Subverting Host Cell P21-Activated Kinase: A Case of Convergent Evolution across Pathogens
Abstract
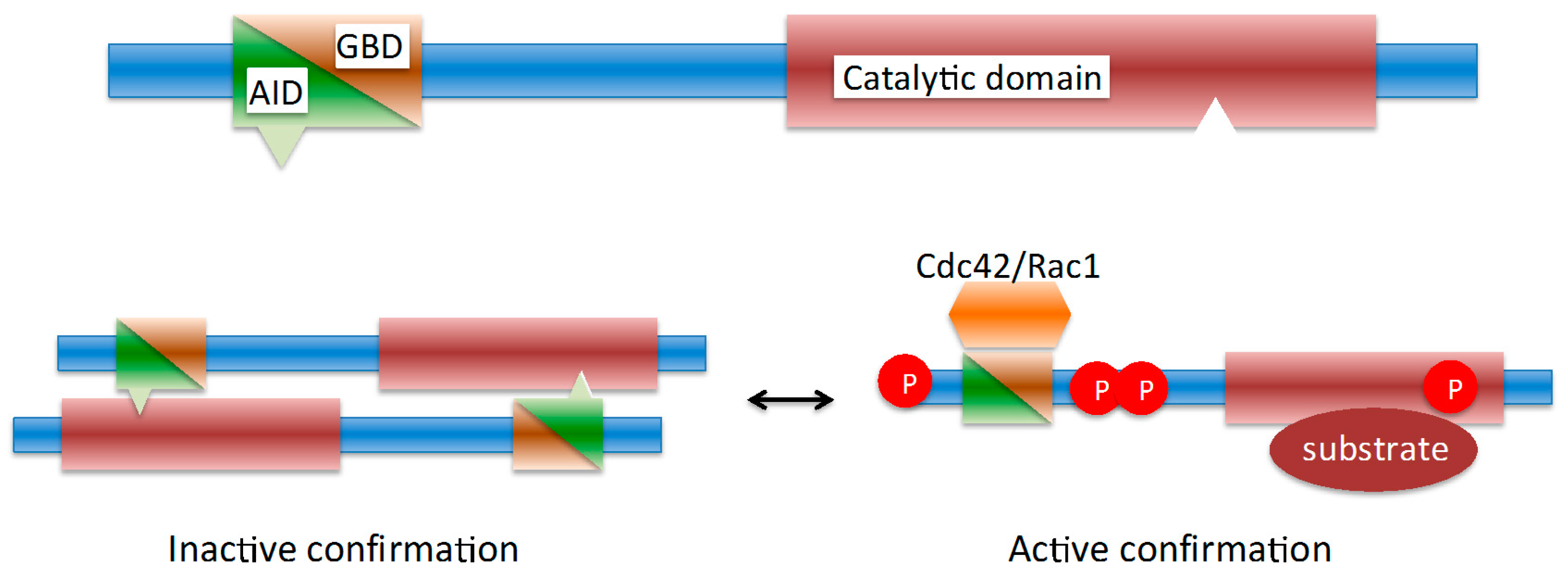
:1. Introduction
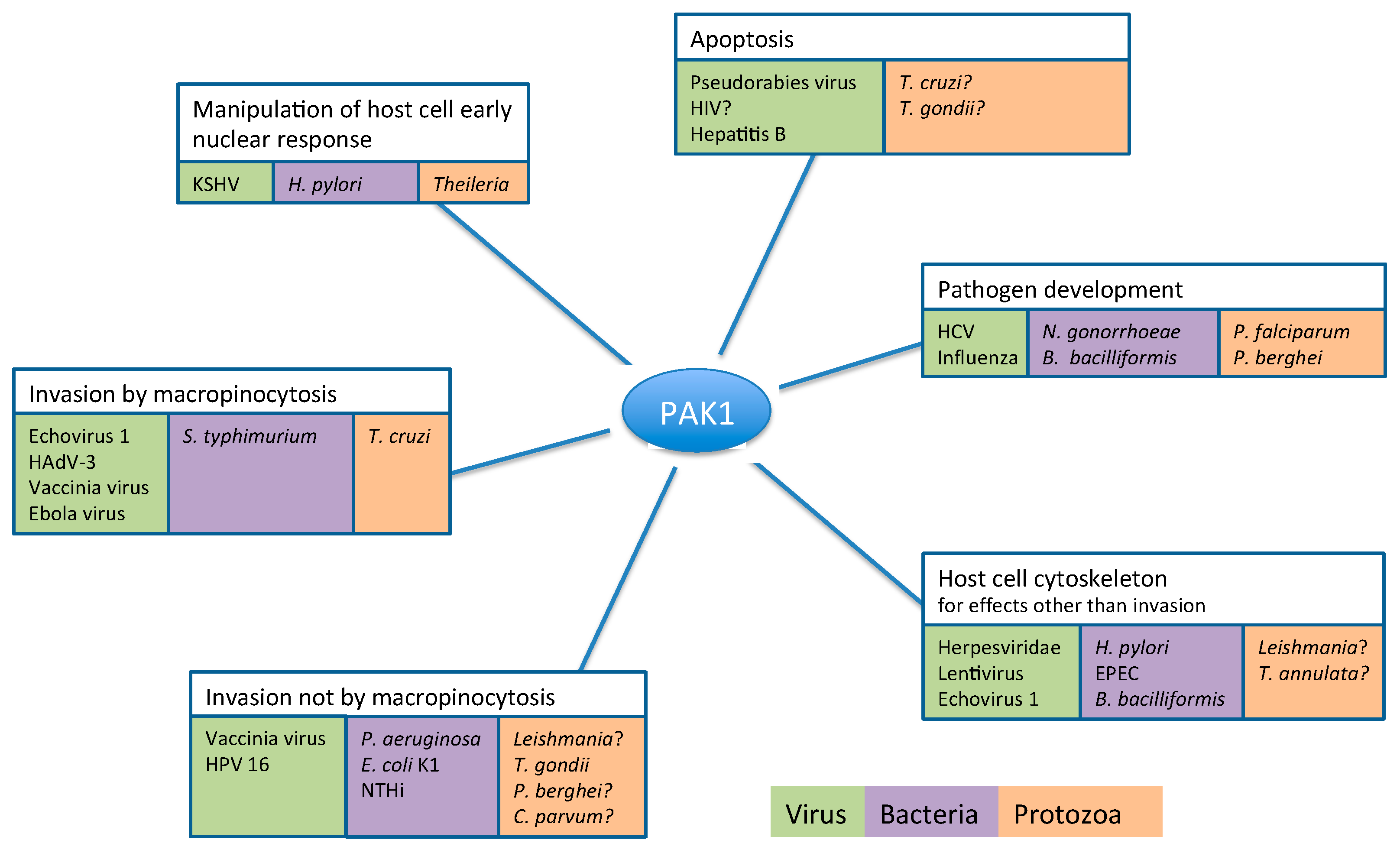
2. Pathogens Subvert Host PAK1 for a Range of Functions
2.1. Role of PAK1 in Pathogen Entry into the Host Cell
2.1.1. Pathogen Entry by Macropinocytosis
1. Viruses
2. Bacteria
3. Parasitic Protists
2.1.2. Pathogen Entry into the Host by Pathways Independent of Macropinocytosis
2.2. PAK1-Dependent Manipulation of Host Cell Cytoskeleton by Pathogens
2.3. PAK1-Dependent Manipulation of Host Cell Apoptosis by Pathogens
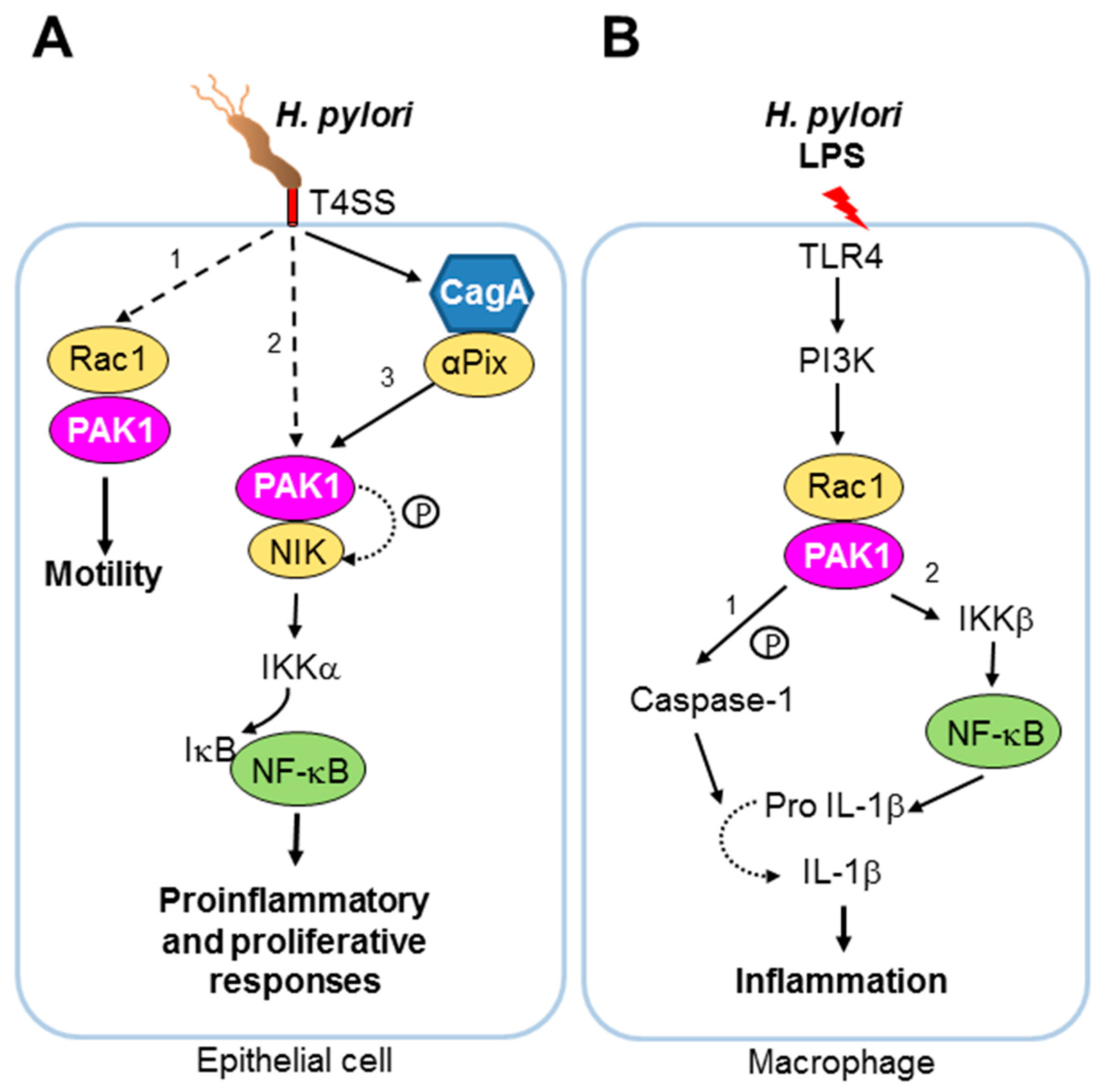
2.4. PAK1-Dependent Manipulation of Host Cell Early Nuclear Response by Pathogens
2.5. Host Cell PAK1 Activity Plays a Crucial Role in Pathogen Replication and Development
3. Conclusions and Future Prospects
Conflicts of Interest
References
- Pacheco, A.; Chernoff, J. Group I p21-activated kinases: Emerging roles in immune function and viral pathogenesis. Int. J. Biochem. Cell Biol. 2010, 42, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Molli, P.R.; Pakala, S.B.; Bui Nguyen, T.M.; Rayala, S.K.; Kumar, R. PAK thread from amoeba to mammals. J. Cell. Biochem. 2009, 107, 579–585. [Google Scholar] [CrossRef] [PubMed]
- Taglieri, D.M.; Ushio-Fukai, M.; Monasky, M.M. P21-activated kinase in inflammatory and cardiovascular disease. Cell. Signal. 2014, 26, 2060–2069. [Google Scholar] [CrossRef] [PubMed]
- Radu, M.; Semenova, G.; Kosoff, R.; Chernoff, J. PAK signalling during the development and progression of cancer. Nat. Rev. Cancer 2014, 14, 13–25. [Google Scholar] [CrossRef] [PubMed]
- Malecka, K.A.; Szentpetery, Z.; Peterson, J.R. Synergistic activation of p21-activated kinase 1 by phosphatidylinositol 4,5-bisphosphate and Rho gtpases. J. Biol. Chem. 2013, 288, 8887–8897. [Google Scholar] [CrossRef] [PubMed]
- Futosi, K.; Fodor, S.; Mocsai, A. Reprint of neutrophil cell surface receptors and their intracellular signal transduction pathways. Int. Immunopharmacol. 2013, 17, 1185–1197. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.D.; Jaffer, Z.M.; Chernoff, J.; Ridley, A.J. PAK1-mediated activation of erk1/2 regulates lamellipodial dynamics. J. Cell Sci. 2008, 121, 3729–3736. [Google Scholar] [CrossRef] [PubMed]
- Allen, J.D.; Jaffer, Z.M.; Park, S.J.; Burgin, S.; Hofmann, C.; Sells, M.A.; Chen, S.; Derr-Yellin, E.; Michels, E.G.; McDaniel, A.; et al. P21-activated kinase regulates mast cell degranulation via effects on calcium mobilization and cytoskeletal dynamics. Blood 2009, 113, 2695–2705. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, J.; Crawford, J.J.; Hoeflich, K.P.; Wang, W. Inhibitors of p21-activated kinases (PAKs). J. Med. Chem. 2015, 58, 111–129. [Google Scholar] [CrossRef] [PubMed]
- Ishida, H.; Li, K.; Yi, M.; Lemon, S.M. P21-activated kinase 1 is activated through the mammalian target of rapamycin/p70 s6 kinase pathway and regulates the replication of Hepatitis C virus in human hepatoma cells. J. Biol. Chem. 2007, 282, 11836–11848. [Google Scholar] [CrossRef] [PubMed]
- Ehrhardt, C.; Kardinal, C.; Wurzer, W.J.; Wolff, T.; von Eichel-Streiber, C.; Pleschka, S.; Planz, O.; Ludwig, S. Rac1 and PAK1 are upstream of ikk-epsilon and tbk-1 in the viral activation of interferon regulatory factor-3. FEBS Lett. 2004, 567, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Martyn, K.D.; Kim, M.J.; Quinn, M.T.; Dinauer, M.C.; Knaus, U.G. P21-activated kinase (PAK) regulates nadph oxidase activation in human neutrophils. Blood 2005, 106, 3962–3969. [Google Scholar] [CrossRef] [PubMed]
- Semblat, J.P.; Doerig, C. PAK in pathogen-host interactions. Cell. Logist. 2012, 2, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Mehlitz, A.; Rudel, T. Modulation of host signaling and cellular responses by chlamydia. Cell Commun. Signal. 2013, 11, 90. [Google Scholar] [CrossRef] [PubMed]
- Ludgate, L.; Adams, C.; Hu, J. Phosphorylation state-dependent interactions of hepadnavirus core protein with host factors. PLoS ONE 2011, 6, e29566. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.M.; Manser, E. PAKs in human disease. Prog. Mol. Biol. Transl. Sci. 2012, 106, 171–187. [Google Scholar] [PubMed]
- Molli, P.R.; Li, D.Q.; Murray, B.W.; Rayala, S.K.; Kumar, R. PAK signaling in oncogenesis. Oncogene 2009, 28, 2545–2555. [Google Scholar] [CrossRef] [PubMed]
- Favoreel, H.W.; Van Minnebruggen, G.; Adriaensen, D.; Nauwynck, H.J. Cytoskeletal rearrangements and cell extensions induced by the US3 kinase of an alphaherpesvirus are associated with enhanced spread. Proc. Natl. Acad. Sci. USA 2005, 102, 8990–8995. [Google Scholar] [CrossRef] [PubMed]
- Van den Broeke, C.; Radu, M.; Deruelle, M.; Nauwynck, H.; Hofmann, C.; Jaffer, Z.M.; Chernoff, J.; Favoreel, H.W. Alphaherpesvirus US3-mediated reorganization of the actin cytoskeleton is mediated by group a p21-activated kinases. Proc. Natl. Acad. Sci. USA 2009, 106, 8707–8712. [Google Scholar] [CrossRef] [PubMed]
- Foster, J.L.; Denial, S.J.; Temple, B.R.; Garcia, J.V. Mechanisms of HIV-1 Nef function and intracellular signaling. J. Neuroimmune Pharmacol. 2011, 6, 230–246. [Google Scholar] [CrossRef] [PubMed]
- Saksela, K.; Cheng, G.; Baltimore, D. Proline-rich (pxxp) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef + viruses but not for down-regulation of CD4. EMBO J. 1995, 14, 484–491. [Google Scholar] [PubMed]
- Nunn, M.F.; Marsh, J.W. Human immunodeficiency virus type 1 Nef associates with a member of the p21-activated kinase family. J. Virol. 1996, 70, 6157–6161. [Google Scholar] [PubMed]
- Nguyen, D.G.; Wolff, K.C.; Yin, H.; Caldwell, J.S.; Kuhen, K.L. “Unpaking” human immunodeficiency virus (HIV) replication: Using small interfering rna screening to identify novel cofactors and elucidate the role of group I PAKs in hiv infection. J. Virol. 2006, 80, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Krautkramer, E.; Giese, S.I.; Gasteier, J.E.; Muranyi, W.; Fackler, O.T. Human immunodeficiency virus type 1 Nef activates p21-activated kinase via recruitment into lipid rafts. J. Virol. 2004, 78, 4085–4097. [Google Scholar] [CrossRef] [PubMed]
- Raney, A.; Shaw, A.Y.; Foster, J.L.; Garcia, J.V. Structural constraints on human immunodeficiency virus type 1 Nef function. Virology 2007, 368, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Rauch, S.; Pulkkinen, K.; Saksela, K.; Fackler, O.T. Human immunodeficiency virus type 1 Nef recruits the guanine exchange factor Vav1 via an unexpected interface into plasma membrane microdomains for association with p21-activated kinase 2 activity. J. Virol. 2008, 82, 2918–2929. [Google Scholar] [CrossRef] [PubMed]
- Wei, B.L.; Arora, V.K.; Raney, A.; Kuo, L.S.; Xiao, G.H.; O'Neill, E.; Testa, J.R.; Foster, J.L.; Garcia, J.V. Activation of p21-activated kinase 2 by human immunodeficiency virus type 1 Nef induces merlin phosphorylation. J. Virol. 2005, 79, 14976–14980. [Google Scholar] [CrossRef] [PubMed]
- Stolp, B.; Abraham, L.; Rudolph, J.M.; Fackler, O.T. Lentiviral Nef proteins utilize PAK2-mediated deregulation of cofilin as a general strategy to interfere with actin remodeling. J. Virol. 2010, 84, 3935–3948. [Google Scholar] [CrossRef] [PubMed]
- Imle, A.; Abraham, L.; Tsopoulidis, N.; Hoflack, B.; Saksela, K.; Fackler, O.T. Association with PAK2 enables functional interactions of lentiviral Nef proteins with the exocyst complex. MBio 2015, 6, e01309–e01315. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.; Witte, V.; Laffert, B.; Blume, K.; Stromer, E.; Trapp, S.; d'Aloja, P.; Schurmann, A.; Baur, A.S. HIV-1 Nef associated PAK and pi3-kinases stimulate akt-independent bad-phosphorylation to induce anti-apoptotic signals. Nat. Med. 2001, 7, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Germane, K.L.; Spiller, B.W. Structural and functional studies indicate that the epec effector, espg, directly binds p21-activated kinase. Biochemistry 2011, 50, 917–919. [Google Scholar] [CrossRef] [PubMed]
- Selyunin, A.S.; Sutton, S.E.; Weigele, B.A.; Reddick, L.E.; Orchard, R.C.; Bresson, S.M.; Tomchick, D.R.; Alto, N.M. The assembly of a gtpase-kinase signalling complex by a bacterial catalytic scaffold. Nature 2011, 469, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Naumann, M.; Rudel, T.; Wieland, B.; Bartsch, C.; Meyer, T.F. Coordinate activation of activator protein 1 and inflammatory cytokines in response to neisseria gonorrhoeae epithelial cell contact involves stress response kinases. J. Exp. Med. 1998, 188, 1277–1286. [Google Scholar] [CrossRef] [PubMed]
- Vorster, P.J.; Guo, J.; Yoder, A.; Wang, W.; Zheng, Y.; Xu, X.; Yu, D.; Spear, M.; Wu, Y. Lim kinase 1 modulates cortical actin and cxcr4 cycling and is activated by HIV-1 to initiate viral infection. J. Biol. Chem. 2011, 286, 12554–12564. [Google Scholar] [CrossRef] [PubMed]
- Geng, M.; Xin, X.; Bi, L.Q.; Zhou, L.T.; Liu, X.H. Molecular mechanism of Hepatitis B virus x protein function in hepatocarcinogenesis. World J. Gastroenterol. 2015, 21, 10732–10738. [Google Scholar] [CrossRef] [PubMed]
- Tse, E.Y.; Ching, Y.P. The role of p21-activated kinases in hepatocellular carcinoma metastasis. J. Mol. Signal. 2014, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, H.; Chen, L.; Wang, S.; Zhou, L.; Yun, X.; Sun, L.; Wen, Y.; Gu, J. Hepatitis B virus x protein confers resistance of hepatoma cells to anoikis by up-regulating and activating p21-activated kinase 1. Gastroenterology 2012, 143, 199–212. [Google Scholar] [CrossRef] [PubMed]
- Dadke, D.; Fryer, B.H.; Golemis, E.A.; Field, J. Activation of p21-activated kinase 1-nuclear factor kappab signaling by kaposi's sarcoma-associated herpes virus G protein-coupled receptor during cellular transformation. Cancer Res. 2003, 63, 8837–8847. [Google Scholar] [PubMed]
- Guilluy, C.; Zhang, Z.; Bhende, P.M.; Sharek, L.; Wang, L.; Burridge, K.; Damania, B. Latent kshv infection increases the vascular permeability of human endothelial cells. Blood 2011, 118, 5344–5354. [Google Scholar] [CrossRef] [PubMed]
- Mori, I. Herpes simplex virus US3 protein kinase regulates host responses and determines neurovirulence. Microbiol. Immunol. 2012, 56, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Deruelle, M.J.; Favoreel, H.W. Keep it in the subfamily: The conserved alphaherpesvirus US3 protein kinase. J. Gen. Virol. 2011, 92, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Van den Broeke, C.; Radu, M.; Nauwynck, H.J.; Chernoff, J.; Favoreel, H.W. Role of group a p21-activated kinases in the anti-apoptotic activity of the pseudorabies virus US3 protein kinase. Virus Res. 2011, 155, 376–380. [Google Scholar] [CrossRef] [PubMed]
- Jacob, T.; Van den Broeke, C.; van Troys, M.; Waterschoot, D.; Ampe, C.; Favoreel, H.W. Alphaherpesviral US3 kinase induces cofilin dephosphorylation to reorganize the actin cytoskeleton. J. Virol. 2013, 87, 4121–4126. [Google Scholar] [CrossRef] [PubMed]
- Pascua, P.N.; Lee, J.H.; Song, M.S.; Park, S.J.; Baek, Y.H.; Ann, B.H.; Shin, E.Y.; Kim, E.G.; Choi, Y.K. Role of the p21-activated kinases (PAKs) in influenza a virus replication. Biochem. Biophys. Res. Commun. 2011, 414, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Ihler, G.M. Activation of Rac, Cdc42 and other downstream signalling molecules by Bartonella bacilliformis during entry into human endothelial cells. Cell. Microbiol. 2002, 4, 557–569. [Google Scholar] [CrossRef] [PubMed]
- Virji, M. Pathogenic neisseriae: Surface modulation, pathogenesis and infection control. Nat. Rev. Microbiol. 2009, 7, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Backert, S.; Tegtmeyer, N.; Selbach, M. The versatility of Helicobacter pylori caga effector protein functions: The master key hypothesis. Helicobacter 2010, 15, 163–176. [Google Scholar] [CrossRef] [PubMed]
- Foryst-Ludwig, A.; Naumann, M. P21-activated kinase 1 activates the nuclear factor kb (NF-kappaB)-inducing kinase-ikb kinases NF-kappaB pathway and proinflammatory cytokines in Helicobacter pylori infection. J. Biol. Chem. 2000, 275, 39779–39785. [Google Scholar] [CrossRef] [PubMed]
- Neumann, M.; Foryst-Ludwig, A.; Klar, S.; Schweitzer, K.; Naumann, M. The PAK1 autoregulatory domain is required for interaction with nik in Helicobacter pylori-induced NF-kappaB activation. Biol. Chem. 2006, 387, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Karin, M.; Greten, F.R. Nf-kb: Linking inflammation and immunity to cancer development and progression. Nat. Rev. Immunol. 2005, 5, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Churin, Y.; Kardalinou, E.; Meyer, T.F.; Naumann, M. Pathogenicity island-dependent activation of Rho gtpases Rac1 and Cdc42 in Helicobacter pylori infection. Mol. Microbiol. 2001, 40, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Rudrabhatla, R.S.; Sukumaran, S.K.; Bokoch, G.M.; Prasadarao, N.V. Modulation of myosin light-chain phosphorylation by p21-activated kinase 1 in escherichia coli invasion of human brain microvascular endothelial cells. Infect. Immun. 2003, 71, 2787–2797. [Google Scholar] [CrossRef] [PubMed]
- Morey, P.; Cano, V.; Marti-Lliteras, P.; Lopez-Gomez, A.; Regueiro, V.; Saus, C.; Bengoechea, J.A.; Garmendia, J. Evidence for a non-replicative intracellular stage of nontypable haemophilus influenzae in epithelial cells. Microbiology 2011, 157, 234–250. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Gomez, A.; Cano, V.; Moranta, D.; Morey, P.; Garcia del Portillo, F.; Bengoechea, J.A.; Garmendia, J. Host cell kinases, A5 and B1 integrins, and Rac1 signalling on the microtubule cytoskeleton are important for non-typable haemophilus influenzae invasion of respiratory epithelial cells. Microbiology 2012, 158, 2384–2398. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, T.; Bokoch, G.M.; Waterman-Storer, C.M. Regulation of microtubule destabilizing activity of op18/stathmin downstream of Rac1. J. Biol. Chem. 2004, 279, 6196–6203. [Google Scholar] [CrossRef] [PubMed]
- Hannemann, S.; Gao, B.; Galan, J.E. Salmonella modulation of host cell gene expression promotes its intracellular growth. PLoS Pathog. 2013, 9, e1003668. [Google Scholar] [CrossRef] [PubMed]
- Chuenkova, M.V.; PereiraPerrin, M. Trypanosoma cruzi targets akt in host cells as an intracellular antiapoptotic strategy. Sci. Signal. 2009, 2, ra74. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, M.; Onishi, K.; Kikuchi, C.; Gotoh, Y. Scaffolding function of PAK in the PDK1-akt pathway. Nat. Cell Biol. 2008, 10, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Sicard, A.; Semblat, J.P.; Doerig, C.; Hamelin, R.; Moniatte, M.; Dorin-Semblat, D.; Spicer, J.A.; Srivastava, A.; Retzlaff, S.; Heussler, V.; et al. Activation of a PAK-mek signalling pathway in malaria parasite-infected erythrocytes. Cell. Microbiol. 2011, 13, 836–845. [Google Scholar] [CrossRef] [PubMed]
- Heussler, V.; Sturm, A.; Langsley, G. Regulation of host cell survival by intracellular plasmodium and theileria parasites. Parasitology 2006, 132 Suppl, S49–S60. [Google Scholar] [CrossRef] [PubMed]
- Durrani, Z.; Weir, W.; Pillai, S.; Kinnaird, J.; Shiels, B. Modulation of activation-associated host cell gene expression by the apicomplexan parasite theileria annulata. Cell. Microbiol. 2012, 14, 1434–1454. [Google Scholar] [CrossRef] [PubMed]
- Frost, J.A.; Swantek, J.L.; Stippec, S.; Yin, M.J.; Gaynor, R.; Cobb, M.H. Stimulation of NF-kappaB activity by multiple signaling pathways requires PAK1. J. Biol. Chem. 2000, 275, 19693–19699. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.P.; Gleeson, P.A. Macropinocytosis: An endocytic pathway for internalising large gulps. Immunol. Cell Biol. 2011, 89, 836–843. [Google Scholar] [CrossRef] [PubMed]
- Liberali, P.; Kakkonen, E.; Turacchio, G.; Valente, C.; Spaar, A.; Perinetti, G.; Bockmann, R.A.; Corda, D.; Colanzi, A.; Marjomaki, V.; et al. The closure of PAK1-dependent macropinosomes requires the phosphorylation of ctbp1/bars. EMBO J. 2008, 27, 970–981. [Google Scholar] [CrossRef] [PubMed]
- Mercer, J.; Helenius, A. Gulping rather than sipping: Macropinocytosis as a way of virus entry. Curr. Opin. Microbiol. 2012, 15, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Haglund, C.M.; Welch, M.D. Pathogens and polymers: Microbe-host interactions illuminate the cytoskeleton. J. Cell Biol. 2011, 195, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Mercer, J.; Helenius, A. Virus entry by macropinocytosis. Nat. Cell Biol. 2009, 11, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, I.; Vilhardt, F. Macropinocytosis is the entry mechanism of amphotropic murine leukemia virus. J. Virol. 2015, 89, 1851–1866. [Google Scholar] [CrossRef] [PubMed]
- Karjalainen, M.; Kakkonen, E.; Upla, P.; Paloranta, H.; Kankaanpaa, P.; Liberali, P.; Renkema, G.H.; Hyypia, T.; Heino, J.; Marjomaki, V. A raft-derived, PAK1-regulated entry participates in alpha2beta1 integrin-dependent sorting to caveosomes. Mol. Biol. Cell 2008, 19, 2857–2869. [Google Scholar] [CrossRef] [PubMed]
- Han, S.C.; Guo, H.C.; Sun, S.Q.; Jin, Y.; Wei, Y.Q.; Feng, X.; Yao, X.P.; Cao, S.Z.; Xiang Liu, D.; Liu, X.T. Productive entry of foot-and-mouth disease virus via macropinocytosis independent of phosphatidylinositol 3-kinase. Sci. Rep. 2016, 6, 19294. [Google Scholar] [CrossRef] [PubMed]
- Nanbo, A.; Imai, M.; Watanabe, S.; Noda, T.; Takahashi, K.; Neumann, G.; Halfmann, P.; Kawaoka, Y. Ebolavirus is internalized into host cells via macropinocytosis in a viral glycoprotein-dependent manner. PLoS Pathog. 2010, 6, e1001121. [Google Scholar] [CrossRef] [PubMed]
- Moller-Tank, S.; Maury, W. Ebola virus entry: A curious and complex series of events. PLoS Pathog. 2015, 11, e1004731. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.F.; Kolokoltsov, A.A.; Albrecht, T.; Davey, R.A. Cellular entry of ebola virus involves uptake by a macropinocytosis-like mechanism and subsequent trafficking through early and late endosomes. PLoS Pathog. 2010, 6, e1001110. [Google Scholar] [CrossRef] [PubMed]
- De Vries, E.; Tscherne, D.M.; Wienholts, M.J.; Cobos-Jimenez, V.; Scholte, F.; Garcia-Sastre, A.; Rottier, P.J.; de Haan, C.A. Dissection of the influenza a virus endocytic routes reveals macropinocytosis as an alternative entry pathway. PLoS Pathog. 2011, 7, e1001329. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Huang, X.; Huang, Y.; Hao, X.; Xu, H.; Cai, M.; Wang, H.; Qin, Q. Entry of a novel marine DNA virus, singapore grouper iridovirus, into host cells occurs via clathrin-mediated endocytosis and macropinocytosis in a ph-dependent manner. J. Virol. 2014, 88, 13047–13063. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.G.; Quintas, A.; Perez-Nunez, D.; Nogal, M.; Barroso, S.; Carrascosa, A.L.; Revilla, Y. African swine fever virus uses macropinocytosis to enter host cells. PLoS Pathog. 2012, 8, e1002754. [Google Scholar] [CrossRef] [PubMed]
- Wolfrum, N.; Greber, U.F. Adenovirus signalling in entry. Cell. Microbiol. 2013, 15, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Amstutz, B.; Gastaldelli, M.; Kalin, S.; Imelli, N.; Boucke, K.; Wandeler, E.; Mercer, J.; Hemmi, S.; Greber, U.F. Subversion of ctbp1-controlled macropinocytosis by human adenovirus serotype 3. EMBO J. 2008, 27, 956–969. [Google Scholar] [CrossRef] [PubMed]
- Kalin, S.; Amstutz, B.; Gastaldelli, M.; Wolfrum, N.; Boucke, K.; Havenga, M.; DiGennaro, F.; Liska, N.; Hemmi, S.; Greber, U.F. Macropinocytotic uptake and infection of human epithelial cells with species B2 adenovirus type 35. J. Virol. 2010, 84, 5336–5350. [Google Scholar] [CrossRef] [PubMed]
- MacLeod, D.T.; Nakatsuji, T.; Wang, Z.; di Nardo, A.; Gallo, R.L. Vaccinia virus binds to the scavenger receptor marco on the surface of keratinocytes. J. Investig. Dermatol. 2015, 135, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Mercer, J.; Knebel, S.; Schmidt, F.I.; Crouse, J.; Burkard, C.; Helenius, A. Vaccinia virus strains use distinct forms of macropinocytosis for host-cell entry. Proc. Natl. Acad. Sci. USA 2010, 107, 9346–9351. [Google Scholar] [CrossRef] [PubMed]
- Villa, N.Y.; Bartee, E.; Mohamed, M.R.; Rahman, M.M.; Barrett, J.W.; McFadden, G. Myxoma and vaccinia viruses exploit different mechanisms to enter and infect human cancer cells. Virology 2010, 401, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Haraga, A.; Ohlson, M.B.; Miller, S.I. Salmonellae interplay with host cells. Nat. Rev. Microbiol. 2008, 6, 53–66. [Google Scholar] [CrossRef] [PubMed]
- Galan, J.E. Molecular genetic bases of salmonella entry into host cells. Mol. Microbiol. 1996, 20, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Hardt, W.D.; Chen, L.M.; Schuebel, K.E.; Bustelo, X.R.; Galan, J.E. S. Typhimurium encodes an activator of Rho gtpases that induces membrane ruffling and nuclear responses in host cells. Cell 1998, 93, 815–826. [Google Scholar] [CrossRef]
- Chen, L.M.; Bagrodia, S.; Cerione, R.A.; Galan, J.E. Requirement of p21-activated kinase (PAK) for Salmonella typhimurium-induced nuclear responses. J. Exp. Med. 1999, 189, 1479–1488. [Google Scholar] [CrossRef] [PubMed]
- Rassi, A., Jr.; Rassi, A.; Marin-Neto, J.A. Chagas disease. Lancet 2010, 375, 1388–1402. [Google Scholar] [CrossRef]
- Barrias, E.S.; Reignault, L.C.; De Souza, W.; Carvalho, T.M. Trypanosoma cruzi uses macropinocytosis as an additional entry pathway into mammalian host cell. Microbes Infect. 2012, 14, 1340–1351. [Google Scholar] [CrossRef] [PubMed]
- Deacon, S.W.; Beeser, A.; Fukui, J.A.; Rennefahrt, U.E.; Myers, C.; Chernoff, J.; Peterson, J.R. An isoform-selective, small-molecule inhibitor targets the autoregulatory mechanism of p21-activated kinase. Chem. Biol. 2008, 15, 322–331. [Google Scholar] [CrossRef] [PubMed]
- Rosestolato, C.T.; Dutra, J.M.; de Souza, W.; de Carvalho, T.M. Participation of host cell actin filaments during interaction of trypomastigote forms of trypanosoma cruzi with host cells. Cell Struct. Funct. 2002, 27, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, T.M.; Ferreira, A.G.; Coimbra, E.S.; Rosestolato, C.T.; De Souza, W. Distribution of cytoskeletal structures and organelles of the host cell during evolution of the intracellular parasitism by trypanosoma cruzi. J. Submicrosc. Cytol. Pathol. 1999, 31, 325–333. [Google Scholar] [PubMed]
- Procopio, D.O.; Barros, H.C.; Mortara, R.A. Actin-rich structures formed during the invasion of cultured cells by infective forms of trypanosoma cruzi. Eur. J. Cell Biol. 1999, 78, 911–924. [Google Scholar] [CrossRef]
- Sells, M.A.; Knaus, U.G.; Bagrodia, S.; Ambrose, D.M.; Bokoch, G.M.; Chernoff, J. Human p21-activated kinase (PAK1) regulates actin organization in mammalian cells. Curr. Biol. 1997, 7, 202–210. [Google Scholar] [CrossRef]
- Ong, C.C.; Jubb, A.M.; Zhou, W.; Haverty, P.M.; Harris, A.L.; Belvin, M.; Friedman, L.S.; Koeppen, H.; Hoeflich, K.P. P21-activated kinase 1- PAK’ED with potential. Oncotarget 2011, 2, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Dutra, J.M.; Bonilha, V.L.; De Souza, W.; Carvalho, T.M. Role of small gtpases in trypanosoma cruzi invasion in mdck cell lines. Parasitol. Res. 2005, 96, 171–177. [Google Scholar] [PubMed]
- Fernandes, A.B.; Mortara, R.A. Invasion of mdck epithelial cells with altered expression of Rho gtpases by trypanosoma cruzi amastigotes and metacyclic trypomastigotes of strains from the two major phylogenetic lineages. Microbes Infect. 2004, 6, 460–467. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.M.; Oghumu, S.; Gupta, G.; McGwire, B.S.; Drew, M.E.; Satoskar, A.R. Mechanisms of cellular invasion by intracellular parasites. Cell. Mol. Life Sci. 2014, 71, 1245–1263. [Google Scholar] [CrossRef] [PubMed]
- Sibley, L.D.; Andrews, N.W. Cell invasion by un-palatable parasites. Traffic 2000, 1, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Bouvard, V.; Baan, R.; Straif, K.; Grosse, Y.; Secretan, B.; El Ghissassi, F.; Benbrahim-Tallaa, L.; Guha, N.; Freeman, C.; Galichet, L.; et al. A review of human carcinogens--part b: Biological agents. Lancet. Oncol. 2009, 10, 321–322. [Google Scholar] [CrossRef]
- Lowy, D.R.; Schiller, J.T. Reducing hpv-associated cancer globally. Cancer Prev. Res. (Phila) 2012, 5, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Bonney, K.M. Chagas disease in the 21st century: A public health success or an emerging threat? Parasite 2014, 21, 11. [Google Scholar] [CrossRef] [PubMed]
- Tretina, K.; Gotia, H.T.; Mann, D.J.; Silva, J.C. Theileria-transformed bovine leukocytes have cancer hallmarks. Trends Parasitol. 2015, 31, 306–314. [Google Scholar] [CrossRef] [PubMed]
- Day, P.M.; Schelhaas, M. Concepts of papillomavirus entry into host cells. Curr. Opin. Virol. 2014, 4, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Schelhaas, M.; Shah, B.; Holzer, M.; Blattmann, P.; Kuhling, L.; Day, P.M.; Schiller, J.T.; Helenius, A. Entry of Human Papillomavirus type 16 by actin-dependent, clathrin- and lipid raft-independent endocytosis. PLoS Pathog. 2012, 8, e1002657. [Google Scholar] [CrossRef] [PubMed]
- Carter, G.C.; Bernstone, L.; Baskaran, D.; James, W. HIV-1 infects macrophages by exploiting an endocytic route dependent on dynamin, Rac1 and PAK1. Virology 2011, 409, 234–250. [Google Scholar] [CrossRef] [PubMed]
- Gobeil, L.A.; Lodge, R.; Tremblay, M.J. Macropinocytosis-like HIV-1 internalization in macrophages is CCR5 dependent and leads to efficient but delayed degradation in endosomal compartments. J. Virol. 2013, 87, 735–745. [Google Scholar] [CrossRef] [PubMed]
- Pielage, J.F.; Powell, K.R.; Kalman, D.; Engel, J.N. Rnai screen reveals an abl kinase-dependent host cell pathway involved in pseudomonas aeruginosa internalization. PLoS Pathog. 2008, 4, e1000031. [Google Scholar] [CrossRef] [PubMed]
- Wittmann, T.; Bokoch, G.M.; Waterman-Storer, C.M. Regulation of leading edge microtubule and actin dynamics downstream of Rac1. J. Cell Biol. 2003, 161, 845–851. [Google Scholar] [CrossRef] [PubMed]
- Peltan, A.; Briggs, L.; Matthews, G.; Sweeney, S.T.; Smith, D.F. Identification of drosophila gene products required for phagocytosis of leishmania donovani. PLoS ONE 2012, 7, e51831. [Google Scholar] [CrossRef] [PubMed]
- Morehead, J.; Coppens, I.; Andrews, N.W. Opsonization modulates Rac-1 activation during cell entry by leishmania amazonensis. Infect. Immun. 2002, 70, 4571–4580. [Google Scholar] [CrossRef] [PubMed]
- Lodge, R.; Descoteaux, A. Phagocytosis of leishmania donovani amastigotes is Rac1 dependent and occurs in the absence of nadph oxidase activation. Eur. J. Immunol. 2006, 36, 2735–2744. [Google Scholar] [CrossRef] [PubMed]
- Doerig, C.; Späth, G.; Wiese, M. Protein phosphorylation in parasites novel targets for antiparasitic intervention. In Drug Discovery in Infectious Diseases; Wiley Blackwell: Weinheim, Germany, 2014. [Google Scholar]
- Laliberte, J.; Carruthers, V.B. Host cell manipulation by the human pathogen toxoplasma gondii. Cell. Mol. Life Sci. 2008, 65, 1900–1915. [Google Scholar] [CrossRef] [PubMed]
- Peixoto, L.; Chen, F.; Harb, O.S.; Davis, P.H.; Beiting, D.P.; Brownback, C.S.; Ouloguem, D.; Roos, D.S. Integrative genomic approaches highlight a family of parasite-specific kinases that regulate host responses. Cell Host Microbe 2010, 8, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Abi Abdallah, D.S.; Lin, C.; Ball, C.J.; King, M.R.; Duhamel, G.E.; Denkers, E.Y. Toxoplasma gondii triggers release of human and mouse neutrophil extracellular traps. Infect. Immun. 2012, 80, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, V.; Combe, A.; David, V.; Malmquist, N.A.; Delorme, V.; Leroy, C.; Blazquez, S.; Menard, R.; Tardieux, I. Host cell entry by apicomplexa parasites requires actin polymerization in the host cell. Cell Host Microbe 2009, 5, 259–272. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, C.V.; da Silva, E.A.; Cruz, M.C.; Chavrier, P.; Mortara, R.A. ARF6, PI3-kinase and host cell actin cytoskeleton in toxoplasma gondii cell invasion. Biochem. Biophys. Res. Commun. 2009, 378, 656–661. [Google Scholar] [CrossRef] [PubMed]
- Na, R.H.; Zhu, G.H.; Luo, J.X.; Meng, X.J.; Cui, L.; Peng, H.J.; Chen, X.G.; Gomez-Cambronero, J. Enzymatically active Rho and Rac small-gtpases are involved in the establishment of the vacuolar membrane after toxoplasma gondii invasion of host cells. BMC Microbiol. 2013, 13, 125. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.E.; Gilson, P.R.; Taechalertpaisarn, T.; Tham, W.H.; de Jong, N.W.; Harvey, K.L.; Fowkes, F.J.; Barlow, P.N.; Rayner, J.C.; Wright, G.J.; et al. Revealing the sequence and resulting cellular morphology of receptor-ligand interactions during plasmodium falciparum invasion of erythrocytes. PLoS Pathog. 2015, 11, e1004670. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.M.; Huang, B.Q.; Splinter, P.L.; Orth, J.D.; Billadeau, D.D.; McNiven, M.A.; LaRusso, N.F. Cdc42 and the actin-related protein/neural wiskott-aldrich syndrome protein network mediate cellular invasion by cryptosporidium parvum. Infect. Immun. 2004, 72, 3011–3021. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Goshima, F.; Daikoku, T.; Takakuwa, H.; Nishiyama, Y. Expression of herpes simplex virus type 2 US3 affects the Cdc42/Rac pathway and attenuates c-Jun N-terminal kinase activation. Genes Cells 2000, 5, 1017–1027. [Google Scholar] [CrossRef] [PubMed]
- Lamote, J.A.; Glorieux, S.; Nauwynck, H.J.; Favoreel, H.W. The US3 protein of pseudorabies virus drives viral passage across the basement membrane in porcine respiratory mucosa explants. J. Virol. 2016, 90, 10945–10950. [Google Scholar] [CrossRef] [PubMed]
- Richerioux, N.; Blondeau, C.; Wiedemann, A.; Remy, S.; Vautherot, J.F.; Denesvre, C. Rho-rock and Rac-PAK signaling pathways have opposing effects on the cell-to-cell spread of marek's disease virus. PLoS ONE 2012, 7, e44072. [Google Scholar] [CrossRef] [PubMed]
- Andrade, L.G.; Albarnaz, J.D.; Mugge, F.L.; David, B.A.; Abrahao, J.S.; da Fonseca, F.G.; Kroon, E.G.; Menezes, G.B.; McFadden, G.; Bonjardim, C.A. Vaccinia virus dissemination requires p21-activated kinase 1. Arch. Virol. 2016, 161, 2991–3002. [Google Scholar] [CrossRef] [PubMed]
- Donnenberg, M.S.; Kaper, J.B.; Finlay, B.B. Interactions between enteropathogenic escherichia coli and host epithelial cells. Trends Microbiol. 1997, 5, 109–114. [Google Scholar] [CrossRef]
- Holm, A.; Tejle, K.; Magnusson, K.E.; Descoteaux, A.; Rasmusson, B. Leishmania donovani lipophosphoglycan causes periphagosomal actin accumulation-correlation with impaired translocation of pkcalpha and defective phagosome maturation. Cell. Microbiol. 2001, 3, 439–447. [Google Scholar] [CrossRef] [PubMed]
- Lerm, M.; Holm, A.; Seiron, A.; Sarndahl, E.; Magnusson, K.E.; Rasmusson, B. Leishmania donovani requires functional Cdc42 and Rac1 to prevent phagosomal maturation. Infect. Immun. 2006, 74, 2613–2618. [Google Scholar] [CrossRef] [PubMed]
- Caldas, L.A.; Seabra, S.H.; Attias, M.; de Souza, W. The effect of kinase, actin, myosin and dynamin inhibitors on host cell egress by toxoplasma gondii. Parasitol. Int. 2013, 62, 475–482. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Baumgartner, M. Filopodia and membrane blebs drive efficient matrix invasion of macrophages transformed by the intracellular parasite theileria annulata. PLoS ONE 2013, 8, e75577. [Google Scholar] [CrossRef] [PubMed]
- Kaminskyy, V.; Zhivotovsky, B. To kill or be killed: How viruses interact with the cell death machinery. J. Intern. Med. 2010, 267, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Kepp, O.; Morselli, E.; Vitale, I.; Senovilla, L.; Pinti, M.; Zitvogel, L.; Kroemer, G. Viral strategies for the evasion of immunogenic cell death. J. Intern. Med. 2010, 267, 526–542. [Google Scholar] [CrossRef] [PubMed]
- Faherty, C.S.; Maurelli, A.T. Staying alive: Bacterial inhibition of apoptosis during infection. Trends Microbiol. 2008, 16, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Ye, D.Z.; Jin, S.; Zhuo, Y.; Field, J. P21-activated kinase 1 (PAK1) phosphorylates bad directly at serine 111 in vitro and indirectly through Raf-1 at serine 112. PLoS ONE 2011, 6, e27637. [Google Scholar] [CrossRef] [PubMed]
- Grauwet, K.; Vitale, M.; De Pelsmaeker, S.; Jacob, T.; Laval, K.; Moretta, L.; Parodi, M.; Parolini, S.; Cantoni, C.; Favoreel, H.W. Pseudorabies virus US3 protein kinase protects infected cells from nk cell-mediated lysis via increased binding of the inhibitory nk cell receptor CD300a. J. Virol. 2015, 90, 1522–1533. [Google Scholar] [CrossRef] [PubMed]
- Payne, T.M.; Molestina, R.E.; Sinai, A.P. Inhibition of caspase activation and a requirement for NF-kappaB function in the toxoplasma gondii-mediated blockade of host apoptosis. J. Cell Sci. 2003, 116, 4345–4358. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Ahn, M.H.; Jun, H.S.; Jung, J.W.; Ryu, J.S.; Min, D.Y. Toxoplasma gondii inhibits apoptosis in infected cells by caspase inactivation and NF-kappaB activation. Yonsei Med. J. 2006, 47, 862–869. [Google Scholar] [CrossRef] [PubMed]
- Baek, H.Y.; Lim, J.W.; Kim, H. Interaction between the Helicobacter pylori caga and a-pix in gastric epithelial ags cells. Ann. N. Y. Acad. Sci. 2007, 1096, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Basak, C.; Pathak, S.K.; Bhattacharyya, A.; Mandal, D.; Pathak, S.; Kundu, M. Nf-kb- and c/ebpb-driven interleukin-1b gene expression and PAK1-mediated caspase-1 activation play essential roles in interleukin-1b release from Helicobacter pylori lipopolysaccharide-stimulated macrophages. J. Biol. Chem. 2005, 280, 4279–4288. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.C.; Harrison, T.; Hamm, H.E.; Lomasney, J.W.; Mohandas, N.; Haldar, K. Erythrocyte G protein as a novel target for malarial chemotherapy. PLoS Med. 2006, 3, e528. [Google Scholar] [CrossRef] [PubMed]
- Harrison, T.; Samuel, B.U.; Akompong, T.; Hamm, H.; Mohandas, N.; Lomasney, J.W.; Haldar, K. Erythrocyte G protein-coupled receptor signaling in malarial infection. Science 2003, 301, 1734–1736. [Google Scholar] [CrossRef] [PubMed]
- Prudencio, M.; Rodrigues, C.D.; Hannus, M.; Martin, C.; Real, E.; Goncalves, L.A.; Carret, C.; Dorkin, R.; Rohl, I.; Jahn-Hoffmann, K.; et al. Kinome-wide rnai screen implicates at least 5 host hepatocyte kinases in plasmodium sporozoite infection. PLoS Pathog. 2008, 4, e1000201. [Google Scholar] [CrossRef] [PubMed]
- Ward, P.; Equinet, L.; Packer, J.; Doerig, C. Protein kinases of the human malaria parasite plasmodium falciparum: The kinome of a divergent eukaryote. BMC Genom. 2004, 5, 79. [Google Scholar] [CrossRef] [PubMed]
- Rudolph, J.; Murray, L.J.; Ndubaku, C.O.; O’Brien, T.; Blackwood, E.; Wang, W.; Aliagas, I.; Gazzard, L.; Crawford, J.J.; Drobnick, J.; et al. Chemically diverse group I p21-activated kinase (PAK) inhibitors impart acute cardiovascular toxicity with a narrow therapeutic window. J. Med. Chem. 2016, 59, 5520–5541. [Google Scholar] [CrossRef] [PubMed]
- Lindvall, M.; Karpov, A.; Bellamacina, C.; Daniel, D.; Fabbro, D.; Fernandez, C.; Jacob, S.; Jahnke, W.; Moebitz, H.; Pecchi, S.; et al. Discovery of novel and highly selective allosteric inhibitors of PAK1. Abstr. Pap. Am. Chem. Soc. 2013, 246. [Google Scholar]
- Coleman, N.; Kissil, J. Recent advances in the development of p21-activated kinase inhibitors. Cell. Logist. 2012, 2, 132–135. [Google Scholar] [CrossRef] [PubMed]
| Name of Pathogen | Pathogen Type | Effect on PAK1 | Pathogen Mediator | Involved Pathway Components | Outcome | Pathogen Process | References |
|---|---|---|---|---|---|---|---|
| HIV | RNA virus | Activation | Nef | PAK1, PAK2, PI3K, BAD | Anti-apoptotic | Pathogen survival | [22,23,24,25,26,27,28,30] |
| Activation | gp120 | Rac1, PAK1, LIMK1 | Actin polymerization | Invasion | [34] | ||
| HBV | DNA virus | Upregulation | HBx | PAK1 | Anti-apoptotic (Anti-Anoikis) | Pathogen survival | [35,36,37] |
| Human herpes virus 8 (HHV-8) | DNA virus | Activation | GPCR | Rac1, Cdc42, PAK1, IKKβ, IκB, NF-κB | Cellular transformation to Kaposi’s sarcoma | Pathogen survival | [38] |
| Not known | Rac1, PAK1, (VE)-cadherin, β-catenin | Disassembly of cell junctions, enhanced vascular permeability | Cytoskeletal changes independent of invasion | [39] | |||
| Alphaherpes-virus (e.g., Herpes simplex virus, Pseudorabies virus) | DNA virus | Activation | US3 | PAK1, Bad, Bid | Anti-apoptotic, protection against NK cells | Pathogen survival | [40,41,42] |
| Activation | US3 | PAK1, cofilin | Breakdown of actin stress fibres, formation of actin dependent cell projections | Cytoskeletal changes independent of invasion | [19,43] | ||
| Influenza A | RNA virus | Activation | Not known | PAK1, MEK1 | Higher viral titres | Pathogen development | [44] |
| EPEC | Bacteria | Activation | T3SS effector protein EspG | PAK1, PAK2 | Not known | Not known | [31,32] |
| B. bacilliformis | Bacteria | Activation | Not known | Rac1, Cdc42, PAK1 | Formation of filopodia and lamellipodia | Cytoskeletal changes | [45] |
| N. gonorrhoeae | Bacteria | Activation | Not known | AP-1, JNK, Rac1, Cdc42, PAK2, PAK1 | Inflammatory response | Host nuclear response | [33,46] |
| H. pylori | Bacteria | Activation | T4SS effector protein CagA | PAK1, NIK, IKKs, NF-kB | Inflammatory responses | Host nuclear response | [47,48,49,50] |
| Activation | T4SS effector(s) | Rac1, PAK1 | Increased motility of host cell | Cytoskeletal changes independent of invasion | [51] | ||
| E. coli K1 | Bacteria | Inactivation | Not known | MLCK, PAK1 | Actin-mediated internalisation | Invasion | [52] |
| Non-typeable H. influenza (NTHi) | Bacteria | Activation | Not known | Rac1, PI3K, PAK1, Op18/stathmin | Microtubule polymerization | Invasion | [53,54,55] |
| S. Typhimurium | Bacteria | Activation | T3SS effectors proteins SopB, SopE, SopE2 | GTPases, PAK1, c-Abl, STAT3 | Intracellular growth of the pathogen | Pathogen development | [56] |
| T. cruzi | Parasite | Activation? | PDNF | PAK1, Akt | Anti-apoptotic | Pathogen survival | [57,58] |
| P. falciparum, P. berghei | Parasite | Activation | Not known | PAK1, MEK1 | Parasite survival | Pathogen development | [59,60] |
| T. annulata | Parasite | upregulation | Not known | IKK, IκB, NFκB, PAK1 | Host cellular transformation | Host nuclear response | [61,62] |
| Pathogen | Type of Pathogen | Cell Factor (Receptor) | Known Pathway Components | Effector of PAK1/Function | References |
|---|---|---|---|---|---|
| A-MLV | RNA virus | Rac1, PAK1, RhoG | [68] | ||
| Echovirus 1 | RNA virus | α2β1 integrin | PI3K, PLC, PKCα, Rac1, PAK1 | CtBP-1/BARS, Macropinosome closure | [64,69] |
| FMDV | RNA virus | αvβ1, αvβ3, αvβ6, αvβ8 integrin | RTK, Rac1, PAK1, myosin II, PKC | [70] | |
| Ebola virus | RNA virus | C-type lectin, phosphotidylserine receptor | PAK1 | bCtBP-1/Bars | [72,73] |
| Influenza A virus | RNA virus | Sialic acids | PAK1, Src | [74] | |
| SGIV | DNA virus | PAK1, Rac1 | [75] | ||
| ASFV | DNA virus | EGFR | PI3K-Akt, Rac1, PAK1 | [76] | |
| HAdV-3 | DNA virus | CD46 and integrins | PAK1 | CtBP-1/fission and stabilization of macropinosome | [77,78,79] |
| Vaccinia virus | DNA virus | MARCO, phosphotidylserine receptor | Cdc42 or Rac1, PAK1 | Actin rearrangement, formation of filopodia, membrane blebbing | [67,80,81] |
| S. Typhimurium | Bacteria | Type III secretion system stimulates macropinocytosis; no host cell entry occurs | SopE (S. Typhimurium protein), JNK, PAK1 | JNK activation, membrane ruffling | [85,86] |
| T. cruzi | Parasite | Rac1, PAK1 | [95,96] |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
John Von Freyend, S.; Kwok-Schuelein, T.; Netter, H.J.; Haqshenas, G.; Semblat, J.-P.; Doerig, C. Subverting Host Cell P21-Activated Kinase: A Case of Convergent Evolution across Pathogens. Pathogens 2017, 6, 17. https://doi.org/10.3390/pathogens6020017
John Von Freyend S, Kwok-Schuelein T, Netter HJ, Haqshenas G, Semblat J-P, Doerig C. Subverting Host Cell P21-Activated Kinase: A Case of Convergent Evolution across Pathogens. Pathogens. 2017; 6(2):17. https://doi.org/10.3390/pathogens6020017
Chicago/Turabian StyleJohn Von Freyend, Simona, Terry Kwok-Schuelein, Hans J. Netter, Gholamreza Haqshenas, Jean-Philippe Semblat, and Christian Doerig. 2017. "Subverting Host Cell P21-Activated Kinase: A Case of Convergent Evolution across Pathogens" Pathogens 6, no. 2: 17. https://doi.org/10.3390/pathogens6020017





