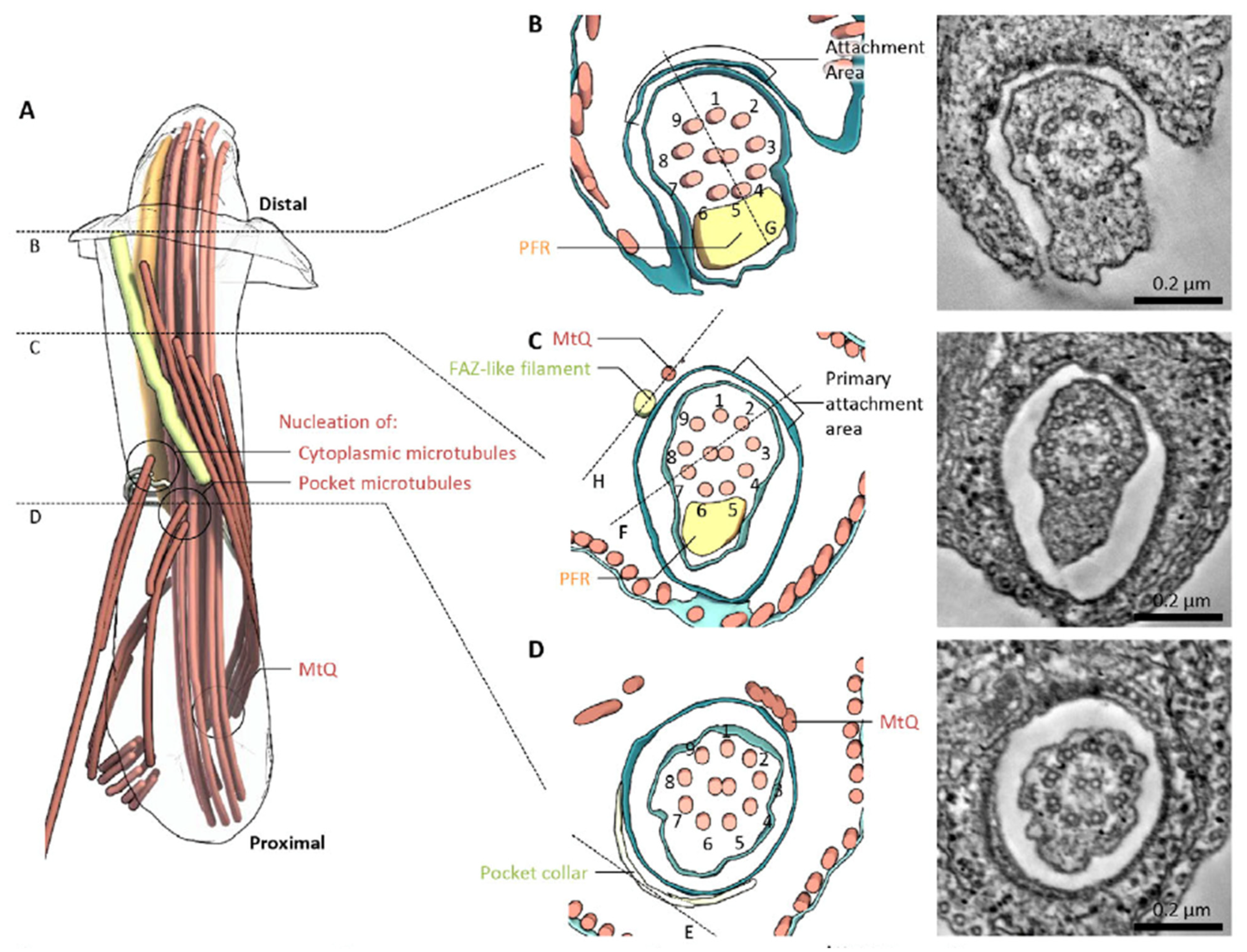
2. Structure of Flagellum throughout the Leishmania Life Cycle
3. Comprehensive Proteome of the Leishmania Flagellum
4. Identification of the Flagellum Attachment Zone
5. Targeting and Functions of Membrane Proteins in the Flagellum
6. Summary and Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Wheway, G.; Nazlamova, L.; Hancock, J.T. Signaling through the primary cilium. Front. Cell Dev. Biol. 2018, 6, 8. [Google Scholar] [CrossRef]
- Long, H.; Huang, K. Transport of Ciliary Membrane Proteins. Front. Cell Dev. Biol. 2019, 7, 381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, F.D.; Sanchez, M.A.; Landfear, S.M. Touching the surface: Diverse roles for the flagellar membrane in Kinetoplastid parasites. Microbiol. Mol. Biol. Rev. 2020, 84, e00079-19. [Google Scholar] [CrossRef] [PubMed]
- Sunter, J.; Gull, K. Shape, form, function and Leishmania pathogenicity: From textbook descriptions to biological understanding. Open Biol. 2017, 7, 170165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Portman, N.; Gull, K. The paraflagellar rod of kinetoplastid parasites: From structure to components and function. Int. J. Parasitol. 2010, 40, 135–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gluenz, E.; Hoog, J.L.; Smith, A.E.; Dawe, H.R.; Shaw, M.K.; Gull, K. Beyond 9+0: Noncanonical axoneme structures characterize sensory cilia from protists to humans. FASEB J. 2010, 24, 3117–3121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Besteiro, S.; Williams, R.A.M.; Coombs, G.H.; Mottram, J.C. Protein turnover and differentiation in Leishmania. Int. J. Parasitol. 2007, 37, 1063–1075. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, R.J.; Sunter, J.D.; Gull, K. Flagellar pocket restructuring through the Leishmania life cycle involves a discrete flagellum attachment zone. J. Cell Sci. 2016, 129, 854–867. [Google Scholar] [CrossRef] [Green Version]
- Beneke, T.; Demay, F.; Hookway, E.; Ashman, N.; Jeffery, H.; Smith, J.; Valli, J.; Becvar, T.; Myskova, J.; Lestinova, T.; et al. Genetic dissection of a Leishmania flagellar proteome demonstrates requirement for directional motility in sand fly infections. PLoS Pathog. 2019, 15, e1007828. [Google Scholar] [CrossRef] [Green Version]
- Beneke, T.; Madden, R.; Makin, L.; Valli, J.; Sunter, J.; Gluenz, E. A CRISPR Cas9 high-throughput genome editing toolkit for kinetoplastids. R. Soc. Open Sci. 2017, 4, 170095. [Google Scholar] [CrossRef] [Green Version]
- Taschner, M.; Lorentzen, E. The intraflagellar transport machinery. Cold Spring Harb. Perspect. Biol. 2016, 8, a028092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beneke, T.; Banecki, K.; Fochler, S.; Gluenz, E. LAX28 is required for the stable assembly of the inner dynein arm f complex, and the tether and tether head complex in Leishmania flagella. J. Cell Sci. 2020, 133, jcs239855. [Google Scholar] [CrossRef] [PubMed]
- Sunter, J.D.; Gull, K. The flagellum attachment zone: ‘The cellular ruler’ of trypanosome morphology. Trends Parasitol. 2016, 32, 309–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sunter, J.D.; Yanase, R.; Wang, Z.; Catta-Preta, C.M.C.; Moreira-Leite, F.; Myskova, J.; Pruzinova, K.; Volf, P.; Mottram, J.C.; Gull, K. Leishmania flagellum attachment zone is critical for flagellar pocket shape, development in the sand fly, and pathogenicity in the host. Proc. Natl. Acad. Sci. USA 2019, 116, 6351–6360. [Google Scholar] [CrossRef] [Green Version]
- Halliday, C.; Yanase, R.; Catta-Preta, C.M.C.; Moreira-Leite, F.; Myskova, J.; Pruzinova, K.; Volf, P.; Mottram, J.C.; Sunter, J.D. Role for the flagellum attachment zone in Leishmania anterior cell tip morphogenesis. PLoS Pathog. 2020, 16, e1008494. [Google Scholar] [CrossRef]
- Corrales, R.M.; Vaselek, S.; Neish, R.; Berry, L.; Brunet, C.D.; Crobu, L.; Kuk, N.; Mateos-Langerak, J.; Robinson, D.R.; Volf, P.; et al. The kinesin of the flagellum attachment zone in Leishmania is required for cell morphogenesis, cell division and virulence in the mammalian host. PLoS Pathog. 2021, 17, e1009666. [Google Scholar] [CrossRef]
- Tran, K.D.; Rodriguez-Contreras, D.; Shinde, U.; Landfear, S.M. Both sequence and context are important for flagellar targeting of a glucose transporter. J. Cell Sci. 2012, 125, 3293–3298. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Contreras, D.; Aslan, H.; Feng, X.; Tran, K.; Yates, P.A.; Kamhawi, S.; Landfear, S. Regulation and biological function of a flagellar glucose transporter in Leishmania mexicana: A potential glucose sensor. FASEB J. 2015, 29, 11–24. [Google Scholar] [CrossRef] [Green Version]
- Tran, K.D.; Rodriguez-Contreras, D.; Vieira, D.P.; Yates, P.A.; David, L.; Beatty, W.; Elferich, J.; Landfear, S.M. KHARON1 mediates flagellar targeting of a glucose transporter in Leishmania mexicana and is critical for viability of infectious intracellular amastigotes. J. Biol. Chem. 2013, 288, 22721–22733. [Google Scholar] [CrossRef] [Green Version]
- Sanchez, M.A.; Tran, K.D.; Valli, J.; Hobbs, S.; Johnson, E.; Gluenz, E.; Landfear, S.M. KHARON is an essential cytoskeletal protein involved in the trafficking of fagellar membrane proteins and cell division in African trypanosomes. J. Biol. Chem. 2016, 291, 19760–19773. [Google Scholar] [CrossRef] [Green Version]
- Dean, S.; Moreira-Leite, F.; Varga, V.; Gull, K. Cilium transition zone proteome reveals compartmentalization and differential dynamics of ciliopathy complexes. Proc. Natl. Acad. Sci. USA 2016, 113, E5135–E5143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephan, A.; Vaughan, S.; Shaw, M.K.; Gull, K.; McKean, P.G. An essential quality control mechanism at the eukaryotic basal body prior to intraflagellar transport. Traffic 2007, 8, 1323–1330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Absalon, S.; Blisnick, T.; Kohl, L.; Toutirais, G.; Dore, G.; Julkowska, D.; Tavenet, A.; Bastin, P. Intraflagellar transport and functional analysis of genes required for flagellum formation in trypanosomes. Mol. Biol. Cell 2008, 19, 929–944. [Google Scholar] [CrossRef]
- Cole, D.G.; Diener, D.R.; Himelblau, A.L.; Beech, P.L.; Fuster, J.C.; Rosenbaum, J.L. Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J. Cell Biol. 1998, 141, 993–1008. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.; Santi-Rocca, J.; Fort, C.; Tinevez, J.-Y.; Schietroma, C.; Bastin, P. Concentration of intraflagellar transport proteins at the ciliary base is required for proper train injection. BioRxiv 2021. [Google Scholar] [CrossRef]
- Nachury, M.V. The molecular machines that traffic signaling receptors into and out of cilia. Curr. Opin. Cell Biol. 2018, 51, 124–131. [Google Scholar] [CrossRef]
- Figarella, K.; Uzcategui, N.L.; Zhou, Y.; LeFurgey, A.; Ouellette, M.; Bhattacharjee, H.; Mukhopadhyay, R. Biochemical characterization of Leishmania major aquaglyceroporin LmAQP1: Possible role in volume regulation and osmotaxis. Mol. Microbiol. 2007, 65, 1006–1017. [Google Scholar] [CrossRef]
- Kelly, F.D.; Tran, K.D.; Hatfield, J.; Schmidt, K.; Sanchez, M.A.; Landfear, S.M. A cytoskeletal protein complex is essential for division of intracellular amastigotes of Leishmania mexicana. J. Biol. Chem. 2020, 295, 13106–13122. [Google Scholar] [CrossRef]
- Mandal, G.; Mandal, S.; Sharma, M.; Charret, K.S.; Papadopoulou, B.; Bhattacharjee, H.; Mukhopadhyay, R. Species-specific antimonial sensitivity in Leishmania is driven by post-transcriptional regulation of AQP1. PLoS Negl. Trop. Dis. 2015, 9, e0003500. [Google Scholar] [CrossRef]
- Plourde, M.; Ubeda, J.M.; Mandal, G.; Monte-Neto, R.L.; Mukhopadhyay, R.; Ouellette, M. Generation of an aquaglyceroporin AQP1 null mutant in Leishmania major. Mol. Biochem. Parasitol. 2015, 201, 108–111. [Google Scholar] [CrossRef]
- Bates, P.A. Leishmania sand fly interaction: Progress and challenges. Curr. Opin. Microbiol. 2008, 11, 340–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldman-Pinkovich, A.; Balno, C.; Strasser, R.; Zeituni-Molad, M.; Bendelak, K.; Rentsch, D.; Ephros, M.; Wiese, M.; Jardim, A.; Myler, P.J.; et al. An arginine deprivation response pathway is induced in Leishmania during macrophage invasion. PLoS Pathog. 2016, 12, e1005494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawar, H.; Puri, M.; Fischer Weinberger, R.; Madhubala, R.; Zilberstein, D. The arginine sensing and transport binding sites are distinct in the human pathogen Leishmania. PLoS Negl. Trop. Dis. 2019, 13, e0007304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tull, D.; Vince, J.E.; Callaghan, J.M.; Naderer, T.; Spurck, T.; McFadden, G.I.; Currie, G.; Ferguson, K.; Bacic, A.; McConville, M.J. SMP-1, a member of a new family of small myristoylated proteins in kinetoplastid parasites, is targeted to the flagellum membrane in Leishmania. Mol. Biol. Cell 2004, 15, 4775–4786. [Google Scholar] [CrossRef] [Green Version]
- Maric, D.; McGwire, B.S.; Buchanan, K.T.; Olson, C.L.; Emmer, B.T.; Epting, C.L.; Engman, D.M. Molecular determinants of ciliary membrane localization of Trypanosoma cruzi flagellar calcium-binding protein. J. Biol. Chem. 2011, 286, 33109–33117. [Google Scholar] [CrossRef] [Green Version]
- Maric, D.; Epting, C.L.; Engman, D.M. Composition and sensory function of the trypanosome flagellar membrane. Curr. Opin. Microbiol. 2010, 13, 466–472. [Google Scholar] [CrossRef] [Green Version]
- Paindavoine, P.; Rolin, S.; Van Assel, S.; Geuskens, M.; Jauniaux, J.; Dinsart, C.; Huet, G.; Pays, E. A gene from the variant surface glycoprotein expression site encodes one of several transmembrane adenylate cyclases located on the flagellum of Trypanosoma brucei. Mol. Cell. Biol. 1992, 12, 1218–1225. [Google Scholar]
- Lopez, M.A.; Saada, E.A.; Hill, K.L. Insect stage-specific adenylate cyclases regulate social motility in African trypanosomes. Eukaryot. Cell 2015, 14, 104–112. [Google Scholar] [CrossRef] [Green Version]
- Salmon, D. Adenylate cyclases of Trypanosoma brucei, environmental sensors and controllers of host innate immune response. Pathogens 2018, 7, 48. [Google Scholar] [CrossRef] [Green Version]
- Saada, E.A.; Kabututu, Z.P.; Lopez, M.; Shimogawa, M.M.; Langousis, G.; Oberholzer, M.; Riestra, A.; Jonsson, Z.O.; Wohlschlegel, J.A.; Hill, K.L. Insect stage-specific receptor adenylate cyclases are localized to distinct subdomains of the Trypanosoma brucei flagellar membrane. Eukaryot. Cell 2014, 13, 1064–1076. [Google Scholar] [CrossRef] [Green Version]
- Findlay, R.C.; Osman, M.; Spence, K.A.; Kaye, P.M.; Walrad, P.B.; Wilson, L.G. High-speed, three-dimensional imaging reveals chemotactic behaviour specific to human-infective Leishmania parasites. Elife 2021, 10, e65051. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Landfear, S.M. New Vistas in the Biology of the Flagellum—Leishmania Parasites. Pathogens 2022, 11, 447. https://doi.org/10.3390/pathogens11040447
Landfear SM. New Vistas in the Biology of the Flagellum—Leishmania Parasites. Pathogens. 2022; 11(4):447. https://doi.org/10.3390/pathogens11040447
Chicago/Turabian StyleLandfear, Scott M. 2022. "New Vistas in the Biology of the Flagellum—Leishmania Parasites" Pathogens 11, no. 4: 447. https://doi.org/10.3390/pathogens11040447




