Oral Microbiota during Childhood and Its Role in Chemotherapy-Induced Oral Mucositis in Children with Cancer
Abstract
:1. Introduction
2. Physiological and Pathological Changes of the Oral Microbiota during Childhood
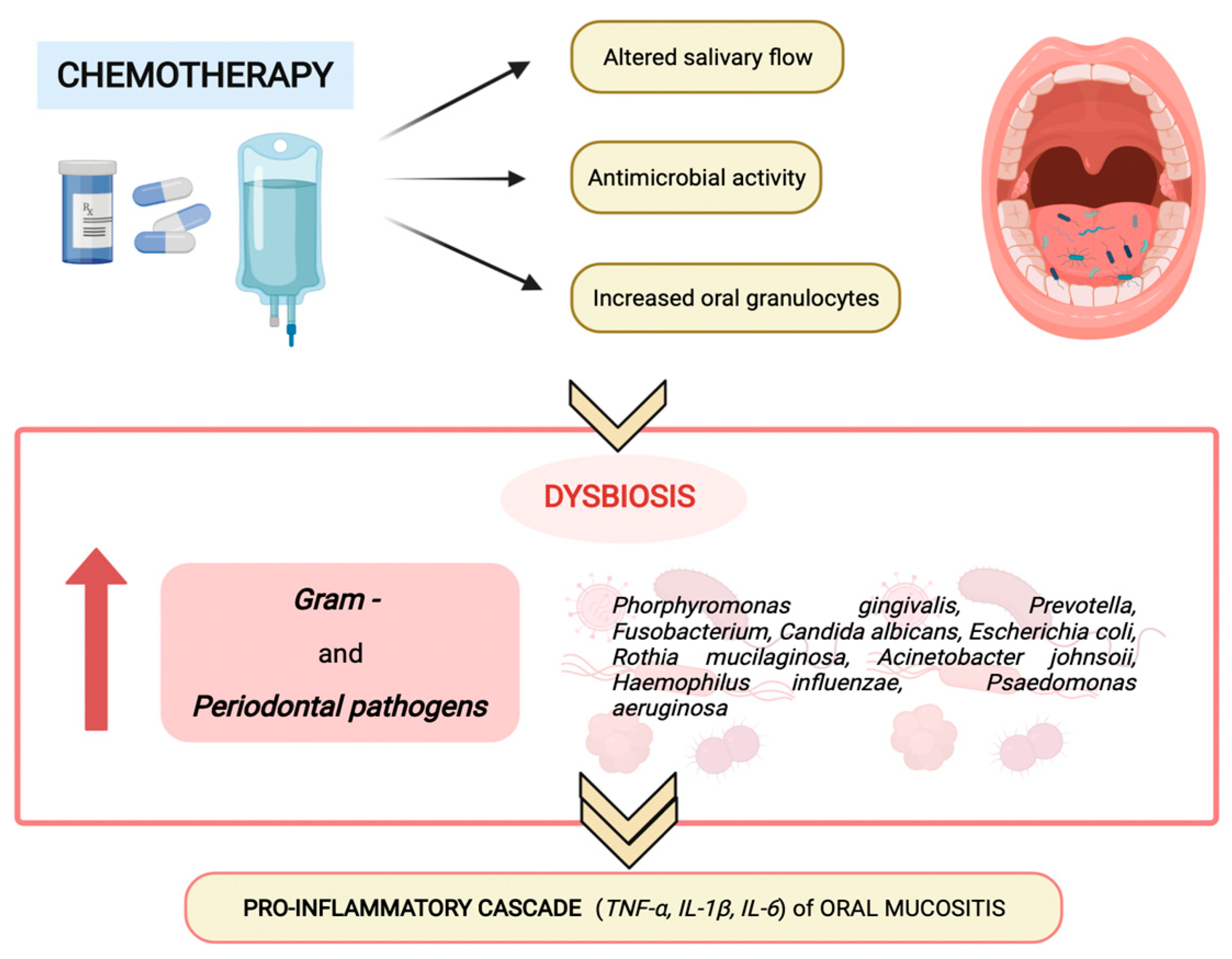
3. Oral microbiota and Chemotherapy-Induced Oral Mucositis in Children
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Turnbaugh, P.J.; Ley, R.E.; Hamady, M.; Fraser-Liggett, C.M.; Knight, R.; Gordon, J.I. The human microbiome project. Nature 2007, 449, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Lederberg, J.; McCray, A.T. ‘Ome Sweet’ Omics—A Genealogical Treasury of Words. Genealogical Treasury of Words. Scientist 2001, 15, 8. [Google Scholar]
- Blaser, M.J. The microbiome revolution. J. Clin. Investig. 2014, 124, 4162–4165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Integrative HMP (iHMP) Research Network Consortium. The Integrative Human Microbiome Project. Nature 2019, 569, 641–648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar] [CrossRef] [Green Version]
- Aas, J.A.; Paster, B.J.; Stokes, L.N.; Olsen, I.; Dewhirst, F.E. Defining the normal bacterial flora of the oral cavity. J. Clin. Microbiol. 2005, 43, 5721–5732. [Google Scholar] [CrossRef] [Green Version]
- Keijser, B.J.; Zaura, E.; Huse, S.M.; van der Vossen, J.M.; Schuren, F.H.; Montijn, R.C.; ten Cate, J.M.; Crielaard, W. Pyrosequencing analysis of the oral microflora of healthy adults. J. Dent. Res. 2008, 87, 1016–1020. [Google Scholar] [CrossRef]
- Lazarevic, V.; Whiteson, K.; Huse, S.; Hernandez, D.; Farinelli, L.; Østerås, M.; Schrenzel, S.; François, P. Metagenomic study of the oral microbiota by Illumina high-throughput sequencing. J. Microbiol. Methods 2009, 79, 266–271. [Google Scholar] [CrossRef] [Green Version]
- Hamady, M.; Knight, R. Microbial community profiling for human microbiome projects: Tools, techniques, and challenges. Genome Res. 2009, 19, 1141–1152. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Yu, W.H.; Izard, J.; Baranova, O.V.; Lakshmanan, A.; Dewhirst, F.E. The Human Oral Microbiome database: A web accessible resource for investigating oral microbe taxonomic and genomic information. Database J. Biol. Database Curation 2010, 2010, baq013. [Google Scholar] [CrossRef]
- Bik, E.M.; Long, C.D.; Armitage, G.C.; Loomer, P.; Emerson, J.; Mongodin, E.F.; Nelson, K.E.; Gill, S.R.; Fraser-Liggett, C.M.; Relman, D.A. Bacterial diversity in the oral cavity of 10 healthy individuals. ISME J. 2010, 4, 962–974. [Google Scholar] [CrossRef]
- Verma, D.; Garg, P.K.; Dubey, A.K. Insights into the human oral microbiome. Arch. Microbiol. 2018, 200, 525–540. [Google Scholar] [CrossRef] [PubMed]
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar] [PubMed]
- Xiao, J.; Fiscella, K.A.; Gill, S.R. Oral microbiome: Possible harbinger for children’s health. Int. J. Oral Sci. 2020, 30, 12. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, K.; Chen, T.; Paster, B.J. A practical guide to the oral microbiome and its relation to health and disease. Oral Dis. 2017, 23, 276–286. [Google Scholar] [CrossRef] [Green Version]
- Gomez, A.; Nelson, K.E. The Oral Microbiome of Children: Development, Disease, and Implications Beyond Oral Health. Microb. Ecol. 2017, 73, 492–503. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhou, X.; Xu, X. Oral microbiota: An overlooked etiology for chemotherapy-induced oral mucositis? J. Formos Med. Assoc. 2015, 114, 297–299. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Wang, X.; Li, H.; Ni, C.; Du, Z.; Yan, F. Human oral microbiota and its modulation for oral health. Biomed. Pharmacother. 2018, 99, 883–893. [Google Scholar] [CrossRef]
- Rotimi, V.O.; Duerden, B.I. The development of the bacterial flora in normal neonates. J. Med. Microbiol. 1981, 14, 51–62. [Google Scholar] [CrossRef] [Green Version]
- Nelson-Filho, P.; Borba, I.G.; Mesquita, K.S.; Silva, R.A.; Queiroz, A.M.; Silva, L.A. Dynamics of microbial colonization of the oral cavity in newborns. Braz. Dent. J. 2013, 24, 415–419. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Mills, D.A.; Underwood, M.A. Surface microbes in the neonatal intensive care unit: Changes with routine cleaning and over time. J. Clin. Microbiol. 2013, 51, 2617–2624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortez, R.V.; Fernandes, A.; Sparvoli, L.G.; Padilha, M.; Feferbaum, R.; Neto, C.M.; Taddei, C.R. Impact of Oropharyngeal Administration of Colostrum in Preterm Newborns’ Oral Microbiome. Nutrients 2021, 13, 4224. [Google Scholar] [CrossRef] [PubMed]
- Mason, M.R.; Chambers, S.; Dabdoub, S.M.; Thikkurissy, S.; Kumar, P.S. Characterizing oral microbial communities across dentition states and colonization niches. Microbiome 2018, 10, 67. [Google Scholar] [CrossRef]
- Kennedy, B.; Peura, S.; Hammar, U.; Vicenzi, S.; Hedman, A.; Almqvist, C.; Andolf, E.; Pershagen, G.; Dicksved, J.; Bertilsson, S.; et al. Oral Microbiota Development in Early Childhood. Sci. Rep. 2019, 9, 19025. [Google Scholar] [CrossRef] [PubMed]
- Burcham, Z.M.; Garneau, N.L.; Comstock, S.S.; Tucker, R.M.; Knight, R.; Metcalf, J.L. Genetics of Taste Lab Citizen Scientists. Patterns of Oral Microbiota Diversity in Adults and Children: A Crowdsourced Population Study. Sci. Rep. 2020, 10, 2133. [Google Scholar] [CrossRef] [Green Version]
- Engberg, E.; Sajan, C.; Rejane, R.; Figueiredo, A.O.; Weiderpass, E.; Rounge, B.; Viljakainen, H. Saliva microbiota differs between children with low and high sedentary screen times. Hum. Microbiome J. 2021, 20, 100080. [Google Scholar] [CrossRef]
- De Palma, G.; Nadal, I.; Medina, M.; Donat, E.; Ribes-Koninckx, C.; Calabuig, M.; Sanz, Y. Intestinal dysbiosis and reduced immunoglobulin-coated bacteria associated with coeliac disease in children. BMC Microbiol. 2010, 10, 63. [Google Scholar] [CrossRef] [Green Version]
- Machiels, K.; Joossens, M.; Sabino, J.; de Preter, V.; Arijs, I.; Eeckhaut, V.; Ballet, V.; Claes, K.; van Immerseel, F.; Verbeke, K.; et al. A decrease of the butyrate-producing species Roseburia hominis and Faecalibacterium prausnitzii defines dysbiosis in patients with ulcerative colitis. Gut 2014, 63, 1275–1283. [Google Scholar] [CrossRef]
- Li, X.; Kolltveit, K.M.; Tronstad, L.; Olsen, I. Systemic diseases caused by oral infection. Clin. Microbiol. Rev. 2000, 13, 547–558. [Google Scholar] [CrossRef]
- Seymour, G.J.; Ford, P.J.; Cullinan, M.P.; Leishman, S.; Yamazaki, K. Relationship between periodontal infections and systemic disease. Clin. Microbiol. Infect. 2007, 13 (Suppl. 4), 3–10. [Google Scholar] [CrossRef] [Green Version]
- Beck, J.D.; Slade, G.; Offenbacher, S. Oral disease, cardiovascular disease and systemic inflammation. Periodontology 2000, 23, 110–120. [Google Scholar] [CrossRef] [PubMed]
- Bottner, A.; He, R.Y.; Sarbu, A.; Nainar, S.M.H.; Dufour, D.; Gong, S.G.; Lévesque, C.M. Streptococcus mutans isolated from children with severe-early childhood caries form higher levels of persisters. Arch. Oral Biol. 2020, 110, 104601. [Google Scholar] [CrossRef] [PubMed]
- Gross, E.L.; Beall, C.J.; Kutsch, S.R.; Firestone, N.D.; Leys, E.J.; Griffen, A.L. Beyond Streptococcus mutans: Dental caries onset linked to multiple species by 16S rRNA community analysis. PLoS ONE 2012, 7, e47722. [Google Scholar] [CrossRef] [PubMed]
- De Angelis, M.; Vannini, L.; Di Cagno, R.; Cavallo, N.; Minervini, F.; Francavilla, R.; Ercolini, D.; Gobbetti, M. Salivary and fecal microbiota and metabolome of celiac children under gluten-free diet. Int. J. Food Microbiol. 2016, 239, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Wang, J.; Wang, Y.; Zhang, J.; Zhao, C.; Shen, N.; Yang, J.; Gai, Z.; Zhang, L. Oral microbiota dysbiosis and its association with Henoch-Schönlein Purpura in children. Int. Immunopharmacol. 2018, 65, 295–302. [Google Scholar] [CrossRef]
- Docktor, M.J.; Paster, B.J.; Abramowicz, S.; Ingram, J.; Wang, Y.E.; Correll, M.; Jiang, H.; Cotton, S.L.; Kokaras, A.S.; Bousvaros, A. Alterations in diversity of the oral microbiome in pediatric inflammatory bowel disease. Inflamm. Bowel Dis. 2012, 18, 935–942. [Google Scholar] [CrossRef]
- Yost, S.; Stashenko, P.; Choi, Y.; Kukuruzinska, M.; Genco, C.A.; Salama, A.; Weinberg, E.O.; Kramer, C.D.; Frias-Lopez, J. Increased virulence of the oral microbiome in oral squamous cell carcinoma revealed by metatranscriptome analyses. Int. J. Oral. Sci. 2018, 10, 32. [Google Scholar] [CrossRef]
- Irfan, M.; Delgado, R.Z.R.; Frias-Lopez, J. The Oral Microbiome and Cancer. Front. Immunol. 2020, 11, 591088. [Google Scholar] [CrossRef]
- Gholizadeh, P.; Eslami, H.; Yousefi, M.; Asgharzadeh, M.; Kafil, H.S. Role of oral microbiome on oral cancers, a review. Biomed. Pharmacother. 2016, 84, 552–558. [Google Scholar] [CrossRef]
- Howlader, N.; Noone, A.M.; Krapcho, M.; Miller, D.; Brest, A.; Yu, M.; Ruhl, J.; Tatalovich, Z.; Mariotto, A.; Lewis, D.R.; et al. SEER Cancer Statistics Review, 1975–2016; National Cancer Institute: Bethesda, MD, USA, 2019. Available online: https://seer.cancer.gov/csr/1975_2016/ (accessed on 9 April 2020).
- Ward, Z.J.; Yeh, J.M.; Bhakta, N.; Frazier, A.L.; Atun, R. Estimating the total incidence of global childhood cancer: A simulation-based analysis. Lancet Oncol. 2019, 20, 483–493. [Google Scholar] [CrossRef]
- Kaatsch, P. Epidemiology of childhood cancer. Cancer Treat. Rev. 2010, 36, 277–285. [Google Scholar] [CrossRef] [PubMed]
- Triarico, S.; Rinninella, E.; Cintoni, M.; Capozza, M.A.; Mastrangelo, S.; Mele, M.C.; Ruggiero, A. Impact of malnutrition on survival and infections among pediatric patients with cancer: A retrospective study. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1165–1175. [Google Scholar] [PubMed]
- Peterson, D.E.; Lalla, R.V. Oral mucositis: The new paradigms. Curr. Opin. Oncol. 2010, 22, 318–322. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Caballero, A.; Torres-Lagares, D.; Robles-García, M.; Pachón-Ibáñez, J.; González-Padilla, D.; Gutiérrez-Pérez, J.L. Cancer treatment-induced oral mucositis: A critical review. Int. J. Oral Maxillofac. Surg. 2012, 41, 225–238. [Google Scholar] [CrossRef]
- Cheng, K.K.; Lee, V.; Li, C.H.; Yuen, H.L.; Epstein, J.B. Oral mucositis in pediatric and adolescent patients undergoing chemotherapy: The impact of symptoms on quality of life. Support. Care Cancer 2012, 20, 2335–2342. [Google Scholar] [CrossRef]
- Attinà, G.; Romano, A.; Maurizi, P.; D’Amuri, S.; Mastrangelo, S.; Capozza, M.A.; Triarico, S.; Ruggiero, A. Management of Oral Mucositis in Children with Malignant Solid Tumors. Front. Oncol. 2021, 11, 599243. [Google Scholar] [CrossRef] [PubMed]
- Karolewska, E.; Konopka, T.; Pupek, M.; Chybicka, A.; Mendak, M. Antibacterial potential of saliva in children with leukemia. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2008, 105, 739–744. [Google Scholar] [CrossRef]
- Hegde, A.M.; Joshi, S.; Rai, K.; Shetty, S. Evaluation of oral hygiene status, salivary characteristics and dental caries experience in acute lymphoblastic leukemic (ALL) children. J. Clin. Pediatr. Dent. 2011, 35, 319–323. [Google Scholar] [CrossRef]
- World Health Organization. Handbook for Reporting Results of Cancer Treatment; Offset publication: Geneva, Switzerland, 1979; Volume 48, pp. 15–22. [Google Scholar]
- Al-Ansari, S.; Zecha, J.A.; Barasch, A.; de Lange, J.; Rozema, F.R.; Raber-Durlacher, J.E. Oral Mucositis Induced by Anticancer Therapies. Curr. Oral Health Rep. 2015, 2, 202–211. [Google Scholar] [CrossRef] [Green Version]
- Pulito, C.; Cristaudo, A.; Porta, C.; Zapperi, S.; Blandino, G.; Morrone, A.; Strano, S. Oral mucositis: The hidden side of cancer therapy. J. Exp. Clin. Cancer Res. 2020, 39, 210. [Google Scholar] [CrossRef]
- Cerchietti, L.C.; Navigante, A.H.; Korte, M.W.; Cohen, A.M.; Quiroga, P.N.; Villaamil, E.C.; Marcelo Bonomi, M.R.; Rothae, B.M. Potential utility of the peripheral analgesic properties of morphine in stomatitis-related pain: A pilot study. Pain 2003, 105, 265–273. [Google Scholar] [CrossRef]
- Mazhari, F.; Shirazi, A.S.; Shabzendehdar, M. Management of oral mucositis in pediatric patients receiving cancer therapy: A systematic review and meta-analysis. Pediatr. Blood Cancer 2019, 66, e27403. [Google Scholar] [CrossRef] [PubMed]
- Sonis, S.T.; Tracey, C.; Shklar, G.; Jenson, J.; Florine, D. An animal model for mucositis induced by cancer chemotherapy. Oral Surg. Oral Med. Oral Pathol. 1990, 69, 437–443. [Google Scholar] [CrossRef]
- Shimamura, Y.; Takeuchi, I.; Terada, H.; Makino, K. A Mouse Model for Oral Mucositis Induced by Cancer Chemotherapy. Anticancer Res. 2018, 38, 307–312. [Google Scholar] [PubMed]
- Takeuchi, I.; Kawamata, R.; Makino, K. A Rat Model of Oral Mucositis Induced by Cancer Chemotherapy for Quantitative Experiments. Anticancer Res. 2020, 40, 2701–2706. [Google Scholar] [CrossRef]
- Sonis, S.T. A biological approach to mucositis. J. Support. Oncol. 2004, 2, 21–32. [Google Scholar]
- Sonis, S.T. The pathobiology of mucositis. Nat. Rev. Cancer 2004, 4, 277–284. [Google Scholar] [CrossRef]
- Logan, R.M.; Stringer, A.M.; Bowen, J.M.; Gibson, R.J.; Sonis, S.T.; Keefe, D.M. Serum levels of NFkappaB and pro-inflammatory cytokines following administration of mucotoxic drugs. Cancer Biol. Ther. 2008, 7, 1139–1145. [Google Scholar] [CrossRef] [Green Version]
- Bian, L.; Han, G.; Zhao, C.W.; Garl, P.J.; Wang, X.J. The role of Smad7 in oral mucositis. Protein Cell 2015, 6, 160–169. [Google Scholar] [CrossRef] [Green Version]
- Mougeot, J.C.; Stevens, C.B.; Morton, D.S.; Brennan, M.T.; Mougeot, F.B. Oral Microbiome and Cancer Therapy-Induced Oral Mucositis. J. Natl. Cancer Inst. Monogr. 2019, 2019, lgz002. [Google Scholar]
- Hong, B.Y.; Sobue, T.; Choquette, L.; Dupuy, A.K.; Thompson, A.; Burleson, J.A.; Salner, A.L.; Schauer, P.K.; Joshi, P.; Fox, E.; et al. Chemotherapy-induced oral mucositis is associated with detrimental bacterial dysbiosis. Microbiome 2019, 7, 66. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xue, J.; Zhou, X.; You, M.; Du, Q.; Yang, X.; He, J.; Zou, J.; Cheng, L.; Li, M.; et al. Oral microbiota distinguishes acute lymphoblastic leukemia pediatric hosts from healthy populations. PLoS ONE 2014, 9, e102116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, Y.; Carlsson, G.; Agholme, M.B.; Wilson, J.A.; Roos, A.; Henriques-Normark, B.; Engstrand, L.; Modéer, T.; Pütsep, K. Oral bacterial community dynamics in paediatric patients with malignancies in relation to chemotherapy-related oral mucositis: A prospective study. Clin. Microbiol. Infect. 2013, 19, E559–E567. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| High Stomatotoxic Effect (Directly Mucosal Breakdown) | Moderate Stomatotoxic Effect | |
|---|---|---|
| Alkylating agents | Melphalan | Busulfan, cyclophosphamide, procarbazine, thiotepa, mechlorethamine |
| Anthracyclines | Doxorubicin | Daunorubicin, epirubicin |
| Antimetabolite agents | Cytarabine, 5-fluoruracil, methotrexate | Hydroxyurea, 6-mercaptopurine, 6-thioguanine |
| Platinum compounds | Cisplatin | Carboplatin, oxaliplatin |
| Antibiotics | - | Actinomycin D, bleomycin, mytomicin |
| Vinca alkaloids | - | Vinblastin, vincristine, vinorelbine |
| Taxanes | - | Docetaxel, paclitaxel |
| Others | Etoposide | Lomustine |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Triarico, S.; Agresti, P.; Rinninella, E.; Mele, M.C.; Romano, A.; Attinà, G.; Maurizi, P.; Mastrangelo, S.; Ruggiero, A. Oral Microbiota during Childhood and Its Role in Chemotherapy-Induced Oral Mucositis in Children with Cancer. Pathogens 2022, 11, 448. https://doi.org/10.3390/pathogens11040448
Triarico S, Agresti P, Rinninella E, Mele MC, Romano A, Attinà G, Maurizi P, Mastrangelo S, Ruggiero A. Oral Microbiota during Childhood and Its Role in Chemotherapy-Induced Oral Mucositis in Children with Cancer. Pathogens. 2022; 11(4):448. https://doi.org/10.3390/pathogens11040448
Chicago/Turabian StyleTriarico, Silvia, Pierpaolo Agresti, Emanuele Rinninella, Maria Cristina Mele, Alberto Romano, Giorgio Attinà, Palma Maurizi, Stefano Mastrangelo, and Antonio Ruggiero. 2022. "Oral Microbiota during Childhood and Its Role in Chemotherapy-Induced Oral Mucositis in Children with Cancer" Pathogens 11, no. 4: 448. https://doi.org/10.3390/pathogens11040448






