Antimicrobial Resistance and Extended-Spectrum Beta-Lactamase Genes in Enterobacterales, Pseudomonas and Acinetobacter Isolates from the Uterus of Healthy Mares
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. Subjects and Criteria Selection
2.3. Uterine Samples, Collection, Isolation, and Identification
2.4. Antimicrobial Susceptibility Testing
2.5. Data Analysis
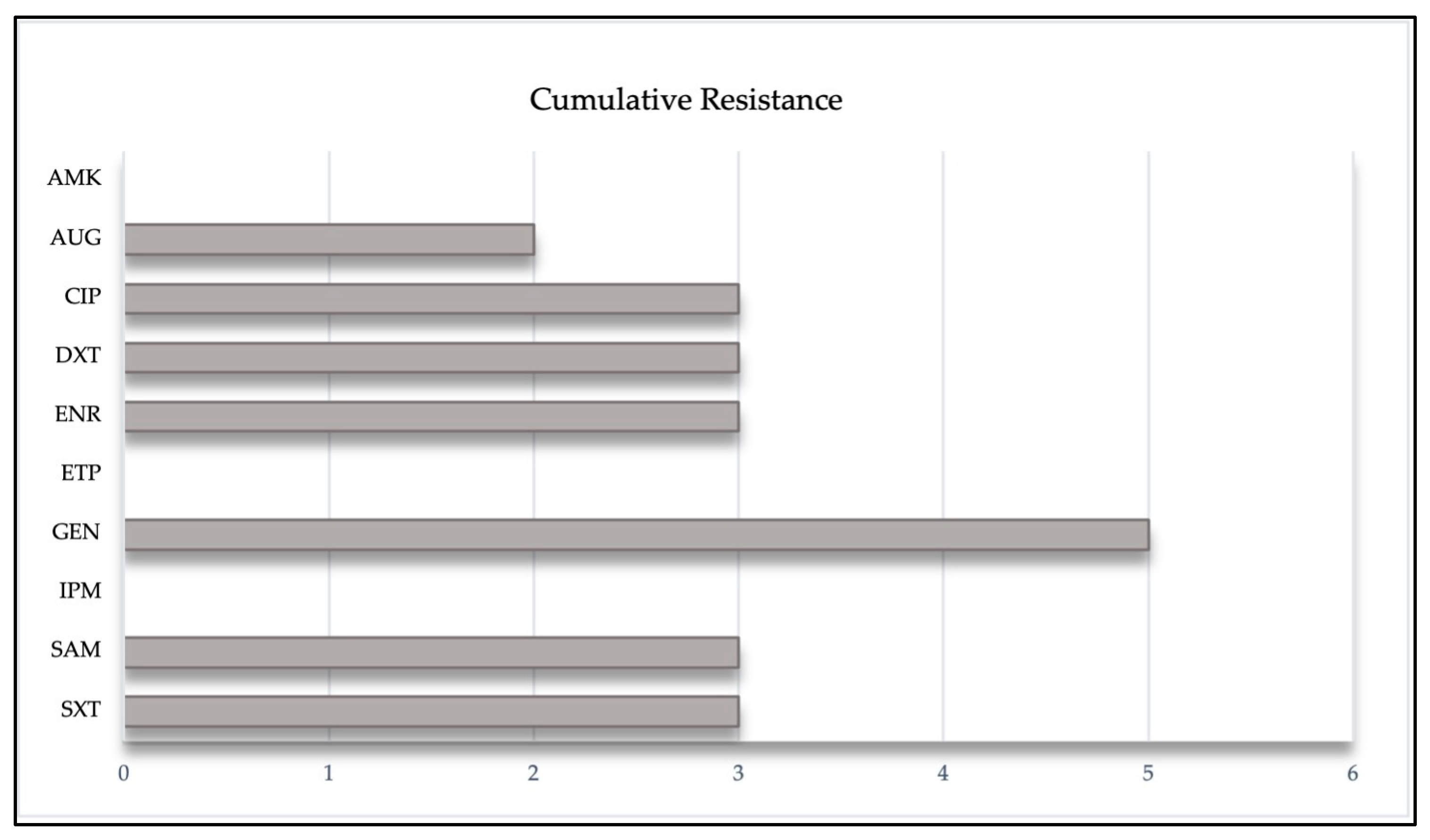
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rock, K.; Love, B.; DeSilva, U.; Rezabek, G.; Carrington, S.; Holyoak, G.; Carroll, B.; Gragg, D. Detectable differences in the endometrial microbiome between normal and susceptible mares using metagenomic profiling and conventional bacterial culture. In Proceedings of the Society of Theriogenology, Milwaukee, WI, USA, 11 August 2011. [Google Scholar]
- Akter, R.; El-Hage, C.M.; Sansom, F.M.; Carrick, J.; Devlin, J.M.; Legione, A.R. Metagenomic investigation of potential abortigenic pathogens in foetal tissues from Australian horses. BMC Genom. 2021, 22, 713. [Google Scholar] [CrossRef] [PubMed]
- Holyoak, G.R. The Equine Endometrial Microbiome: A Brief Review. Am. J. Biomed. Sci. Res. 2021, 11, 532–534. [Google Scholar] [CrossRef]
- Holyoak, G.R.; Premathilake, H.U.; Lyman, C.C.; Sones, J.L.; Gunn, A.; Wieneke, X.; DeSilva, U. The healthy equine uterus harbors a distinct core microbiome plus a rich and diverse microbiome that varies with geographical location. Sci. Rep. 2022, 12, 14790. [Google Scholar] [CrossRef] [PubMed]
- Thomson, P.; Pareja, J.; Núñez, A.; Santibáñez, R.; Castro, R. Characterization of microbial communities and predicted metabolic pathways in the uterus of healthy mares. Open Vet. J. 2022, 12, 797–805. [Google Scholar] [CrossRef]
- Wang, J.; Li, Z.; Ma, X.; Du, L.; Jia, Z.; Cui, X.; Yu, L.; Yang, J.; Xiao, L.; Zhang, B.; et al. Translocation of vaginal microbiota is involved in impairment and protection of uterine health. Nat. Commun. 2021, 12, 4191. [Google Scholar] [CrossRef] [PubMed]
- Sathe, S.; Leiken, A.; Plummer, P. Metagenomic sequencing of the uterine microbial environment during estrus and early pregnancy in mares. Clin. Theriogenol. 2017, 9, 453. [Google Scholar]
- Crha, I.; Ventruba, P.; Žáková, J.; Ješeta, M.; Pilka, R.; Lousová, E.; Papíková, Z. Uterine microbiome and endometrial receptivity. Ceska Gynekol. 2019, 84, 49–54. [Google Scholar]
- Heil, B.A.; Paccamonti, D.L.; Sones, J.L. Role for the mammalian female reproductive tract microbiome in pregnancy outcomes. Physiol. Genom. 2019, 51, 390–399. [Google Scholar] [CrossRef]
- Hemberg, E.; Lundeheim, N.; Einarsson, S. Retrospective study on vulvar conformation in relation to endometrial cytology and fertility in thoroughbred mares. J. Vet. Med. Ser. A Physiol. Pathol. Clin. Med. 2005, 52, 474–477. [Google Scholar] [CrossRef]
- Davis, H.A.; Stanton, M.B.; Thungrat, K.; Boothe, D.M. Uterine bacterial isolates from mares and their resistance to antimicrobials: 8296 cases (2003–2008). J. Am. Vet. Med. Assoc. 2013, 242, 977–983. [Google Scholar] [CrossRef]
- Leblanc, M.; Causey, R. Clinical and subclinical endometritis in the mare: Both threats to fertility. Reprod. Domest. Anim. 2009, 44, 10–22. [Google Scholar] [CrossRef] [PubMed]
- Katila, T. Evaluation of diagnostic methods in equine endometritis. Reprod. Biol. 2016, 16, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Troedsson, M.H.T.; Woodward, E.M. Our current understanding of the pathophysiology of equine endometritis with an emphasis on breeding-induced endometritis. Reprod. Biol. 2016, 16, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Frontoso, R.; De Carlo, E.; Pasolini, M.; van der Meulen, K.; Pagnini, U.; Iovane, G.; De Martino, L. Retrospective study of bacterial isolates and their antimicrobial susceptibilities in equine uteri during fertility problems. Res. Vet. Sci. 2008, 84, 1–6. [Google Scholar] [CrossRef]
- Benko, T.; Boldizar, M.; Novotny, F.; Hura, V.; Valocky, I.; Dudrikova, K.; Karamanova, M.; Petrovic, V. Incidence of bacterial pathogens in equine uterine swabs, their antibiotic resistance patterns, and selected reproductive indices in English thoroughbred mares during the foal heat cycle. Vet. Med. 2015, 60, 613–620. [Google Scholar] [CrossRef]
- Muñoz, P.C.M.; Sánchez, R.A.C. Estimación de la integridad uterina en yeguas Pura Raza Chilena y su asociación con edad y número de partos. Rev. Investig. Vet. Perú 2018, 29, 565–574. [Google Scholar] [CrossRef]
- Ferrer, M.S.; Palomares, R. Aerobic uterine isolates and antimicrobial susceptibility in mares with post-partum metritis. Equine Vet. J. 2018, 50, 202–207. [Google Scholar] [CrossRef]
- Ravaioli, V.; Raffini, E.; Tamburini, M.; Galletti, G.; Frasnelli, M. Infectious Endometritis in Mares: Microbiological Findings in Field Samples. J. Equine Vet. Sci. 2022, 112, 103913. [Google Scholar] [CrossRef]
- Goncagul, G.; Gocmen, H.; Yendim, S.K.; Yılmaz, K.; Intas, K.S. Bacteriologic and Cytologic Examination Results of Mares with Pneumovagina in Bursa Region. Int. J. Vet. Sci. 2016, 5, 295–298. [Google Scholar]
- Nocera, F.P.; Papulino, C.; Del Prete, C.; Palumbo, V.; Pasolini, M.P.; De Martino, L. Endometritis associated with Enterococcus casseliflavus in a mare: A case report. Asian Pac. J. Trop. Biomed. 2017, 7, 760–762. [Google Scholar] [CrossRef]
- Pisello, L.; Rampacci, E.; Stefanetti, V.; Beccati, F.; Hyatt, D.R.; Coletti, M.; Passamonti, F. Temporal efficacy of antimicrobials against aerobic bacteria isolated from equine endometritis: An Italian retrospective analysis (2010–2017). Vet. Rec. 2019, 185, 598. [Google Scholar] [CrossRef]
- Balamurugan, K.; Subapriya, S.; Dinesh, N.M.; Partheban, P. Antibiotic sensitivity test on pathogens causing reproductive tract infection in thoroughbred mares. J. Entomol. Zool. Stud. 2020, 8, 913–915. [Google Scholar]
- Wright, J.G.; Jung, S.; Holman, R.C.; Marano, N.N.; McQuiston, J.H. Infection control practices and zoonotic disease risks among veterinarians in the United States. J. Am. Vet. Med. Assoc. 2008, 232, 1863–1872. [Google Scholar] [CrossRef] [PubMed]
- Weese, J.S. Infection control in veterinary practice; the time is now. J. Small Anim. Pract. 2011, 52, 507–508. [Google Scholar] [CrossRef]
- Maddox, T.W.; Clegg, P.D.; Diggle, P.J.; Wedley, A.L.; Dawson, S.; Pinchbeck, G.L.; Williams, N.J. Cross-sectional study of antimicrobial-resistant bacteria in horses. Part 1: Prevalence of antimicrobial-resistant Escherichia coli and methicillin-resistant Staphylococcus aureus. Equine Vet. J. 2012, 44, 289–296. [Google Scholar] [CrossRef]
- Morley, P.S. Evidence-based infection control in clinical practice: If you buy clothes for the emperor, will he wear them? J. Vet. Intern. Med. 2013, 27, 430–438. [Google Scholar] [CrossRef]
- Walther, B.; Lübke-Becker, A.; Stamm, I.; Gehlen, H.; Barton, A.K.; Janßen, T.; Wieler, L.; Guenther, S. Fallbericht über vermutlich nosokomiale Infektionen durch multiresistente E. coli in einer pferdeklinik einschließlich extended-spectrum beta-lactamase (ESBL)-produzierender Isolate. Berl. Munch. Tierarztl. Wochenschr. 2014, 127, 11–12. [Google Scholar]
- Walther, B.; Tedin, K.; Lübke-Becker, A. Multidrug-resistant opportunistic pathogens challenging veterinary infection control. Vet. Microbiol. 2017, 200, 71–78. [Google Scholar] [CrossRef]
- Walther, B.; Klein, K.-S.; Barton, A.-K.; Semmler, T.; Huber, C.; Wolf, S.A.; Tedin, K.; Merle, R.; Mitrach, F.; Guenther, S.; et al. Extended-spectrum beta-lactamase (ESBL)producing Escherichia coli and Acinetobacter baumannii among horses entering a veterinary teaching hospital: The contemporary “Trojan Horse”. PLoS ONE 2018, 13, e0191873. [Google Scholar] [CrossRef]
- Perestrelo, S.; Correia Carreira, G.; Valentin, L.; Fischer, J.; Pfeifer, Y.; Werner, G.; Schmiedel, J.; Falgenhauer, L.; Imirzalioglu, C.; Chakraborty, T.; et al. Comparison of approaches for source attribution of ESBL-producing Escherichia coli in Germany. PLoS ONE 2022, 17, e0271317. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Guidelines for the Prevention and Control of Carbapenem-Resistant Enterobacteriaceae, Acinetobacter baumannii and Pseudomonas aeruginosa in Health Care Facilities; World Health Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Rajendran, N.B.; Mutters, N.; Marasca, G.; Conti, M.; Sifakis, F.; Vuong, C.; Voss, A.; Baño, J.; Tacconelli, E. Mandatory surveillance, and outbreaks reporting of the WHO priority pathogens for research & discovery of new antibiotics in European countries. Clin. Microbiol. Infect. 2020, 26, 943.e1–943.e6. [Google Scholar]
- Mancuso, G.; Midiri, A.; Gerace, E.; Biondo, C. Bacterial antibiotic resistance: The most critical pathogens. Pathogens 2021, 10, 1310. [Google Scholar] [CrossRef]
- Endimiani, A.; Hujer, K.M.; Hujer, A.M.; Bertschy, I.; Rossano, A.; Koch, C.; Gerber, V.; Francey, T.; Bonomo, R.A.; Perreten, V. Acinetobacter baumannii isolates from pets and horses in Switzerland: Molecular characterization and clinical data. J. Antimicrob. Chemother. 2011, 66, 2248–2254. [Google Scholar] [CrossRef] [PubMed]
- Müller, S.; Janßen, T.; Wieler, L.H. Multidrug resistant Acinetobacter baumannii in veterinary medicine-emergence of an underestimated pathogen? Multiresistente Acinetobacter baumannii in der Veterinär-medizin-Vormarsch eines unterschätzten Krankheits-erregers? Berl. Münch. Tierärztl. Wochenschr 2014, 127, 435–446. [Google Scholar] [PubMed]
- Van der Kolk, J.H.; Endimiani, A.; Graubner, C.; Gerber, V.; Perreten, V. Acinetobacter in veterinary medicine, with an emphasis on Acinetobacter baumannii. J. Glob. Antimicrob. Resist. 2019, 16, 59–71. [Google Scholar] [CrossRef] [PubMed]
- Maboni, G.; Seguel, M.; Lorton, A.; Sanchez, S. Antimicrobial resistance patterns of Acinetobacter spp. of animal origin reveal high rate of multidrug resistance. Vet. Microbiol. 2020, 245, 108702. [Google Scholar] [CrossRef] [PubMed]
- Isgren, C.M.; Williams, N.J.; Fletcher, O.D.; Timofte, D.; Newton, R.J.; Maddox, T.W.; Clegg, P.D.; Pinchbeck, G.L. Antimicrobial resistance in clinical bacterial isolates from horses in the UK. Equine Vet. J. 2022, 54, 390–414. [Google Scholar] [CrossRef] [PubMed]
- Anyanwu, M.U.; Jaja, I.F.; Nwobi, O.C.; Mgbeahuruike, A.C.; Ikpendu, C.N.; Okafor, N.A.; Oguttu, J.W. Epidemiology and Traits of Mobile Colistin Resistance (mcr) Gene-Bearing Organisms from Horses. Microorganisms 2022, 10, 1499. [Google Scholar] [CrossRef]
- Higuera-Llantén, S.; Vásquez-Ponce, F.; Barrientos-Espinoza, B.; Mardones, F.O.; Marshall, S.H.; Olivares-Pacheco, J. Extended antibiotic treatment in salmon farms select multiresistant gut bacteria with a high prevalence of antibiotic resistance genes. PLoS ONE 2018, 13, e0203641. [Google Scholar] [CrossRef]
- Tchamba, C.N.; Rao, A.; Boyen, F.; Haesebrouck, F.; Duprez, J.; Théron, L.; Thiry, D.; Mainil, J. Comparison of quantitative PCR and MALDI-TOF mass spectrometry assays for identification of bacteria in milk samples from cows with subclinical mastitis. J. Appl. Microbiol. 2019, 127, 683–692. [Google Scholar] [CrossRef]
- Uchida-Fujii, E.; Niwa, H.; Kinoshita, Y.; Nukada, T. Matrix-Assisted Laser Desorption Ionization–Time of Flight Mass Spectrometry (MALDI-TOF MS) for Identification of Bacterial Isolates from Horses. J. Equine Vet. Sci. 2020, 95, 103276. [Google Scholar] [CrossRef] [PubMed]
- Kolínská, R.; Španělová, P.; Dřevínek, M.; Hrabák, J.; Žemličková, H. Species identification of strains belonging to genus Citrobacter using the biochemical method and MALDI-TOF mass spectrometry. Folia Microbiol. 2015, 60, 53–59. [Google Scholar] [CrossRef]
- CLSI VET01S; Performance Standards for Antimicrobial Disk and Dilution Susceptibility Test for Bacteria Isolate from Animals. Clinical and Laboratory Standards Institute: Philadelphia, PA, USA, 2020.
- CLSI M100; Performance Standards for Antimicrobial Susceptibility Testing; Supplement M100. Clinical and Laboratory Standards Institute: Philadelphia, PA, USA, 2023.
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant, and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Bogaerts, P.; Rezende de Castro, R.; de Mendonça, R.; Huang, T.D.; Denis, O.; Glupczynski, Y. Validation of carbapenemase and extended-spectrum β-lactamase multiplex endpoint PCR assays according to ISO 15189. J. Antimicrob. Chemother. 2013, 68, 1576–1582. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Caxito, M.; Benavides, J.A.; Munita, J.M.; Rivas, L.; García, P.; Listoni, F.J.; Moreno-Switt, A.I.; Paes, A.C. Risk factors associated with faecal carriage of extended-spectrum cephalosporin-resistant Escherichia coli among dogs in Southeast Brazil. Prev. Vet. Med. 2021, 190, 105316. [Google Scholar] [CrossRef]
- Eisenberg, R.L.; Sotman, T.E.; Czum, J.M.; Montner, S.M.; Meyer, C.A. Prevalence of Burnout among Cardiothoracic Radiologists: Stress Factors, Career Satisfaction, and Modality-specific Imaging Volumes. J. Thorac. Imaging 2022, 37, 194–200. [Google Scholar] [CrossRef]
- Martin, B.D.; Schwab, E. Current Usage of Symbiosis and Associated Terminology. Int. J. Biol. 2012, 5, 32–54. [Google Scholar] [CrossRef]
- Nielsen, J.M.; Troedsson, M.H.; Pedersen, M.R.; Bojesen, A.M.; Lehn-Jensen, H.; Zent, W.W. Diagnosis of Endometritis in the Mare Based on Bacteriological and Cytological Examinations of the Endometrium: Comparison of Results Obtained by Swabs and Biopsies. J. Equine Vet. Sci. 2010, 30, 27–30. [Google Scholar] [CrossRef]
- Yang, X.; Cheng, G.; Li, C.; Yang, J.; Li, J.; Chen, D.; Zou, W.; Jin, S.; Zhang, H.; Li, D.; et al. The normal vaginal and uterine bacterial microbiome in giant pandas (Ailuropoda melanoleuca). Microbiol. Res. 2017, 199, 1–9. [Google Scholar] [CrossRef]
- Christoffersen, M.; Brandis, L.; Samuelsson, J.; Bojesen, A.M.; Troedsson, M.H.T.; Petersen, M.R. Diagnostic double-guarded low-volume uterine lavage in mares. Theriogenology 2015, 83, 222–227. [Google Scholar] [CrossRef]
- Gallego Rodríguez, R.S.; Ruiz Jaramillo, A.F.; Ruiz Buitrago, J.D. Frecuencia del aislamiento bacteriano y patrones de sensibilidad en yeguas criollas colombianas diagnosticadas con endometritis. Rev. Med. Vet. 2020, 1, 13–21. [Google Scholar] [CrossRef]
- Omar, H.; Hambidge, M.; Firmanes, B.; Shabandri, A.M.; Wilsher, S. Bacteria Isolated from Equine Uteri in the United Arab Emirates: A Retrospective Study. J. Equine Vet. Sci. 2022, 115, 104029. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, M.M.; Magsig, J.; Stromberg, A.J. Use of a low-volume uterine flush for diagnosing endometritis in chronically infertile mares. Theriogenology 2007, 68, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Hoyles, L.; Ortman, K.; Cardew, S.; Foster, G.; Rogerson, F.; Falsen, E. Corynebacterium uterequi sp. nov., a non-lipophilic bacterium isolated from urogenital samples from horses. Vet. Microbiol. 2013, 165, 469–474. [Google Scholar] [CrossRef]
- Verstraelen, H.; Vilchez-Vargas, R.; Desimpel, F.; Jauregui, R.; Vankeirsbilck, N.; Weyers, S.; Verhelst, R.; De Sutter, P.; Pieper, D.H.; Van De Wiele, T. Characterisation of the human uterine microbiome in non-pregnant women through deep sequencing of the V1-2 region of the 16S rRNA gene. PeerJ 2016, 4, e1602. [Google Scholar] [CrossRef]
- Moore, S.G.; Ericsson, A.C.; Poock, S.E.; Melendez, P.; Lucy, M.C. Hot topic: 16S rRNA gene sequencing reveals the microbiome of the virgin and pregnant bovine uterus. J. Dairy Sci. 2017, 100, 4953–4960. [Google Scholar] [CrossRef]
- DeSilva, U.; Lyman, C.C.; Holyoak, G.R.; Meinkoth, K.; Wieneke, X.; Chillemi, K.A. Canine endometrial and vaginal microbiomes reveal distinct and complex ecosystems. PLoS ONE 2019, 14, e0210157. [Google Scholar]
- Malaluang, P.; Wilén, E.; Lindahl, J.; Hansson, I.; Morrell, J.M. Antimicrobial Resistance in Equine Reproduction. Animals 2021, 11, 3035. [Google Scholar] [CrossRef]
- Díaz-Bertrana Sánchez, M.L. Estudio Microbiológico de Infertilidad en Yeguas; Universidad de Las Palmas de Gran Canaria: Las Palmas de Gran Canaria, Spain, 2013. [Google Scholar]
- Pyörälä, S.; Taponen, J.; Katila, T. Use of antimicrobials in the treatment of reproductive diseases in cattle and horses. Reprod. Domest. Anim. 2014, 49, 16–26. [Google Scholar] [CrossRef]
- Thomson, K.; Eskola, K.; Eklund, M.; Suominen, K.; Määttä, M.; Junnila, J.; Nykäsenoja, S.; Niinistö, K.; Grönthal, T.; Rantala, M. Characterisation of and risk factors for extended-spectrum β-lactamase producing Enterobacterales (ESBL-E) in an equine hospital with a special reference to an outbreak caused by Klebsiella pneumoniae ST307:CTX-M-1. Acta Vet. Scand. 2022, 64, 4. [Google Scholar] [CrossRef]
- Arafa, A.A.; Hedia, R.H.; Dorgham, S.M.; Ibrahim, E.S.; Bakry, M.A.; Abdalhamed, A.M.; Abuelnaga, A.S.M. Determination of extended-spectrum β-lactamase-producing Klebsiella pneumoniae isolated from horses with respiratory manifestation. Vet. World 2022, 15, 827–833. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, T.; Xu, L.; Wang, Q.; Li, H.; Wang, X. Extracellular superoxide produced by Enterococcus faecalis reduces endometrial receptivity via inflammatory injury. Am. J. Reprod. Immunol. 2021, 86, e13453. [Google Scholar] [CrossRef]
- Vaneechoutte, M.; Devriese, L.A.; Dijkshoorn, L.; Lamote, B.; Deprez, P.; Verschraegen, G.; Haesebrouck, F. Acinetobacter baumannii-infected vascular catheters collected from horses in an equine clinic. J. Clin. Microbiol. 2000, 38, 4280–4281. [Google Scholar] [CrossRef] [PubMed]
- Doi, Y.; Murray, G.L.; Peleg, A.Y. Acinetobacter baumannii: Evolution of antimicrobial resistance-treatment options. Semin. Respir. Crit. Care Med. 2015, 36, 85–98. [Google Scholar] [PubMed]
- Lupo, A.; Haenni, M.; Madec, J.-Y. Antimicrobial Resistance in Acinetobacter spp. and Pseudomonas spp. Microbiol. Spectr. 2018, 6, 377–393. [Google Scholar] [CrossRef]
- Köhne, M.; Tönissen, A.; Unruh, C.; Pruß, D.; Sieme, H. Occurrence of Intrauterine Purulent Concernments in a Maiden Mare-A Case Report. J. Equine Vet. Sci. 2020, 95, 103278. [Google Scholar] [CrossRef]
- Guidi, E.E.A.; Thomas, A.; Cadoré, J.L.; Smith, A.B. Citrobacter freundii induced endocarditis in a yearling colt. Can. Vet. J. 2016, 57, 767. [Google Scholar]
- Malaluang, P.; Wilén, E.; Frosth, S.; Lindahl, J.; Hansson, I.; Morrell, J.M. Vaginal Bacteria in Mares and the Occurrence of Antimicrobial Resistance. Microorganisms 2022, 10, 2204. [Google Scholar] [CrossRef]
- Rice, L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef]
- Bassetti, M.; Ginocchio, F.; Mikulska, M. New treatment options against gram-negative organisms. Crit. Care 2011, 15, 215. [Google Scholar] [CrossRef]
- Aslam, B.; Khurshid, M.; Arshad, M.I.; Muzammil, S.; Rasool, M.; Yasmeen, N.; Shah, T.; Chaudhry, T.H.; Rasool, M.H.; Shahid, A.; et al. Antibiotic Resistance: One Health One World Outlook. Front. Cell. Infect. Microbiol. 2021, 11, 1153. [Google Scholar] [CrossRef] [PubMed]
- González, C.; Moreno, L.; Fumuso, E.; García, J.; Rivulgo, M.; Confalonieri, A.; Sparo, M.; Bruni, S.S. Enrofloxacin-based therapeutic strategy for the prevention of endometritis in susceptible mares. J. Vet. Pharmacol. Ther. 2010, 33, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Dorph, K.; Haughan, J.; Robinson, M.; Redding, L.E. Critically important antimicrobials are frequently used on equine racetracks. J. Am. Vet. Med. Assoc. 2022, 260, 774–779. [Google Scholar] [CrossRef] [PubMed]
- Haenni, M.; Bour, M.; Châtre, P.; Madec, J.Y.; Plésiat, P.; Jeannot, K. Resistance of animal strains of Pseudomonas aeruginosa to carbapenems. Front. Microbiol. 2017, 8, 1847. [Google Scholar] [CrossRef] [PubMed]
- Petrina, M.A.B.; Cosentino, L.A.; Wiesenfeld, H.C.; Darville, T.; Hillier, S.L. Susceptibility of endometrial isolates recovered from women with clinical pelvic inflammatory disease or histological endometritis to antimicrobial agents. Anaerobe 2019, 56, 61–65. [Google Scholar] [CrossRef]
- Eliasi, U.L.; Sebola, D.; Oguttu, J.W.; Qekwana, D.N. Antimicrobial resistance patterns of Pseudomonas aeruginosa isolated from canine clinical cases at a veterinary academic hospital in South Africa. J. S. Afr. Vet. Assoc. 2020, 91, 6. [Google Scholar] [CrossRef]
- Dégi, J.; Moțco, O.-A.; Dégi, D.M.; Suici, T.; Mareș, M.; Imre, K.; Cristina, R.T. Antibiotic Antibiotic Susceptibility Profile of Pseudomonas aeruginosa Canine Isolates from a Multicentric Study in Romania. Antibiotics 2021, 10, 846. [Google Scholar] [CrossRef]
- Tragesser, L.A.; Wittum, T.E.; Funk, J.A.; Winokur, P.L.; Rajala-Schultz, P.J. Association between ceftiofur use and isolation of Escherichia coli with reduced susceptibility to ceftriaxone from fecal samples of dairy cows. Am. J. Vet. Res. 2006, 67, 1696–1700. [Google Scholar] [CrossRef]
- Elias, L.; Gillis, D.C.; Gurrola-Rodriguez, T.; Jeon, J.H.; Lee, J.H.; Kim, T.Y.; Lee, S.H.; Murray, S.A.; Ohta, N.; Scott, H.M.; et al. The Occurrence and Characterization of Extended-Spectrum-Beta-Lactamase-Producing Escherichia coli Isolated from Clinical Diagnostic Specimens of Equine Origin. Animals 2019, 10, 28. [Google Scholar] [CrossRef]
- Von Dollen, K.A.; Jones, M.; Beachler, T.; Harris, T.L.; Papich, M.G.; Lyle, S.K.; Bailey, C.S. Antimicrobial Activity of Ceftiofur and Penicillin with Gentamicin against Escherichia coli and Streptococcus equi Subspecies zooepidemicus in an Ex Vivo Model of Equine Postpartum Uterine Disease. J. Equine Vet. Sci. 2019, 79, 121–126. [Google Scholar] [CrossRef]
- Pradella, G.D.; Taschetto, P.M.; Duarte, C.A.; da Silva Azevedo, M.; Góss, G.C. Ceftiofur Side Effect in a Mare-Case Report. J. Equine Vet. Sci. 2020, 95, 103295. [Google Scholar] [CrossRef]
- Cantón, R.; Coque, T.M. The CTX-M beta-lactamase pandemic. Curr. Opin. Microbiol. 2006, 9, 466–475. [Google Scholar] [CrossRef]
- Athanasakopoulou, Z.; Reinicke, M.; Diezel, C.; Sofia, M.; Chatzopoulos, D.C.; Braun, S.D.; Reissig, A.; Spyrou, V.; Monecke, S.; Ehricht, R.; et al. Antimicrobial Resistance Genes in ESBL-Producing Escherichia coli Isolates from Animals in Greece. Antibiotics 2021, 10, 389. [Google Scholar] [CrossRef]
- Wongtawan, T.; Narinthorn, R.; Sontigun, N.; Sansamur, C.; Petcharat, Y.; Fungwithaya, P.; Saengsawang, P.; Blackall, P.J.; Thomrongsuwannakij, T. Characterizing the antimicrobial resistance profile of Escherichia coli found in sport animals (fighting cocks, fighting bulls, and sport horses) and soils from their environment. Vet. World 2022, 15, 2673–2680. [Google Scholar] [CrossRef] [PubMed]
- Samir, A.; Abdel-Moein, K.A.; Zaher, H.M. The Public Health Burden of Virulent Extended-Spectrum β-Lactamase-Producing Klebsiella pneumoniae Strains Isolated from Diseased Horses. Vector Borne Zoonotic Dis. 2022, 22, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Teshager, T.; Domínguez, L.; Moreno, M.A.; Saénz, Y.; Torres, C.; Cardeñosa, S. solation of an SHV-12 beta-lactamase-producing Escherichia coli strain from a dog with recurrent urinary tract infections. Antimicrob. Agents Chemother. 2000, 44, 3483–3484. [Google Scholar] [CrossRef] [PubMed]
- Sukmawinata, E.; Uemura, R.; Sato, W.; Thu Htun, M.; Sueyoshi, M. Multidrug-Resistant ESBL/AmpC-Producing Klebsiella pneumoniae Isolated from Healthy Thoroughbred Racehorses in Japan. Animals 2020, 10, 369. [Google Scholar] [CrossRef] [PubMed]
- Meijs, A.P.; Gijsbers, E.F.; Hengeveld, P.D.; Dierikx, C.M.; de Greeff, S.C.; van Duijkeren, E. ESBL/pAmpC-producing Escherichia coli and Klebsiella pneumoniae carriage among veterinary healthcare workers in the Netherlands. Antimicrob Resist. Infect. Control 2021, 10, 147. [Google Scholar] [CrossRef] [PubMed]
- Johansson, V.; Nykäsenoja, S.; Myllyniemi, A.L.; Rossow, H.; Heikinheimo, A. Genomic characterization of ESBL/AmpC-producing and high-risk clonal lineages of Escherichia coli and Klebsiella pneumoniae in imported dogs with shelter and stray background. J. Glob. Antimicrob. Resist. 2022, 30, 183–190. [Google Scholar] [CrossRef]
- Nossair, M.A.; Abd El Baqy, F.A.; Rizk, M.S.Y.; Elaadli, H.; Mansour, A.M.; Abd El-Aziz, A.H.; Alkhedaide, A.; Soliman, M.M.; Ramadan, H.; Shukry, M.; et al. Prevalence and Molecular Characterization of Extended-Spectrum β-Lactamases and AmpC β-lactamase-Producing Enterobacteriaceae among Human, Cattle, and Poultry. Pathogens 2022, 11, 852. [Google Scholar] [CrossRef]
- Veloo, Y.; Thahir, S.S.A.; Rajendiran, S.; Hock, L.K.; Ahmad, N.; Muthu, V.; Shaharudin, R. Multidrug-Resistant Gram-Negative Bacteria and Extended-Spectrum β-Lactamase-Producing Klebsiella pneumoniae from the Poultry Farm Environment. Microbiol Spectr. 2022, 10, e0269421. [Google Scholar] [CrossRef] [PubMed]
- Leinyuy, J.F.; Ali, I.M.; Ousenu, K.; Tume, C.B. Molecular characterization of antimicrobial resistance related genes in E. coli, Salmonella and Klebsiella isolates from broilers in the West Region of Cameroon. PLoS ONE. 2023, 18, e0280150. [Google Scholar] [CrossRef]
- Shnaiderman-Torban, A.; Paitan, Y.; Arielly, H.; Kondratyeva, K.; Tirosh-Levy, S.; Abells-Sutton, G.; Navon-Venezia, S.; Steinman, A. Extended-Spectrum β-Lactamase-Producing Enterobacteriaceae in Hospitalized Neonatal Foals: Prevalence, Risk Factors for Shedding and Association with Infection. Animals 2019, 9, 600. [Google Scholar] [CrossRef] [PubMed]
- Damborg, P.; Marskar, P.; Baptiste, K.E.; Guardabassi, L. Faecal shedding of CTX-M-producing Escherichia coli in horses receiving broad-spectrum antimicrobial prophylaxis after hospital admission. Vet. Microbiol. 2012, 154, 298–304. [Google Scholar] [CrossRef]
- Maddox, T.W.; Williams, N.J.; Clegg, P.D.; O’Donnell, A.J.; Dawson, S.; Pinchbeck, G.L. Longitudinal study of antimicrobial-resistant commensal Escherichia coli in the faeces of horses in an equine hospital. Prev. Vet. Med. 2011, 100, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Adler, A.; Sturlesi, N.A.; Fallach, N.; Zilberman-Barzilai, D.; Hussein, O.; Blum, S.E.; Klement, E.; Schwaber, M.J.; Carmeli, Y. Prevalence, Risk Factors, and Transmission Dynamics of Extended-Spectrum-β-Lactamase-Producing Enterobacteriaceae: A National Survey of Cattle Farms in Israel in 2013. J. Clin. Microbiol. 2015, 53, 3515–3521. [Google Scholar] [CrossRef] [PubMed]
- Jawad, A.; Heritage, J.; Snelling, A.M.; Gascoyne-Binzi, D.M.; Hawkey, P.M. Influence of relative humidity and suspending menstrua on survival of Acinetobacter spp. on dry surfaces. J. Clin. Microbiol. 1996, 34, 2881–2887. [Google Scholar] [CrossRef]
- Kramer, A.; Schwebke, I.; Kampf, G. How long do nosocomial pathogens persist on inanimate surfaces? A systematic review. BMC Infect. Dis. 2006, 6, 130. [Google Scholar] [CrossRef]
- Winters, A.D.; Romero, R.; Gervasi, M.T.; Gomez-Lopez, N.; Tran, M.R.; Garcia-Flores, V.; Pacora, P.; Jung, E.; Hassan, S.S.; Hsu, C.D.; et al. Does the endometrial cavity have a molecular microbial signature? Sci. Rep. 2019, 9, 9905. [Google Scholar] [CrossRef]
- Neely, A.N. A survey of gram-negative bacteria survival on hospital fabrics and plastics. J. Burn. Care Rehabil. 2000, 21, 523–527. [Google Scholar] [CrossRef]
- Bălan, G.G.; Roșca, I.; Ursu, E.-L.; Doroftei, F.; Bostănaru, A.-C.; Hnatiuc, E.; Năstasă, V.; Șandru, V.; Ștefănescu, G.; Trifan, A.; et al. Plasma-activated water: A new and effective alternative for duodenoscope reprocessing. Infect. Drug Resist. 2018, 11, 727–733. [Google Scholar] [CrossRef] [PubMed]

| β-Lactamase Gene | Primer Sequence (5′ → 3′) | Product Length (bp) | T° Annealing |
|---|---|---|---|
| blaCTX-M | MA1: SCSATGTGCAGYACCAGTAA | 554 | 57 °C |
| MA2: CCGCRATATGRTTGGTGGTG | |||
| blaSHV | SHV F: TCGGCCTTCACTCAAGGATG | 785 | 57 °C |
| SHV R: ATGCCGCCGCCAGTCATATC | |||
| blaTEM | TEM F: TTAGACGTCAGGTGGCACTT | 972 | 52 °C |
| TEM R: GGACCGGAGTTACCAATGCT | |||
| blaPER | PER F: AAAGAGCAAATTGAATCCATAGTC | 835 | 57 °C |
| PER R: GTTAATTTGGGCTTAGGGCAG | |||
| blaGES | GES F: ATGCGCTTCATTCACGCAC | 864 | 57 °C |
| GES R: CTATTTGTCCGTGCTCAGG |
| Isolate | Specific Antibiotic Resistance |
|---|---|
| Acinetobacter lwoffii | AMP |
| Acinetobacter lwoffii | - |
| Acinetobacter spp. | - |
| Acinetobacter spp. | AMP |
| Acinetobacter spp. | AMP |
| Acinetobacter spp. | AMP |
| Acinetobacter spp. | - |
| Acinetobacter spp. | - |
| Acinetobacter johnsonii | - |
| Citrobacter spp. | CAZ-SAM-CRO |
| Enterobacter cloacae | CAZ-GM-CRO |
| Enterobacter cloacae | SAM-CIP-SXT-GM-CRO-ENR |
| Enterobacter spp. | CAZ-GM |
| Escherichia coli | CAZ-CIP-SXT-GM-CRO-AMP-ENR-AUG-DXT |
| Escherichia coli | DXT |
| Escherichia coli | - |
| Escherichia coli | - |
| Escherichia coli | - |
| Escherichia coli | - |
| Escherichia coli | - |
| Escherichia coli | - |
| Escherichia coli | - |
| Escherichia coli | - |
| Escherichia coli | - |
| Escherichia coli | - |
| Escherichia coli | - |
| Escherichia coli | - |
| Escherichia coli | - |
| Escherichia coli | - |
| Escherichia coli | - |
| Klebsiella pneumoniae | CAZ-SAM-CIP-SXT-GM-CRO-ENR-AUG-DXT |
| Pseudomonas spp. | - |
| Species | ESBL | bla Genes |
|---|---|---|
| Acinetobacter lwoffii | − | − |
| Acinetobacter spp. | + | blaSHV |
| Acinetobacter spp. | + | − |
| Acinetobacter spp. | + | − |
| Citrobacter sp. | + | blaCTX-M, blaTEM, blaSHV |
| Enterobacter cloacae | − | − |
| Enterobacter cloacae | − | − |
| Escherichia coli | + | blaSHV |
| Klebsiella pneumoniae | + | blaCTX-M, blaTEM, blaSHV |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thomson, P.; García, P.; Río, C.d.; Castro, R.; Núñez, A.; Miranda, C. Antimicrobial Resistance and Extended-Spectrum Beta-Lactamase Genes in Enterobacterales, Pseudomonas and Acinetobacter Isolates from the Uterus of Healthy Mares. Pathogens 2023, 12, 1145. https://doi.org/10.3390/pathogens12091145
Thomson P, García P, Río Cd, Castro R, Núñez A, Miranda C. Antimicrobial Resistance and Extended-Spectrum Beta-Lactamase Genes in Enterobacterales, Pseudomonas and Acinetobacter Isolates from the Uterus of Healthy Mares. Pathogens. 2023; 12(9):1145. https://doi.org/10.3390/pathogens12091145
Chicago/Turabian StyleThomson, Pamela, Patricia García, Camila del Río, Rodrigo Castro, Andrea Núñez, and Carolina Miranda. 2023. "Antimicrobial Resistance and Extended-Spectrum Beta-Lactamase Genes in Enterobacterales, Pseudomonas and Acinetobacter Isolates from the Uterus of Healthy Mares" Pathogens 12, no. 9: 1145. https://doi.org/10.3390/pathogens12091145
APA StyleThomson, P., García, P., Río, C. d., Castro, R., Núñez, A., & Miranda, C. (2023). Antimicrobial Resistance and Extended-Spectrum Beta-Lactamase Genes in Enterobacterales, Pseudomonas and Acinetobacter Isolates from the Uterus of Healthy Mares. Pathogens, 12(9), 1145. https://doi.org/10.3390/pathogens12091145








