A Flow Cytometry Study of the Binding and Stimulation Potential of Inactivated Trypanosoma evansi toward Dromedary Camel Leukocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animal Use and Blood Sample Collection
2.2. Separation of Mononuclear Cells from Camel Blood
2.3. Separation of Granulocytes from Camel Blood
2.4. Labeling of T. evansi with Propidium Iodide
2.5. In Vitro Stimulation of Camel Neutrophils and PBMCs with T. evansi
2.6. Analysis of Stimulation-induced Shape Change in Neutrophils
2.7. Generation of Reactive Oxygen Species (ROS)
2.8. Cell Vitality Assay
2.9. Statistical Analyses
3. Results
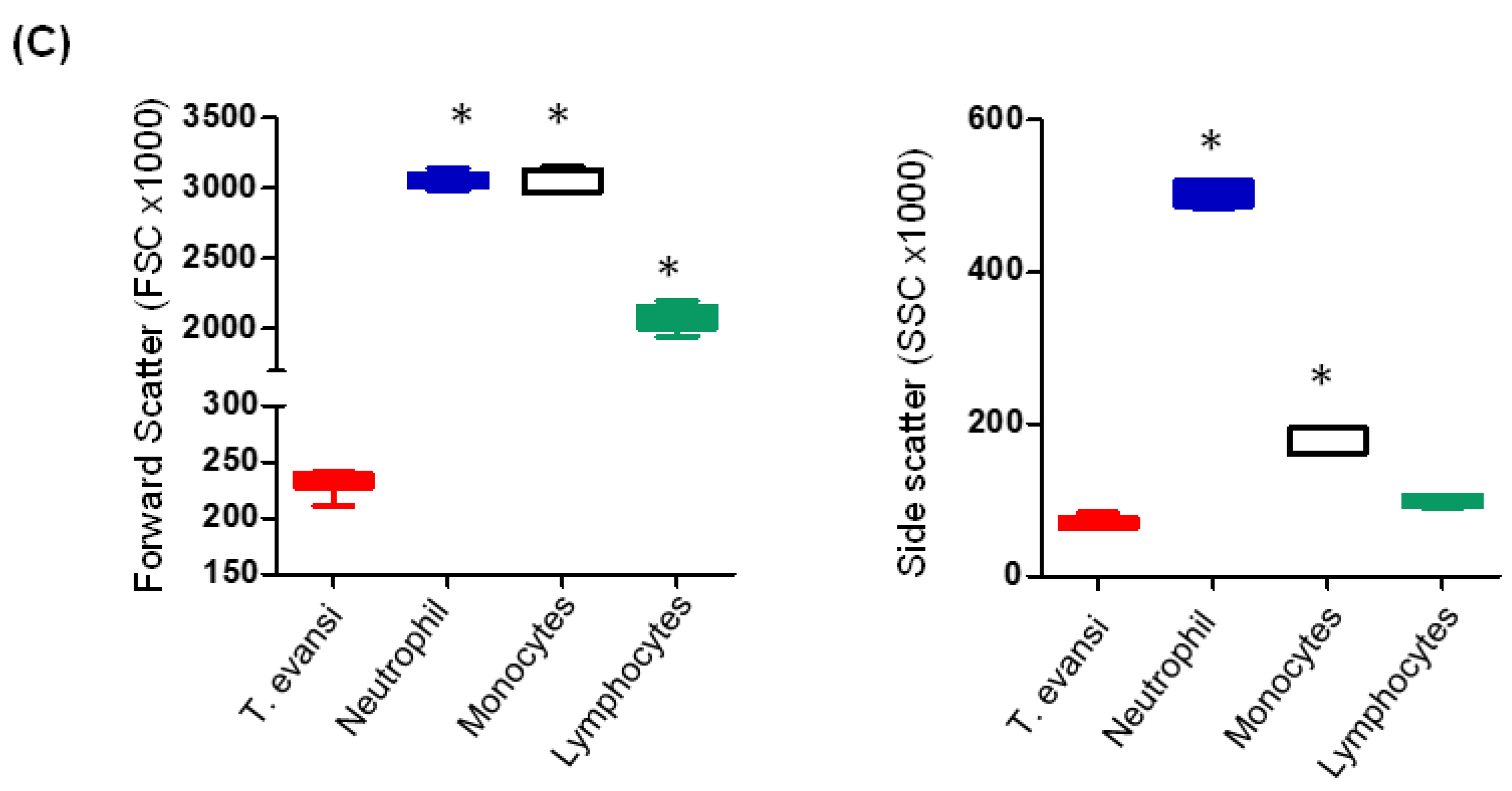
3.1. Flow Cytometric Light Scatter Properties of T. evansi
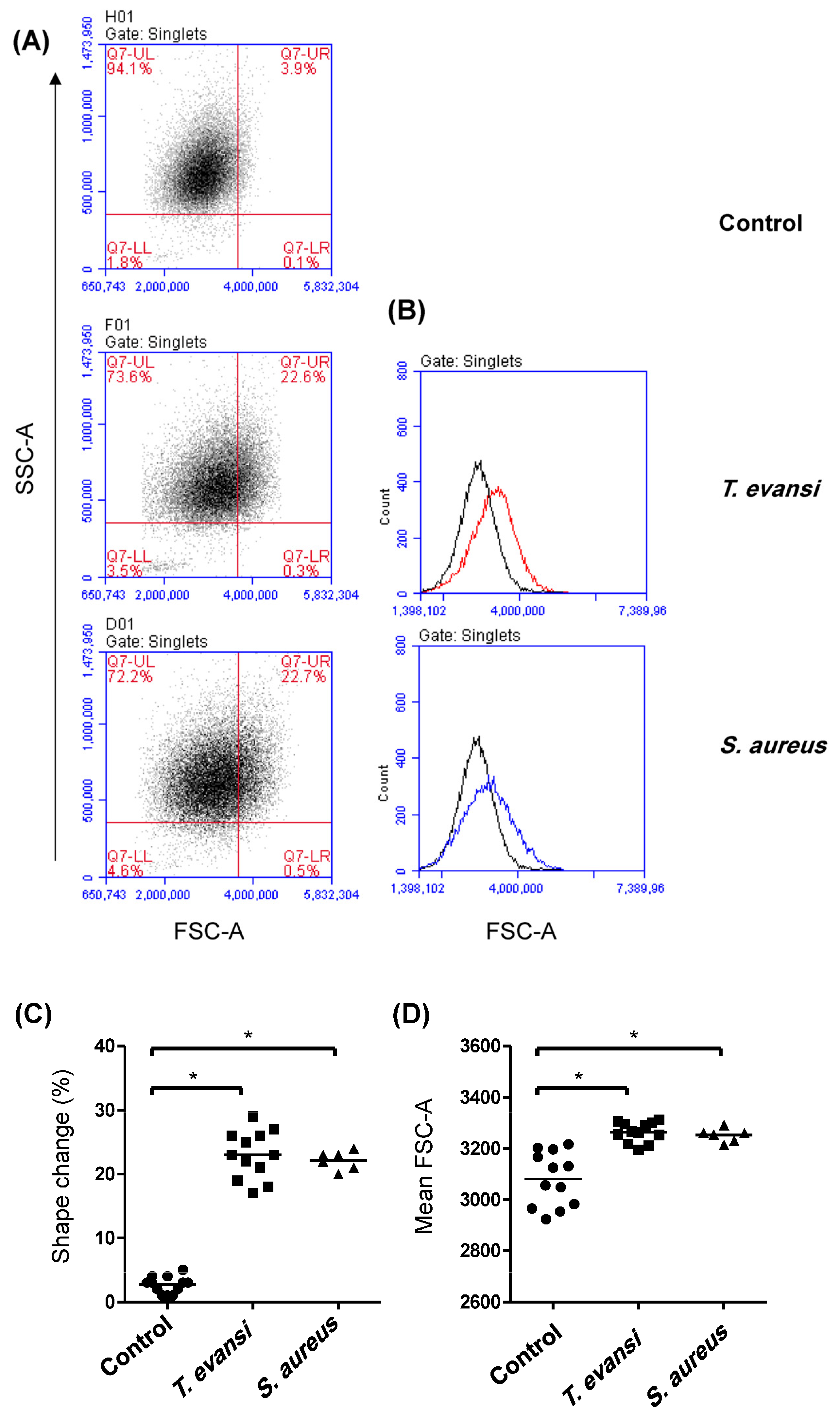
3.2. Binding Potential of T. evansi to Camel Granulocytes and Mononuclear Cells
3.3. Stimulation Potential of T. evansi on Camel Neutrophils
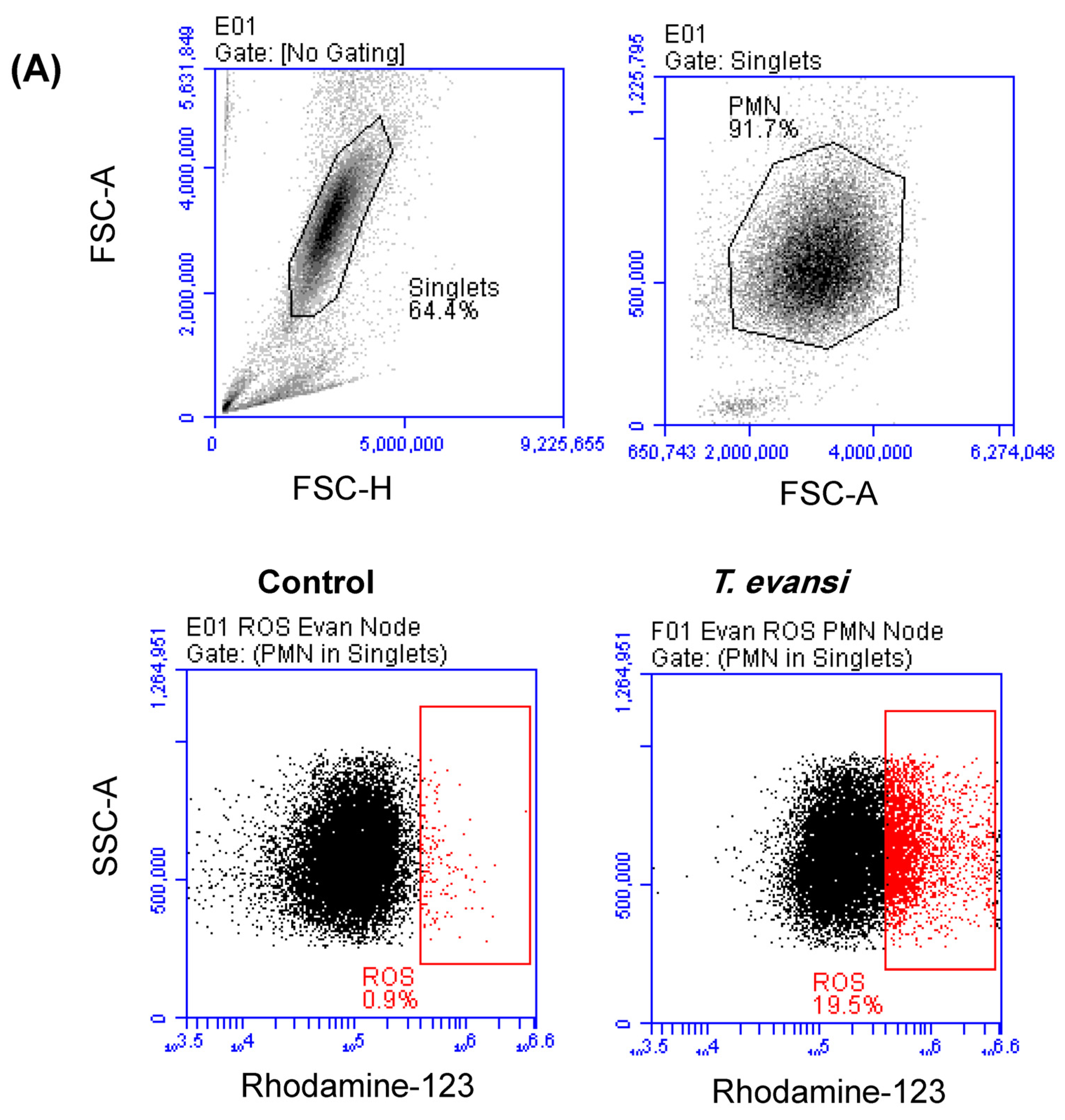
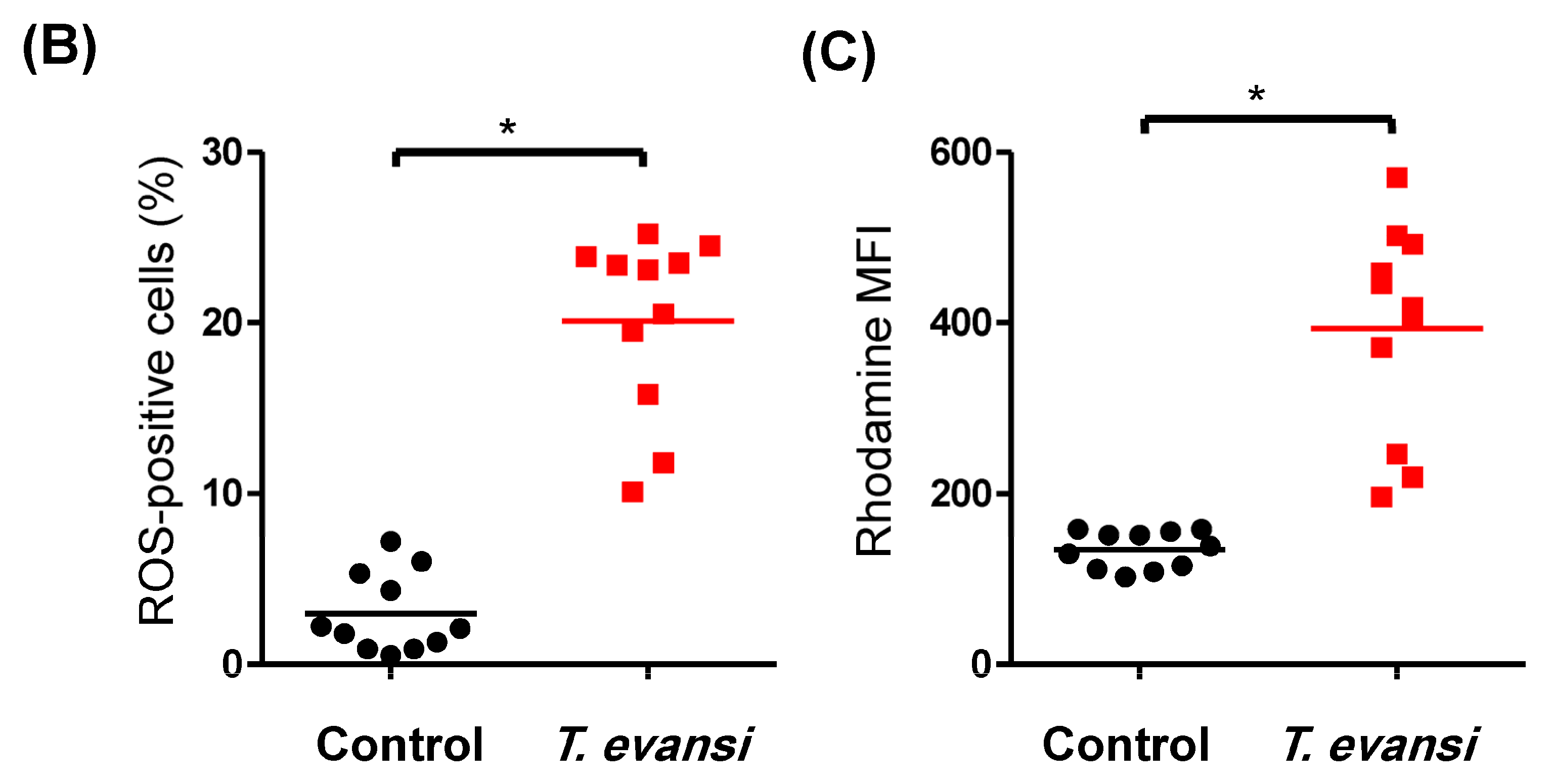
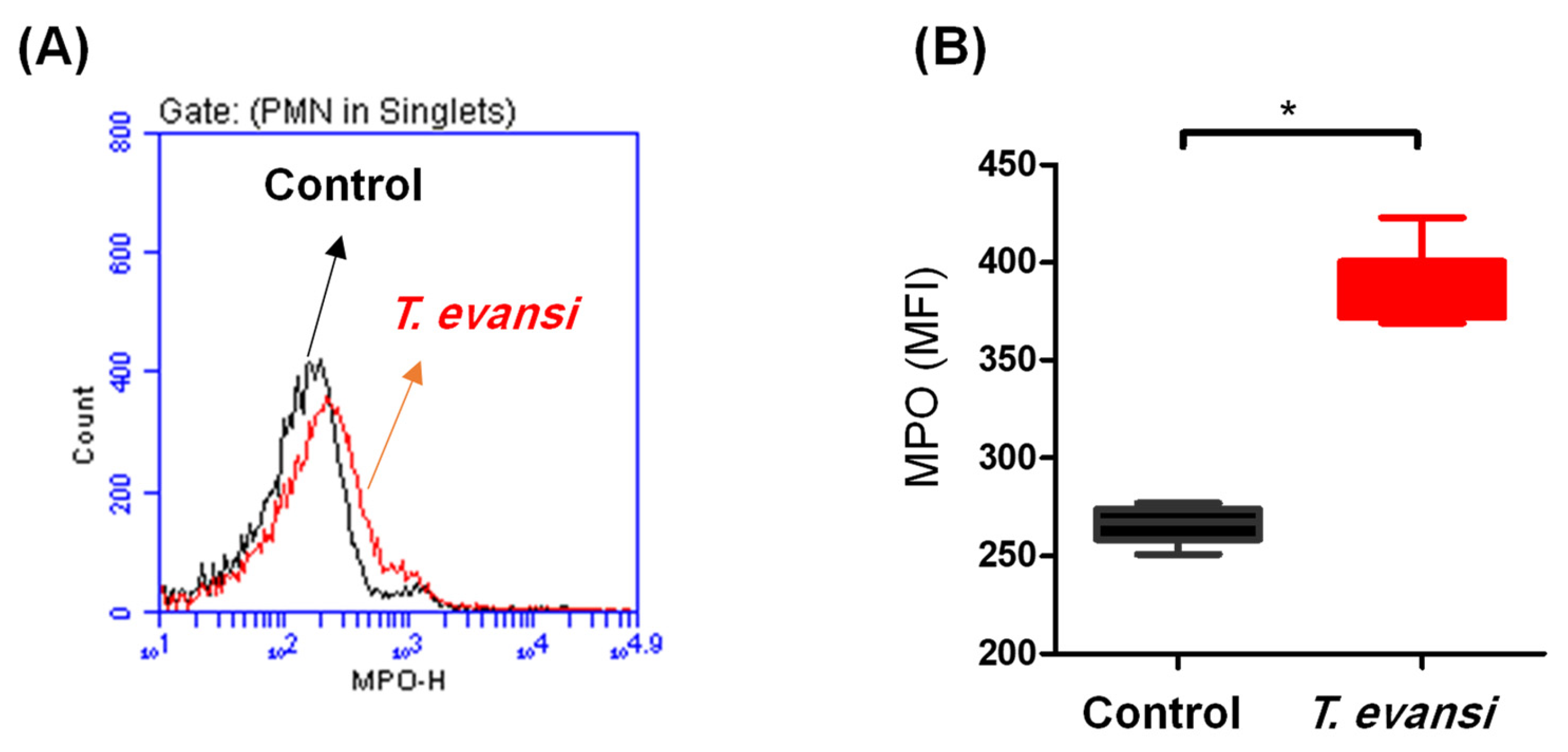
3.4. In Vitro Stimulation with T. evansi-Induced ROS Production in Camel Neutrophils
3.5. In Vitro Stimulation with T. evansi-Induced Neutrophil Extracellular Traps (NET)-Formation in Camel Neutrophils
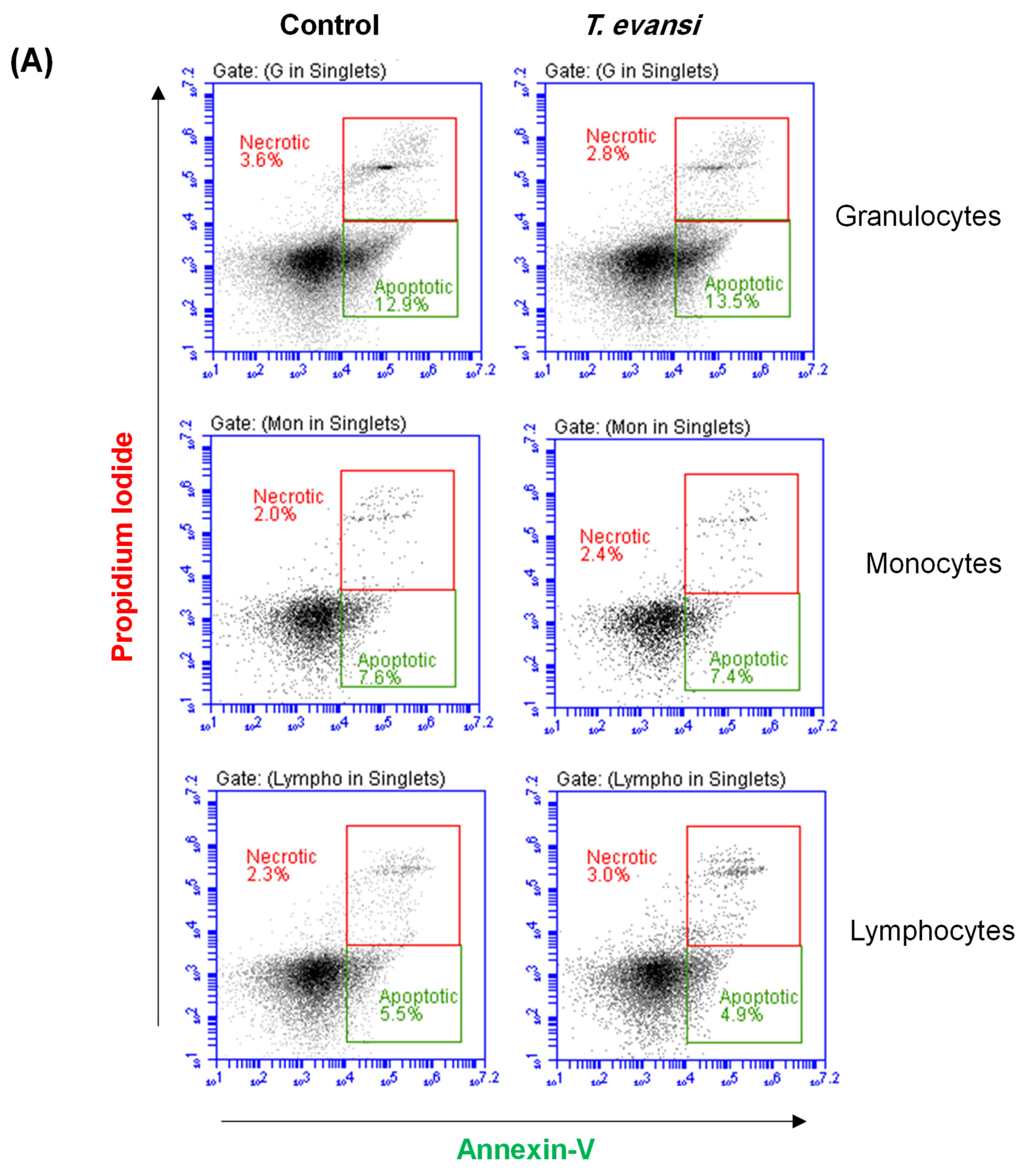
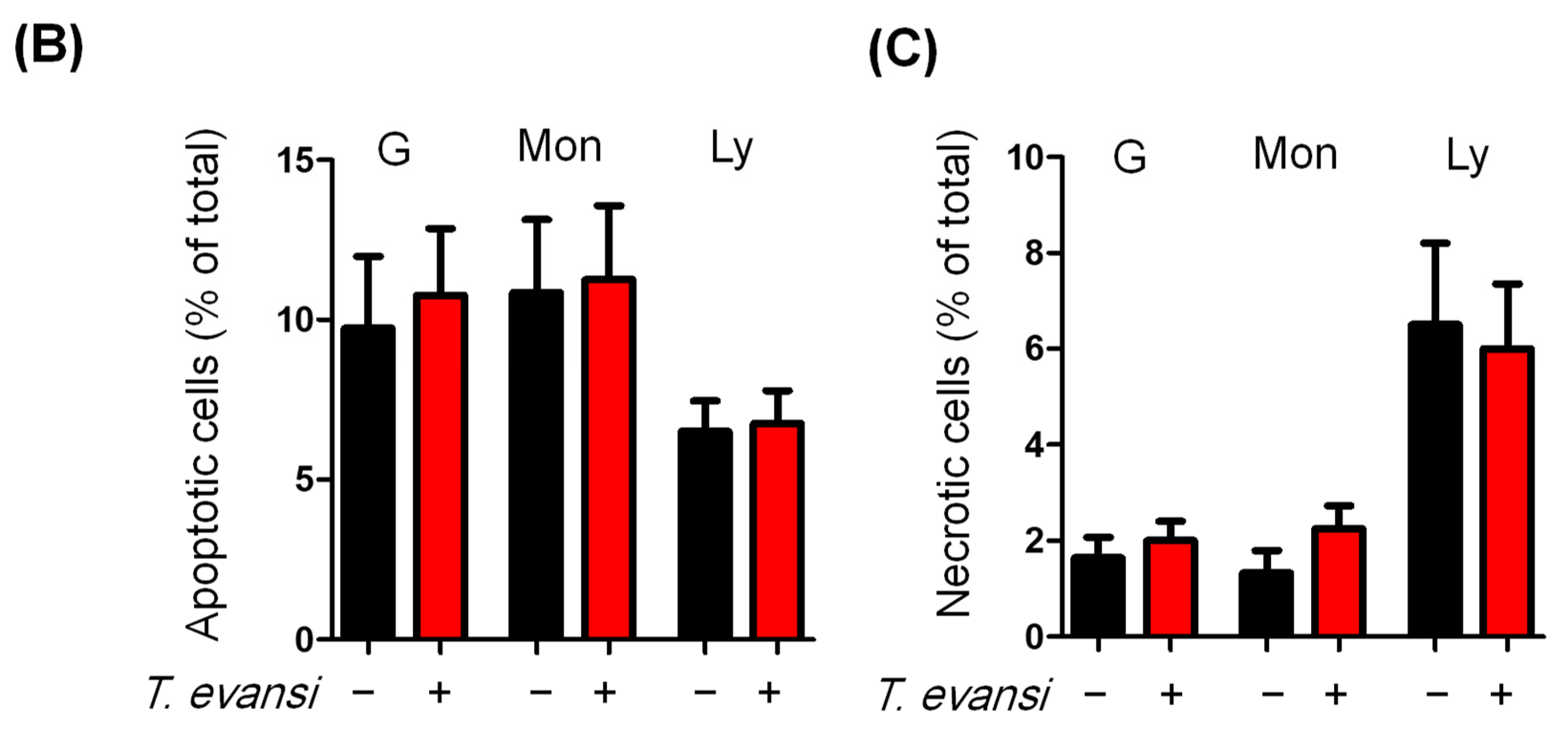
3.6. Stimulation with T. evansi Did Not Impact the Vitality of Camel Neutrophils
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Moreira, R.S.; Calomeno, N.A.; das Neves, G.B.; do Nascimento, L.F.N.; Filho, V.B.; Wagner, G.; Miletti, L.C. Trypanosoma evansi secretome carries potential biomarkers for Surra diagnosis. J. Proteom. 2023, 272, 104789. [Google Scholar] [CrossRef] [PubMed]
- Diall, O.; Desquesnes, M.; Faye, B.; Dia, M.L.; Jacquiet, P.; Sazmand, A.; Otranto, D.; Touratier, L. Development of a control strategy towards elimination of Trypanosoma evansi infection (surra) in camels in Africa. Acta Trop. 2022, 234, 106583. [Google Scholar] [CrossRef] [PubMed]
- Birhanu, H.; Gebrehiwot, T.; Goddeeris, B.M.; Buscher, P.; Van Reet, N. New Trypanosoma evansi Type B Isolates from Ethiopian Dromedary Camels. PLoS Negl. Trop. Dis. 2016, 10, e0004556. [Google Scholar] [CrossRef] [PubMed]
- Hunter, A.G. Urine odour in a camel suffering from surra (T. evansi infection). Trop. Anim. Health Prod. 1986, 18, 146–148. [Google Scholar] [CrossRef] [PubMed]
- Hassan-Kadle, A.A.; Ibrahim, A.M.; Nyingilili, H.S.; Yusuf, A.A.; Vieira, T.; Vieira, R.F.C. Parasitological, serological and molecular survey of camel trypanosomiasis in Somalia. Parasit. Vectors 2019, 12, 598. [Google Scholar] [CrossRef] [PubMed]
- Khalafalla, A.I. Zoonotic diseases transmitted from the camels. Front. Vet. Sci. 2023, 10, 1244833. [Google Scholar] [CrossRef] [PubMed]
- Ranking, G. Horse Surra. Ind. Med. Gaz. 1893, 28, 430. [Google Scholar]
- Salah, A.A.; Robertson, I.; Mohamed, A. Estimating the economic impact of Trypanosoma evansi infection on production of camel herds in Somaliland. Trop. Anim. Health Prod. 2015, 47, 707–714. [Google Scholar] [CrossRef]
- Desquesnes, M.; Gonzatti, M.; Sazmand, A.; Thevenon, S.; Bossard, G.; Boulange, A.; Gimonneau, G.; Truc, P.; Herder, S.; Ravel, S.; et al. A review on the diagnosis of animal trypanosomoses. Parasit. Vectors 2022, 15, 64. [Google Scholar] [CrossRef]
- Lai, D.H.; Hashimi, H.; Lun, Z.R.; Ayala, F.J.; Lukes, J. Adaptations of Trypanosoma brucei to gradual loss of kinetoplast DNA: Trypanosoma equiperdum and Trypanosoma evansi are petite mutants of T. brucei. Proc. Natl. Acad. Sci. USA 2008, 105, 1999–2004. [Google Scholar] [CrossRef]
- Carnes, J.; Anupama, A.; Balmer, O.; Jackson, A.; Lewis, M.; Brown, R.; Cestari, I.; Desquesnes, M.; Gendrin, C.; Hertz-Fowler, C.; et al. Genome and phylogenetic analyses of Trypanosoma evansi reveal extensive similarity to T. brucei and multiple independent origins for dyskinetoplasty. PLoS Negl. Trop. Dis. 2015, 9, e3404. [Google Scholar] [CrossRef]
- Kocher, A.; Desquesnes, M.; Kamyingkird, K.; Yangtara, S.; Leboucher, E.; Rodtian, P.; Dargantes, A.; Jittapalapong, S. Evaluation of an Indirect-ELISA Test for Trypanosoma evansi Infection (Surra) in Buffaloes and Its Application to a Serological Survey in Thailand. Biomed. Res. Int. 2015, 2015, 361037. [Google Scholar] [CrossRef] [PubMed]
- Desquesnes, M.; Sazmand, A.; Gonzatti, M.; Boulange, A.; Bossard, G.; Thevenon, S.; Gimonneau, G.; Truc, P.; Herder, S.; Ravel, S.; et al. Diagnosis of animal trypanosomoses: Proper use of current tools and future prospects. Parasit. Vectors 2022, 15, 235. [Google Scholar] [CrossRef] [PubMed]
- Faye, B. How many large camelids in the world? A synthetic analysis of the world camel demographic changes. Pastoralism 2020, 10, 25. [Google Scholar] [CrossRef]
- Blackwell, P.J. East Africa’s pastoralist emergency: Is climate change the straw that breaks the camel’s back? Third World Q. 2010, 31, 1321–1338. [Google Scholar] [CrossRef] [PubMed]
- Hussen, J.; Schuberth, H.J. Recent Advances in Camel Immunology. Front. Immunol. 2020, 11, 614150. [Google Scholar] [CrossRef]
- Hussen, J.; Alkuwayti, M.A.; Falemban, B.; Al-Sukruwah, M.A.; Alhojaily, S.M.; Humam, N.A.A.; Adwani, S.A. Immunomodulatory Effects of Bacterial Toll-like Receptor Ligands on the Phenotype and Function of Milk Immune Cells in Dromedary Camel. Biology 2023, 12, 276. [Google Scholar] [CrossRef]
- Soehnlein, O.; Lindbom, L. Phagocyte partnership during the onset and resolution of inflammation. Nat. Rev. Immunol. 2010, 10, 427–439. [Google Scholar] [CrossRef]
- McDaniel, M.M.; Meibers, H.E.; Pasare, C. Innate control of adaptive immunity and adaptive instruction of innate immunity: Bi-directional flow of information. Curr. Opin. Immunol. 2021, 73, 25–33. [Google Scholar] [CrossRef]
- Stijlemans, B.; Caljon, G.; Van Den Abbeele, J.; Van Ginderachter, J.A.; Magez, S.; De Trez, C. Immune Evasion Strategies of Trypanosoma brucei within the Mammalian Host: Progression to Pathogenicity. Front. Immunol. 2016, 7, 233. [Google Scholar] [CrossRef]
- Magez, S.; Pinto Torres, J.E.; Obishakin, E.; Radwanska, M. Infections With Extracellular Trypanosomes Require Control by Efficient Innate Immune Mechanisms and Can Result in the Destruction of the Mammalian Humoral Immune System. Front. Immunol. 2020, 11, 382. [Google Scholar] [CrossRef] [PubMed]
- Harris, T.H.; Mansfield, J.M.; Paulnock, D.M. CpG oligodeoxynucleotide treatment enhances innate resistance and acquired immunity to African trypanosomes. Infect. Immun. 2007, 75, 2366–2373. [Google Scholar] [CrossRef] [PubMed]
- Radwanska, M.; Guirnalda, P.; De Trez, C.; Ryffel, B.; Black, S.; Magez, S. Trypanosomiasis-induced B cell apoptosis results in loss of protective anti-parasite antibody responses and abolishment of vaccine-induced memory responses. PLoS Pathog. 2008, 4, e1000078. [Google Scholar] [CrossRef] [PubMed]
- Al-Harrasi, M.; Al-Habsi, K.; Ali, H.; Al-Marzooqi, W.; Al-Kharousi, K.; Al-Hamrashdi, A.; El-Tahir, H.A.; Elshafie, E.I. Circulation of Trypanosoma evansi antibodies and risk variables among dromedary camels in Al Batinah governorates, Sultanate of Oman. Vet. Parasitol. Reg. Stud. Rep. 2023, 40, 100863. [Google Scholar] [CrossRef] [PubMed]
- Habeeba, S.; Khan, R.A.; Zackaria, H.; Yammahi, S.; Mohamed, Z.; Sobhi, W.; AbdelKader, A.; Alhosani, M.A.; Muhairi, S.A. Comparison of Microscopy, Card Agglutination Test for Trypanosoma evansi, and Real-time PCR in The Diagnosis of Trypanosomosis in Dromedary Camels of The Abu Dhabi Emirate, UAE. J. Vet. Res. 2022, 66, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Hussen, J.; Duvel, A.; Koy, M.; Schuberth, H.J. Inflammasome activation in bovine monocytes by extracellular ATP does not require the purinergic receptor P2X7. Dev. Comp. Immunol. 2012, 38, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Barr, D.A.; Omollo, C.; Mason, M.; Koch, A.; Wilkinson, R.J.; Lalloo, D.G.; Meintjes, G.; Mizrahi, V.; Warner, D.F.; Davies, G. Flow cytometry method for absolute counting and single-cell phenotyping of mycobacteria. Sci. Rep. 2021, 11, 18661. [Google Scholar] [CrossRef]
- McGrath, J.S.; Honrado, C.; Spencer, D.; Horton, B.; Bridle, H.L.; Morgan, H. Analysis of Parasitic Protozoa at the Single-cell Level using Microfluidic Impedance Cytometry. Sci. Rep. 2017, 7, 2601. [Google Scholar] [CrossRef]
- Hussen, J.; Alkuwayti, M.A.; Falemban, B.; Alhojaily, S.M.; Adwani, S.A.; Hassan, E.A.E.; Al-Mubarak, A.I. Impact of Selected Bacterial and Viral Toll-like Receptor Agonists on the Phenotype and Function of Camel Blood Neutrophils. Vet. Sci. 2023, 10, 154. [Google Scholar] [CrossRef]
- Desquesnes, M.; Holzmuller, P.; Lai, D.H.; Dargantes, A.; Lun, Z.R.; Jittaplapong, S. Trypanosoma evansi and surra: A review and perspectives on origin, history, distribution, taxonomy, morphology, hosts, and pathogenic effects. Biomed. Res. Int. 2013, 2013, 194176. [Google Scholar] [CrossRef]
- EFSA Panel on Animal Health and Welfare (AHAW); More, S.; Botner, A.; Butterworth, A.; Calistri, P.; Depner, K.; Edwards, S.; Garin-Bastuji, B.; Good, M.; Schmidt, C.G.; et al. Assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law (Regulation (EU) No 2016/429): Trypanosoma evansi infections (including Surra). EFSA J. 2017, 15, e04892. [Google Scholar] [CrossRef] [PubMed]
- LaRock, D.L.; Johnson, A.F.; Wilde, S.; Sands, J.S.; Monteiro, M.P.; LaRock, C.N. Group A Streptococcus induces GSDMA-dependent pyroptosis in keratinocytes. Nature 2022, 605, 527–531. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, K.M. Flow Cytometry: An Overview. Curr. Protoc. Immunol. 2018, 120, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Hiraiwa, P.M.; de Aguiar, A.M.; Avila, A.R. Fluorescence-based assay for accurate measurement of transcriptional activity in trypanosomatid parasites. Cytom. Part A 2018, 93, 727–736. [Google Scholar] [CrossRef] [PubMed]
- Martins-Filho, O.A.; Pereira, M.E.; Carvalho, J.F.; Cancado, J.R.; Brener, Z. Flow cytometry, a new approach to detect anti-live trypomastigote antibodies and monitor the efficacy of specific treatment in human Chagas’ disease. Clin. Diagn. Lab. Immunol. 1995, 2, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Shadomy, S.V.; Waring, S.C.; Martins-Filho, O.A.; Oliveira, R.C.; Chappell, C.L. Combined use of enzyme-linked immunosorbent assay and flow cytometry to detect antibodies to Trypanosoma cruzi in domestic canines in Texas. Clin. Diagn. Lab. Immunol. 2004, 11, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Schulz, D.; Mugnier, M.R.; Boothroyd, C.E.; Papavasiliou, F.N. Detection of Trypanosoma brucei Variant Surface Glycoprotein Switching by Magnetic Activated Cell Sorting and Flow Cytometry. J. Vis. Exp. 2016, 116, 54715. [Google Scholar] [CrossRef]
- Miranda, C.G.; Solana, M.E.; Curto, M.L.A.; Lammel, E.M.; Schijman, A.G.; Alba Soto, C.D. A flow cytometer-based method to simultaneously assess activity and selectivity of compounds against the intracellular forms of Trypanosoma cruzi. Acta Trop. 2015, 152, 8–16. [Google Scholar] [CrossRef]
- Kurup, S.P.; Tarleton, R.L. Perpetual expression of PAMPs necessary for optimal immune control and clearance of a persistent pathogen. Nat. Commun. 2013, 4, 2616. [Google Scholar] [CrossRef]
- Kamyingkird, K.; Pothipongsathorn, T.; Chalermwong, P.; Chimnoi, W.; Kengradonkij, C.; Ngasaman, R.; Inpankaew, T.; Desquesnes, M. Trypanosoma evansi was found as vesicle like form in the cytoplasm of nucleated cells of horses, Sukhothai, Thailand. In Proceedings of the 26th International Conference of the World Association for the Advancement of Veterinary Parasitology, Kuala Lumpur, Malaysia, 4–8 September 2017. [Google Scholar]
- Dos-Santos, A.L.; Carvalho-Kelly, L.F.; Dick, C.F.; Meyer-Fernandes, J.R. Innate immunomodulation to trypanosomatid parasite infections. Exp. Parasitol. 2016, 167, 67–75. [Google Scholar] [CrossRef]
- Fox, S.; Leitch, A.E.; Duffin, R.; Haslett, C.; Rossi, A.G. Neutrophil apoptosis: Relevance to the innate immune response and inflammatory disease. J. Innate Immun. 2010, 2, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Abi Abdallah, D.S.; Denkers, E.Y. Neutrophils cast extracellular traps in response to protozoan parasites. Front. Immunol. 2012, 3, 382. [Google Scholar] [CrossRef] [PubMed]
- Deleeuw, V.; Pham, H.T.T.; De Poorter, I.; Janssens, I.; De Trez, C.; Radwanska, M.; Magez, S. Trypanosoma brucei brucei causes a rapid and persistent influx of neutrophils in the spleen of infected mice. Parasite Immunol. 2019, 41, e12664. [Google Scholar] [CrossRef] [PubMed]
- Caljon, G.; Mabille, D.; Stijlemans, B.; De Trez, C.; Mazzone, M.; Tacchini-Cottier, F.; Malissen, M.; Van Ginderachter, J.A.; Magez, S.; De Baetselier, P.; et al. Neutrophils enhance early Trypanosoma brucei infection onset. Sci. Rep. 2018, 8, 11203. [Google Scholar] [CrossRef]
- Reshetnyak, T.; Nurbaeva, K.; Ptashnik, I.; Kudriaeva, A.; Belogurov, A., Jr.; Lila, A.; Nasonov, E. Markers of NETosis in Patients with Systemic Lupus Erythematosus and Antiphospholipid Syndrome. Int. J. Mol. Sci. 2023, 24, 9210. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussen, J.; AL-Jabr, O.A.; Alkuwayti, M.A.; Alrabiah, N.A.; Falemban, B.; Alouffi, A.; Al Salim, W.S.; Kamyingkird, K.; Desquesnes, M. A Flow Cytometry Study of the Binding and Stimulation Potential of Inactivated Trypanosoma evansi toward Dromedary Camel Leukocytes. Pathogens 2024, 13, 21. https://doi.org/10.3390/pathogens13010021
Hussen J, AL-Jabr OA, Alkuwayti MA, Alrabiah NA, Falemban B, Alouffi A, Al Salim WS, Kamyingkird K, Desquesnes M. A Flow Cytometry Study of the Binding and Stimulation Potential of Inactivated Trypanosoma evansi toward Dromedary Camel Leukocytes. Pathogens. 2024; 13(1):21. https://doi.org/10.3390/pathogens13010021
Chicago/Turabian StyleHussen, Jamal, Omar A. AL-Jabr, Mayyadah Abdullah Alkuwayti, Noof Abdulrahman Alrabiah, Baraa Falemban, Abdulaziz Alouffi, Waleed S. Al Salim, Ketsarin Kamyingkird, and Marc Desquesnes. 2024. "A Flow Cytometry Study of the Binding and Stimulation Potential of Inactivated Trypanosoma evansi toward Dromedary Camel Leukocytes" Pathogens 13, no. 1: 21. https://doi.org/10.3390/pathogens13010021







