IgG Avidity Test in Congenital Toxoplasmosis Diagnoses in Newborns
Abstract
:1. Introduction
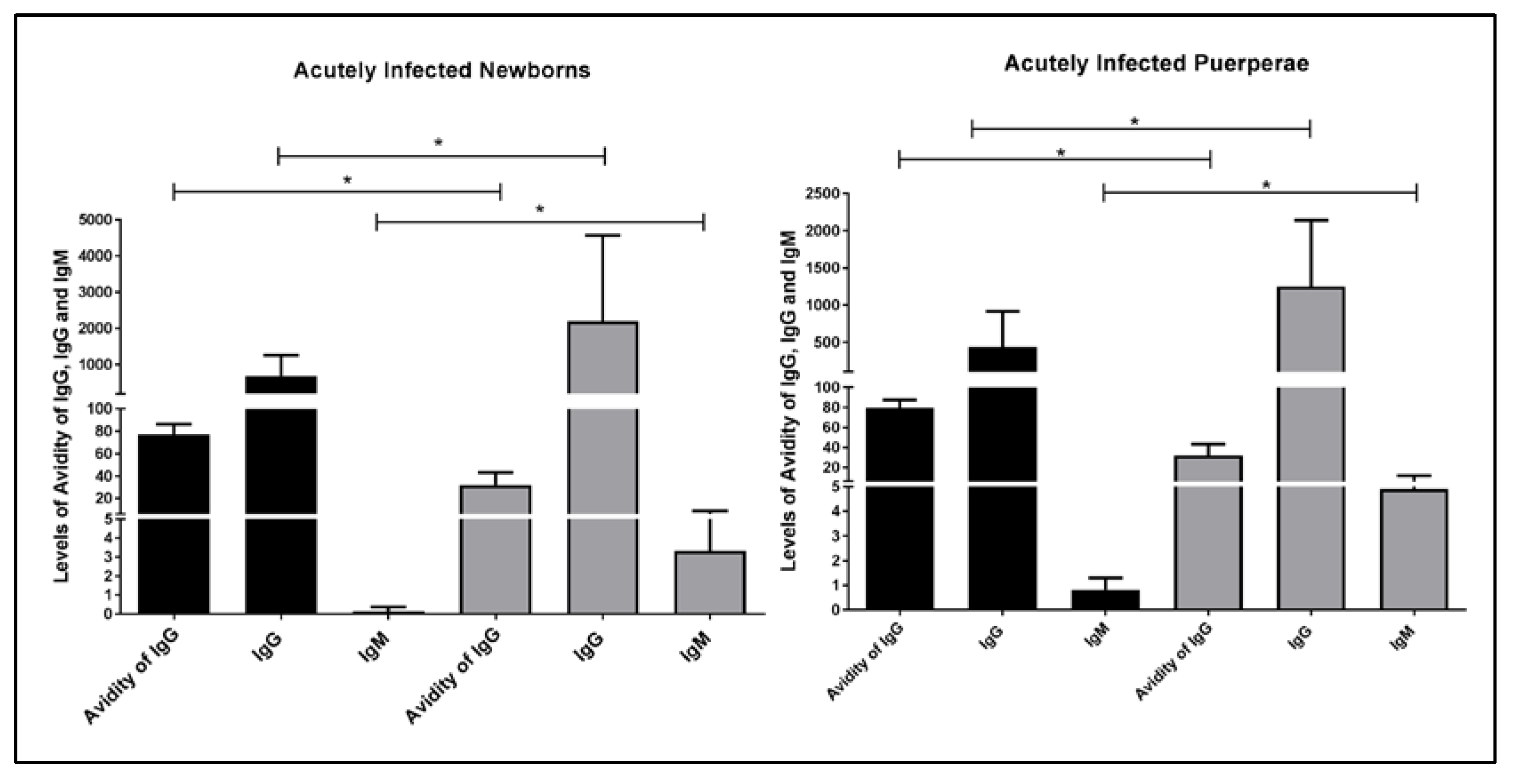
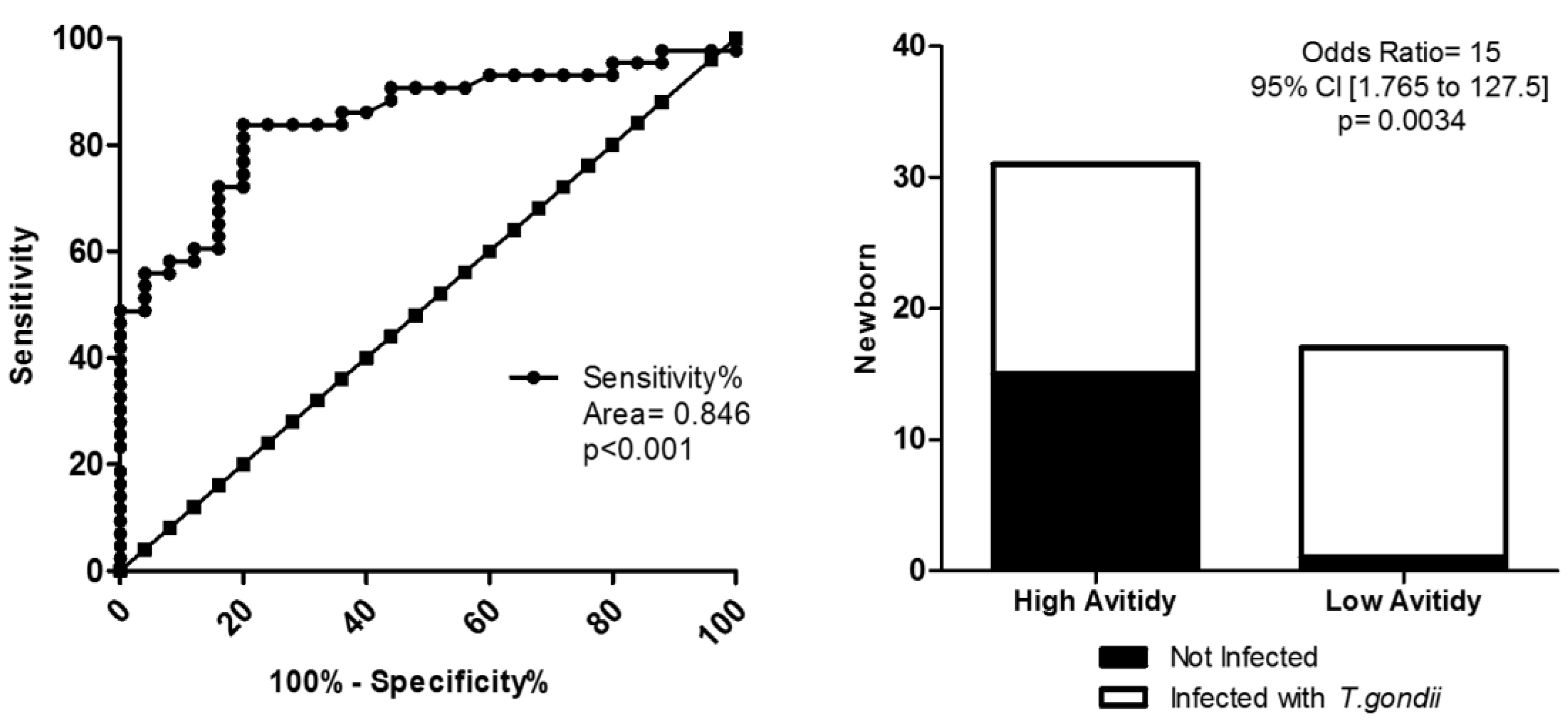
2. Results
3. Discussion
4. Materials and Methods
4.1. Type of Study and Population
4.2. Ethical and Legal Aspects
4.3. Inclusion Criteria
- Presence of anti-T. gondii IgM and/or IgA in any concentration (above the cut-off) after the fifth day of birth. These immunoglobulins can pass to a NB during labor, but they do not cross the placental barrier and their encounter after the fifth day of birth (average lifespan) is diagnosed as congenital infection;
- High anti-T. gondii IgG (greater than or equal to 300 IU/mL), associated with clinical alterations compatible with congenital toxoplasmosis;
- Cerebrospinal fluid abnormalities, such as the presence of antibodies of the IgG class (measured thought indirect immunofluorescence), the identification of Toxoplama gondii by PCR, and the inoculation of the CSF of the newborns suspected of congenital infection in mice;
- Increase in IgG concentration or maintenance of elevated levels for more than three months with or without clinical infection manifestations;
- IgG presence in greater concentration than that from the mother, in at least four dilutions;
- Protozoa identification by PCR.
4.4. Laboratory Tests
4.4.1. Detection of IgG and IgM Serum Levels and IgG Avidity Tests
4.4.2. Fundoscopy
4.4.3. Cranial Ultrasound
4.4.4. Cerebrospinal Fluid Analysis
4.5. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dubey, J.P. The history of Toxoplasma gondii—The first 100 years. J. Eukaryot. Microbiol. 2008, 55, 467–475. [Google Scholar] [CrossRef] [PubMed]
- McAuley, J.B. Congenital toxoplasmosis. J. Pediatric Infect. Dis. Soc. 2014, 3 (Suppl. 1), 30–35. [Google Scholar]
- Derouin, F.; Pelloux, H. Prevention of toxoplasmosis in transplant patients. Clin. Microbiol. Infect. 2008, 14, 1089–1101. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.L.; Dargelas, V.; Roberts, J.; Press, C.; Remington, J.S.; Montoya, J.G. Risk factors for Toxoplasma gondii infection in the United States. Clin. Infect. Dis. 2009, 49, 878–884. [Google Scholar] [CrossRef] [PubMed]
- Esch, K.J.; Petersen, C.A. Transmission and epidemiology of zoonotic protozoal diseases of companion animals. Clin. Microbiol. Rev. 2013, 26, 58–85. [Google Scholar] [CrossRef] [PubMed]
- Gelaye, W.; Kebede, T.; Hailu, A. High prevalence of anti-toxoplasma antibodies and absence of Toxoplasma gondii infection risk factors among pregnant women attending routine antenatal care in two Hospitals of Addis Ababa, Ethiopia. Int. J. Infect. Dis. 2015, 34, 41–45. [Google Scholar] [CrossRef] [PubMed]
- Rocha, D.d.S.; Moura, R.L.d.S.; Maciel, B.M.; Guimarães, L.A.; O’Dwyer, H.N.S.; Munhoz, A.D.; Albuquerque, G.R. Detection of Toxoplasma gondii DNA in naturally infected shees milk. Genet. Mol. Res. 2015, 14, 8658–8662. [Google Scholar] [CrossRef] [PubMed]
- Chaudhry, S.A.; Gad, N.; Koren, G. Toxoplasmosis and pregnancy. Can. Fam. Physician 2014, 60, 334–336. [Google Scholar] [PubMed]
- Vetrivel, U.; Muralikumar, S.; Mahalakshm, B.; Therese, K.L.; Madhavan, H.N.; Alameen, M.; Thirumudi, I. Multilevel Precision-Based Rational Design of Chemical Inhibitors Targeting the Hydrophobic Cleft of Toxoplasma gondii Apical Membrane Antigen 1 (AMA1). Genom. Inf. 2016, 14, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Jenum, P.A.; Stray-Pedersen, B.; Gundersen, A.G. Improved diagnosis of primary Toxoplasma gondii infection in early pregnancy by determination of antitoxoplasma immunoglobulin G avidity. J. Clin. Microbiol. 1997, 35, 1972–1977. [Google Scholar] [PubMed]
- Evengård, B.; Petersson, K.; Engman, M.L.; Wiklund, S.; Ivarsson, S.A.; Teär-Fahnehjelm, K.; Forsgren, M.; Gibert, R.; Malm, G. Low incidence of toxoplasma infection during pregnancy and in newborns in Sweden. Epidemiol. Infect. 2001, 127, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Figueiró-Filho, E.A.; Lopes, A.H.A.; Senefonte, F.R.D.A.; Júnior, V.G.d.S.; Botelho, C.A.; Figueiredo, M.S.; Duarte, G. Toxoplasmose aguda: Estudo da freqüência, taxa de transmissão vertical e relação entre os testes diagnósticos materno-fetais em gestantes em estado da Região Centro-Oeste do Brasil. Rev. Bras. Ginecol. Obset. 2005, 27, 442–449. [Google Scholar] [CrossRef]
- Dubey, J.P.; Lago, E.G.; Gennari, S.M.; Jones, J.L. Toxoplasmosis in humans and animals in Brazil: High prevalence, high burden of disease, and epidemiology. Parasitology 2012, 139, 1375–1424. [Google Scholar] [CrossRef] [PubMed]
- Avelino, M.M.; Campos, D.; Parada, J.B.D.; Castro, A.M.D. Risk factors for Toxoplasma gondii infection in women of childbearing age. Braz. J. Infect. Dis. 2004, 8, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Avelino, M.M.; Amaral, W.N.; Rodrigues, I.M.X.; Rassi, A.R.; Gomes, M.B.; Costa, T.L.; Castro, A.M. Congenital toxoplasmosis and prenatal care state programs. BMC Infect. Dis. 2014, 14, 33. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.L.; Silva, R.A.; Fux, B.; Madureira, A.P.; Sousa, F.F.; Margonari, C. Aspectos epidemiológicos da toxoplasmose e avaliação de sua soroprevalência em gestantes. Rev. Soc. Bras. Med. Trop. 2012, 45, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Câmara, J.T.; Silva MG, D.A.; Castro, A.M.D. Prevalência de toxoplasmose em gestantes atendidas em dois centros de referência em uma cidade do Nordeste, Brasil. Rev. Bras. Ginecol. Obset. 2015, 37, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, M.G.; Vinaud, M.C.; De Castro, A.M. Prevalence of toxoplasmosis in pregnant women and vertical transmission of Toxoplasma gondii in patients from basic units of health from Gurupi, Tocantins, Brazil, from 2012 to 2014. PLoS ONE 2015, 10, e0141700. [Google Scholar]
- Yamada, H.; Nishikawa, A.; Yamamoto, T.; Mizue, Y.; Yamada, T.; Morizane, M.; Tairaku, S.; Nishihira, J. Prospective study of congenital toxoplasmosis screening with use of IgG avidity and multiplex nested PCR methods. J. Clin. Microbiol. 2011, 49, 2552–2556. [Google Scholar] [CrossRef] [PubMed]
- English, E.D.; Adomako-Ankomah, Y.; Boyle, J.P. Secreted effectors in Toxoplasma gondii and related species: Determinants of host range and pathogenesis? Parasite Immunol. 2015, 37, 127–140. [Google Scholar] [CrossRef] [PubMed]
- De Souza-e-Silva, C.H.; Vasconcelos-Santos, D.V.; de Andrade, G.Q.; Carellos, E.V.M.; de Castro Romanelli, R.M.; de Resende, L.M.; Januário, J.N.; Carneiro, M.; de Aguiar Vasconcelos Carneiro, A.C.; de Almeida Vitor, R.W. Association between IgG subclasses against Toxoplasma gondii and clinical signs in newborns with congenital toxoplasmosis. Pediatr. Infect. Dis. J. 2013, 32, 13–16. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Wang, Z.; Huang, S.; Zhu, X. Diagnosis of toxoplasmosis and typing of Toxoplasma gondii. Parasites Vectors 2015, 1, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Halonen, S.K.; Weiss, L.M. Toxoplasmosis. Handb. Clin. Neurol. 2013, 114, 125–145. [Google Scholar]
- Villard, O.; Cimon, B.; L’Ollivier, C.; Fricker-Hidalgo, H.; Godineau, N.; Houze, S.; Paris, L.; Pelloux, H.; Villena, I.; Candolfi, E. Serological diagnosis of Toxoplasma gondii infection. Recommendations from the French National Reference Center for Toxoplasmosis. Diagn. Microbiol. Infect. Dis. 2016, 84, 22–33. [Google Scholar] [CrossRef] [PubMed]
- Rabilloud, M.; Wallon, M.; Peyron, F. In utero and at birth diagnosis of congenital toxoplasmosis: Use of likelihood ratios for clinical management. Pediatr. Infect. Dis. J. 2010, 29, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Liesenfeld, O.; Montoya, J.G.; Kinney, S.; Press, C.; Remington, J.S. Effect of testing for IgG avidity in the diagnosis of Toxoplasma gondii infection in pregnant women: Experience in a US reference laboratory. J. Infect. Dis. 2001, 183, 1248–1253. [Google Scholar] [CrossRef] [PubMed]
- Montoya, J.G.; Rosso, F. Diagnosis and management of toxoplasmosis. Clin. Perinatol. 2005, 32, 705–726. [Google Scholar] [CrossRef] [PubMed]
- Remington, J.S.; Thulliez, P.; Montoya, J.G. Recent Developments for Diagnosis of Toxoplasmosis MINIREVIEW Recent Developments for Diagnosis of Toxoplasmosis. J. Clin. Microbiol. 2004, 42, 941–945. [Google Scholar] [CrossRef] [PubMed]
- L’Ollivier, C.; Wallon, M.; Faucher, B.; Piarroux, R.; Peyron, F.; Franck, J. Comparison of mother and child antibodies that target high-molecular-mass Toxoplasma gondii antigens by immunoblotting improves neonatal diagnosis of congenital toxoplasmosis. Clin. Vaccine Immunol. 2012, 19, 1326–1328. [Google Scholar] [CrossRef] [PubMed]
- Rorman, E.; Zamir, C.S.; Rilkis, I.; Ben-David, H. Congenital toxoplasmosis—Prenatal aspects of Toxoplasma gondii infection. Reprod. Toxicol. 2006, 21, 458–472. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, L.; Lin, G.; Sun, Y.; Zhang, R.; Xie, J.; Li, J. Results of the national external quality assessment for toxoplasmosis serological testing in China. PLoS ONE 2015, 10, e0130003. [Google Scholar] [CrossRef] [PubMed]
- Olariu, T.R.; Remington, J.S.; McLeod, R.; Alam, A.; Montoya, J.G. Severe congenital toxoplasmosis in the United States: Clinical and serologic findings in untreated infants. Pediatr. Infect. Dis. J. 2011, 30, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Eida, O.M.; Eida, M.M.; Ahmed, A.B. Evaluation of polymerase chain reaction on amniotic fluid for diagnosis of congenital toxoplasmosis. J. Egypt. Soc. Parasitol. 2009, 39, 541–550. [Google Scholar] [PubMed]
- Belaz, S.; Gangneux, J.P.; Dupretz, P.; Guiguen, C.; Robert-Gangneux, F. A 10-year retrospective comparison of two target sequences, REP-529 and B1, for Toxoplasma gondii detection by quantitative PCR. J. Clin. Microbiol. 2015, 53, 1294–1300. [Google Scholar] [CrossRef] [PubMed]
- Murat, J.B.; L’Ollivier, C.; Hidalgo, H.F.; Franck, J.; Pelloux, H.; Piarroux, R. Evaluation of the new Elecsys Toxo IgG avidity assay for toxoplasmosis and new insights into the interpretation of avidity results. Clin. Vaccine Immunol. 2012, 19, 1838–1843. [Google Scholar] [CrossRef] [PubMed]
- Prusa, A.R.; Hayde, M.; Pollak, A.; Herkner, K.R.; Kasper, D.C. Evaluation of the liaison automated testing system for diagnosis of congenital toxoplasmosis. Clin. Vaccine Immunol. 2012, 19, 1859–1863. [Google Scholar] [CrossRef] [PubMed]
- Hedman, K.; Lappalainen, M.; Seppaia, I.; Mäkelä, O. Recent primary toxoplasma infection indicated by a low avidity of specific IgG. J. Infect. Dis. 1989, 159, 736–740. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.; Hedman, K.; Luyasu, V.; Zufferey, J.; Bessières, M.H.; Blatz, R.M.; Candolfi, E.; Decoster, A.; Enders, G.; Gross, U. Multicenter evaluation of strategies for serodiagnosis of primary infection with Toxoplasma gondii. Eur. J. Clin. Microbiol. Infect. Dis. 2001, 20, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Emelia, O.; Rahana, A.R.; Mohamad Firdaus, A.; Cheng, H.S.; Nursyairah, M.S.; Fatinah, A.S.; Azmawati, M.N.; Siti, N.A.; Aisah, M.Y. IgG avidity assay: A tool for excluding acute toxoplasmosis in prolonged IgM titer sera from pregnant women. Trop. Biomed. 2014, 31, 633–640. [Google Scholar] [PubMed]
- Haeri, M.R.; Jalalizadegan, B.; Tabatabaie, F. Recognition of acute toxoplasmosis with IgG avidity ELISA test in the pregnant women (the first trimester) in Qom Province, Iran, during two years (2012-2013). Am. J. Life Sci. 2012, 2, 18–21. [Google Scholar]
- Candolfi, E.; Pastor, R.; Huber, R.; Filisetti, D.; Villard, O. IgG avidity assay firms up the diagnosis of acute toxoplasmosis on the first serum sample in immunocompetent pregnant women. Diagn. Microbiol. Infect. Dis. 2007, 58, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Buffolano, W.; Lappalainen, M.; Hedman, L.; Ciccimarra, F.; Del Pezzo, M.; Rescaldani, R.; Gargano, N.; Hedman, K. Delayed maturation of IgG avidity in congenital toxoplasmosis. Eur. J. Clin. Microbiol. Infect. Dis. 2004, 23, 825–830. [Google Scholar] [CrossRef] [PubMed]
- Mukaka, M.M. Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med. J. 2012, 24, 69–71. [Google Scholar] [PubMed]
- Reis, M.M.; Tessaro, M.M.; D’Azevedo, P.A. Toxoplasma-IgM and IgG-avidity in single samples from areas with a high infection rate can determine the risk of mother-to-child transmission. Rev. Inst. Med. Trop. Sao Paulo 2006, 48, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Flori, P.; Tardy, L.; Patural, H.; Bellete, B.; Varlet, M.N.; Hafid, J.; Raberin, H.; Sung, R.T. Reliability of immunoglobulin G antitoxoplasma avidity test and effects of treatment on avidity indexes of infants and pregnant women. Clin. Diagn. Lab. Immunol. 2004, 11, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Sensini, A.; Pascoli, S.; Marchetti, D.; Castronari, R.; Marangi, M.; Sbaraglia, G.; Cimmino, C.; Favero, A.; Castelletto, M.; Mottola, A. IgG avidity in the serodiagnosis of acute Toxoplasma gondii infection: A multicenter study. Clin. Microbiol. Infect. 1996, 2, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Mattos, C.d.C.B.; Spegiorin, L.L.C.J.F.; Meira, C.d.S.; Silva, T.C.; FerreirA, A.I.C.; Nakashima, F.; Pereira-Chioccola, V.L.; Mattos, L.C. Anti-Toxoplasma gondii antibodies in pregnant women and their newborn infants in the region of São José do Rio Preto, São Paulo, Brazil. Sao Paulo Med. 2011, 129, 261–266. [Google Scholar] [CrossRef]
- Murat, J.B.; Souvignet, A.; Fricker-Hidalgo, H.; Brenier-Pinchart, M.P.; Bost-Bru, C.; Pelloux, H. Assessment of the IgA immunosorbent agglutination assay for the diagnosis of congenital toxoplasmosis on a series of 145 toxoplasmic seroconversions. Clin. Vaccine Immunol. 2015, 22, 456–458. [Google Scholar] [CrossRef] [PubMed]
- Findal, G.; Stray-Pedersen, B.; Holter, E.K.; Berge, T.; Jenum, P.A. Persistent low toxoplasma IgG avidity is common in pregnancy: Experience from antenatal testing in Norway. PLoS ONE 2015, 10, e0145519. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.E.; Freeman, K.; Lago, E.G.; Bahia-Oliveira, L.M.; Tan, H.K.; Wallon, M.; Buffolano, W.; Stanford, M.R.; Petersen, E.; European Multicentre Study on Congenital Toxoplasmosis (EMSCOT). Ocular sequelae of congenital toxoplasmosis in Brazil compared with Europe. PLoS Negl. Trop. Dis. 2008, 2, e277. [Google Scholar] [CrossRef] [PubMed]
- Melamed, J. Contributions to the history of ocular toxoplasmosis in Southern Brazil. Mem. Inst. Oswaldo Cruz 2009, 104, 358–363. [Google Scholar] [CrossRef] [PubMed]
| Newborn | Puerperae | |||||
|---|---|---|---|---|---|---|
| LA | HA | GZ | LA | HA | GZ | |
| NB from AIP with LA | 100% (n = 17) | 0% (n = 0) | 0% (n = 0) | 88% (n = 15) | 0% (n = 0) | 22% (n = 2) |
| NB from AIP with HA | 0% (n = 0) | 96.7% (n = 30) | 3.33% (n = 1) | 0% (n = 0) | 100% (n = 31) | 0% (n = 0) |
| NB from CIP | 0% (n = 0) | 100% (n = 40) | 0% (n = 0) | 0% (n = 0) | 100% (n = 40) | 0% (n = 0) |
| Total | 19.32% (n = 17) | 79.54% (n = 70) | 1.14% (n = 1) | 17.94% (n = 15) | 80.68% (n = 71) | 2.27% (n = 2) |
| Puerperae IgG | NB IgM | Puerperae IgM | IgG Avidity in NB | IgG Avidity in Puerperae | ||
|---|---|---|---|---|---|---|
| NB IgG | R | 0.809 | 0.460 | 0.179 | –0.170 | –0.450 |
| p | <0.001 * | 0.001 * | 0.224 | 0.113 | <0.001 * | |
| Puerperae IgG | R | 0.274 | 0.177 | –0.199 | –0.535 | |
| p | 0.059 | 0.230 | 0.064 | <0.001 * | ||
| NB IgM | R | 0.835 | –0.559 | –0.535 | ||
| p | <0.001 * | <0.001 | <0.001 * | |||
| Puerperae IgM | R | –0.568 | –0.574 | |||
| p | <0.001 * | <0.001 * | ||||
| IgG Avidity in NB | R | 0.256 | ||||
| p | 0.016 * | |||||
| Clinical Manifestations | NB with Low Avidity of IgG | NB with High Avidity of IgG | p Value |
|---|---|---|---|
| Absence | 43.8% (7/16) | 68.7% (11/16) | 0.0006 * |
| Presence | 56.2% (9/16) | 31.3% (5/16) | |
| Cortical-subcortical Dysfunction | 11.1% (1/9) | – | |
| Cerebral Calcification | 22.2% (2/9) | 80.0% (4/5) | |
| Prematurity | 11.1% (1/9) | – | |
| Hydrocephaly | 44.4% (4/9) | – | |
| Systemic Toxoplasmosis | 11.1% (1/9) | – | |
| Microcephaly | 11.1% (1/9) | – | |
| Hepatoesplenomegaly | 22.2% (2/9) | – | |
| Blindness | 22.2% (2/9) | – | |
| Generalized Lymph Node Form | – | 20.0% (1/5) | |
| Neuro-optic Form | 22.2% (2/9) | – |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fonseca, Z.C.; Rodrigues, I.M.X.; Melo, N.C.e.; Avelar, J.B.; Castro, A.M.; Avelino, M.M. IgG Avidity Test in Congenital Toxoplasmosis Diagnoses in Newborns. Pathogens 2017, 6, 26. https://doi.org/10.3390/pathogens6020026
Fonseca ZC, Rodrigues IMX, Melo NCe, Avelar JB, Castro AM, Avelino MM. IgG Avidity Test in Congenital Toxoplasmosis Diagnoses in Newborns. Pathogens. 2017; 6(2):26. https://doi.org/10.3390/pathogens6020026
Chicago/Turabian StyleFonseca, Zulmirene Cardoso, Isolina Maria Xavier Rodrigues, Natália Cruz e Melo, Juliana Boaventura Avelar, Ana Maria Castro, and Mariza Martins Avelino. 2017. "IgG Avidity Test in Congenital Toxoplasmosis Diagnoses in Newborns" Pathogens 6, no. 2: 26. https://doi.org/10.3390/pathogens6020026




