Abstract
Aggregatibacter actinomycetemcomitans is a Gram-negative bacterium that is part of the oral microbiota. The aggregative nature of this pathogen or pathobiont is crucial to its involvement in human disease. It has been cultured from non-oral infections for more than a century, while its portrayal as an aetiological agent in periodontitis has emerged more recently. A. actinomycetemcomitans is one species among a plethora of microorganisms that constitute the oral microbiota. Although A. actinomycetemcomitans encodes several putative toxins, the complex interplay with other partners of the oral microbiota and the suppression of host response may be central for inflammation and infection in the oral cavity. The aim of this review is to provide a comprehensive update on the clinical significance, classification, and characterisation of A. actinomycetemcomitans, which has exclusive or predominant host specificity for humans.
1. Introduction
Aggregatibacter actinomycetemcomitans is the type species of genus Aggregatibacter, which is part of bacterial family Pasteurellaceae. [Bacterium actinomycetem comitans] was cultured from actinomycotic lesions of humans in the early 20th century. The absence of related microorganisms rendered it difficult to classify this Gram-negative, fastidious rod, and isolates cultured from invasive infections were referred to national reference institutions. The expanding field of oral microbiology with a focus on periodontitis, particularly the localized, severe form that affects adolescents, caused a renewed interest in the bacterial species. In 2006, the current species name was adopted, and A. actionomycetemcomtians became type species of a new bacterial genus, Aggregatibacter. Influential events in the narrative of A. actinomycetemcomitans are listed in Table 1.

Table 1.
Seminal events in the history of Aggregatibacter actinomycetemcomitans.
A. actinomycetemcomitans is one species among a plethora of microorganisms that constitute the oral microbiota. It has been estimated that at least 500 different bacterial species colonise the oral cavity [13,14,15], and half of these may have been cultivated and validly named because of vigorous efforts directed to the cultivation of oral bacteria. Analysis of a large number of 16S rRNA gene clones from studies of the oral microbiota increased the number of taxa to 619 [16], and the number is steadily increasing (www.homd.org). Bacterial species cannot be validly named in the absence of a cultured type strain [17]. Although “taxa”, “phylotypes” or “operative taxonomic units” revealed by deep sequencing of polymerase chain reaction (PCR)-amplified 16S rRNA genes have relevance for recognition of microbial fluctuations in health and disease, only cultivable microbiota can be made subject to extensive characterisation, including adherence, animal experiments, antimicrobial susceptibility, co-culture, generation of mutants, and growth characteristics.
Carriage of A. actinomycetemcomitans appears to be highly host-specific. Although the spread and dissemination of bacterial clones occur, these are not frequent events; hosts tend to carry their strain from teething to edentulous old age [18]. Yet, the species encompasses properties that sometimes reveal its significance in human disease. Particularly, a single serotype b clonal lineage designated the JP2 clone is associated with a severe form of localised periodontitis and tooth loss in adolescents [12]. But rather than being the causative agent of aggressive periodontitis, A. actinomycetemcomitans may be necessary for the action of a consortium of bacterial partners by suppressing host defences [19]. It may be classified as a low abundance oral pathobiont, defined as a member of the microbiota that exerts specific effects on the host’s mucosal immune system associated with the development of disease [20]. Although A. actinomycetemcomitans may accompany (comitans) Actinomyces, the narrative of a pathobiont is not valid for other invasive infections such as infectious endocarditis, where A. actinomycetemcomitans—when identified—is detected as the sole pathogen by culture and/or PCR on removed heart valves. Severe periodontitis and infective endocarditis are two prominent diseases of very different prevalence, symptoms, and outcome. Although they may share a causative microorganism, a number of conditions is still unknown, and host factors, oral hygiene, and incidental circumstances may be instrumental.
The aim of the present review is to provide a comprehensive update on the characterisation, classification and clinical significance of A. actinomycetemcomitans with a particular focus on selected clinical entities. Adhesion, persistence, and inactivation of immune cells are probably essential for the understanding of the intimate association with the host, and these factors are detailed for the purpose of the elucidation of pathogenicity. A number of relevant publications and reviews of other important biochemical mechanisms of this bacterial species are listed in the relevant sections.
2. Taxonomy, Classification, Serotype (St) and Population Structure
More than 100 years ago, [Bacterium actinomycetem comitans] was co-isolated with Actinomyces from actinomycotic lesions of humans [1] (Actinomyces, ray fungus, referring to the radial arrangement of filaments in Actinomyces bovis sulfur granules; actinomycosis, a chronic disease characterised by hard granulomatous masses). Ample changes have occurred in the classification and nomenclature of this species. Despite the limited similarity with Actinobacillus lignieresii, it was reclassified as [Actinobacillus actinomycetemcomitans] in a seminal textbook from 1929 [2]. According to Cowan [21], the bacterium was placed in this genus because ‘neither Topley nor Wilson could think where to put it’. In 1962 the phenotypic resemblance of [Actinobacillus actinomycetemcomitans] with [Haemophilus aphrophilus] was described [3], and a subsequent relocation of [Actinobacillus actinomycetemcomitans] to genus Haemophilus occurred [22]. Nomenclatural classification as [Haemophilus actinomycetemcomitans] within the genus Haemophilus permitted antimicrobial susceptibility testing according to standards outlined by the US Clinical and Laboratory Standards Institute. Disk diffusion could be performed and interpreted on Haemophilus test medium (HTM) in 5% CO2, and HTM broth microdilution testing was carried out in ambient air [23]. However, the nomenclatural relocation did not result in a satisfying classification, because neither [Actinobacillus actinomycetemcomitans] nor [Haemophilus aphrophilus] are adequately related to Haemophilus influenzae, the type species of the genus Haemophilus. Finally, in 2006 the new genus Aggregatibacter was created to accomodate Aggregatibacter actinomycetemcomitans, Aggregatibacter aphrophilus and Aggregatibacter segnis [11]. A fourth Aggregatibacter species, Aggregatibacter kilianii, has recently been named (Figure 1) [24].
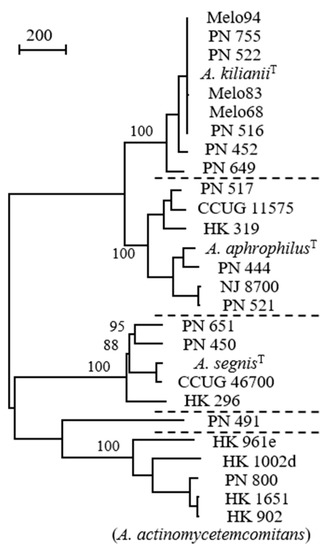
Figure 1.
Comparison of Aggregatibacter strains by whole genome sequences; distinct species are separated by dotted lines (strain PN_491 is unclustered). A total of 3261 positions with single nucleotide polymorphism (SNP) are included in the dataset. Values at nodes are percentages of bootstrap replications supporting the node (500 replicates). Bar represents 200 SNPs. Reprinted from Journal of Clinical Microbiology [24] with permission.
In the early 1980s, three distinct surface antigens of A. actinomycetemcomitans were identified [9], while six serotypes (a through f) were recognised by 2001. The serological specificity is defined by structurally and antigenically distinct O-polysaccharide components of their respective lipopolysaccharide molecules. A seventh St, designated St(g), with a 1:1 ratio of 2,4-di-O-methyl-rhamnose and 2,3,6-tri-O-methyl-glucose, was recently added [25]. St(a), St(b), and St(c) are globally dominant [26], but the distribution may vary according to ethnicity and geography. In Scandinavia, the three dominant serotypes are equally prevalent, while predominance of St(c) is observed in Chinese, Japanese, Korean, Thai and Vietnamese populations [27,28,29,30,31]; a noticeable high prevalence of St(e) has been reported among Japanese periodontitis patients [32]. Assessment of serotype-specific antibodies supports these findings, as all early-onset periodontitis patients from Turkey and Brazil had elevated antibody levels to St(c) and St(a), while St(b) levels were higher in the US [33,34].
An initial characterisation of the population structure of the species was published in 1994, using multi-locus enzyme electrophoresis [35]. Two large and four small divisions were identified, with division I (St(a) and St(d)) and III (St(b) and St(c)) encompassing 34% and 58% of the 97 strains analysed, respectively. Two St(e) strains occupied separate divisions (II and VI), one St(c) strain constituted electrophoretic division IV, while division V was composed of two St(a) and one non-serotypable strain. Sequencing of a 16S rRNA gene fragment from 35 strains suggested a different structure with three major clusters [36]. RNA cluster I included 12 strains of four serotypes (a, d, e, and f), all 10 St(b) strains belonged to RNA cluster II, while RNA cluster III only included St(c) strains (N = 10). Strains of particular serotypes were not exclusively confined to specific RNA clusters; one St(a) strain belonged to the St(b) cluster (II), and two divergent RNA clusters were composed of single strains, namely a St(c) (RNA cluster IV) and a St(e) strain (RNA cluster V), respectively [36].
One study attempted to establish a multi-locus sequence typing (MLST) scheme for A. actinomycetemcomitans [37]. Six gene fragments from the Haemophilus influenzae MLST scheme were used. The investigation focused on the JP2 clone, which contributed 66 of 82 strains. MLST has insufficient power to reveal dissemination patterns of clonally related strains, and point mutations of two pseudogenes present in the JP2 clone were more versatile in this respect [37]. Moreover, a MLST web site was not organised and, therefore, the benefits of a portable typing scheme were not corroborated. But MLST of 16 non-JP2 strains carefully selected from the enzyme electrophoresis study [35] suggested the existence of four phylogenetic clusters, rooted by an outgroup consisting of an uncommon St(e) strain. Two related clusters were composed of St(b) and St(c) strains, respectively, while a more distinct cluster encompassed strains of St(a), St(d) and St(e) [37].
Restriction fragment length polymorphism using various restriction enzymes and arbitrarily-primed PCR has been used to differentiate types of A. actinomycetemcomitans cultured from patients with severe periodontitis and healthy controls [30,37,38,39,40,41,42]. The method is versatile and discriminative, but lacks portability and a common nomenclature; thus, it is of value for individual studies of specific strains, but lacks general applicability and descriptive significance.
Finally, whole genome sequencing has been introduced for characterisation of the species [43,44,45]. In the largest study, sequences from two human strains of Aggregatibacter aphrophilus, 30 human A. actinomycetemcomitans strains, and one St(b) strain isolated from a rhesus macaque Old World monkey were used for selection of 397 core genes which were concatenated and trimmed to produce a single alignment of 335,400 bp [45]. Five clades were recognised, designated clade b, clade c, clade e/f, clade a/d and clade e’. Although the analysis clearly separated six strains of serotype b from six strains of serotype c, a close similarity was observed between these two clades, as well as between clade a/d and e/f. In contrast, the clade designated e’, encompassing four St(e) strains, was phylogenetically distinct. The open reading frames necessary for expression of St(e) antigen were highly conserved between clade e and clade e’ strains, but e’ strains were found to be missing the genomic island that carries genes encoding the cytolethal distending toxin. Moreover, the clade e’ strains were more related to an Old World primate strain and carried the unusual 16S rRNA type V sequence (RNA types as defined by Kaplan et al. [36]). Although bacterial species are not defined by DNA sequence, average nucleotide identity (ANI) values locate whole genome sequences from this group/clade outside the species boundary [44]. Thus, strains belonging to the so-called clade e’ (as well as the rhesus macaque monkey strain) may possibly be transferred to new species, and A. actinomycetemcomitans may be restricted to strains with exclusive host specificity for humans.
A recent study compared whole genome sequences of strains from blood stream infections supplemented with oral reference strains [46]. Exclusion of so-called clade e’ strains increased the number of core genes present in all strains from 1146 to 1357. Strains of A. actinomycetemcomitans are basically divided into three lineages (numbering of lineages differs from reference [44]). Lineage I encompasses the type strain and consists of two groups (St(b) and St(c), respectively). Lineage II consists of St(a) plus St(d)-(g). In contrast to lineage I, many strains of different serotypes from this lineage are competent for natural transformation, and the average size of genomes is approximately 10% larger than in lineage I. Lineage III also expresses St(a) membrane O polysaccharide, and the genome size is comparable to lineage II. However, all six investigated strains were incompetent for transformation due to inactivation of multiple competence genes [46].
In conclusion, St designations are valuable for initial typing of clinical strains, but insufficient for characterisation. Recognition of a general MLST scheme could be helpful, and whole genome sequences could be used for MLST and in silico serotyping, as well as further characterisation and epidemiologic investigations. The species description consisting of three separate lineages is figurative, but more knowledge on the new lineage III is needed to disclose the relevance for phenotype, host specificity and pathogenicity.
3. General Characteristics
A. actinomycetemcomitans is a fastidious, facultatively anaerobic, non-motile, small Gram-negative rod, 0.4–0.5 µm × 1.0–1.5 µm in size. Microscopically, the cells may appear as cocci in broth and in clinical samples. It grows poorly in ambient air, but well in 5% CO2 [47]. Colonies on chocolate agar are small, with a diameter of ≤0.5 mm after 24 h, but may exceed 1–2 mm after 48 h [48]. Primary colonies are rough-textured and adhere strongly to the surface of agar plates (Figure 2).

Figure 2.
(A) Tenacious, rough-textured colonies of A. actinomycetemcomitans strain HK1651 on chocolate agar. Diameter of colonies did not reach 2 mm after 3 days incubation in 5% CO2. (B) Clinical isolate incubated on TSBV (tryptic soy-serum-bacitracin-vancomycin) agar for 4 days in 5% CO2. Expression of the distinctive “star-shaped” colony is facilitated by growth on TSBV agar. Pictures by courtesy of Jan Berg Gertsen and Rolf Claesson.
3.1. Recovery, Phenotype, and Molecular Detection
Relevant sites in the oral cavity for sampling of A. actinomycetemcomitans are periodontal pockets, the mucosa, and saliva. Sampling techniques include use of sterile paper points to be inserted in periodontal pockets, cotton swab for the mucosa, and chewing on a piece of paraffin for the collection of stimulated saliva. For transport of paper points, the VMGAIII-medium is recommended [49]; samples collected with cotton swap can be transported in a salt buffer or in TE-buffer [50]. Saliva can be transported in tubes without additives. For short-time transportation, saliva can be transported in tubes without additives. Otherwise, it can be frozen or stored at room temperature in a Saliva DNA Preservation Buffer. Proteomic analysis of gingival crevicular fluid and saliva is an expanding diagnostic field that may require improvements in standardised collection techniques and devices [51,52].
The selective medium TSBV (tryptic soy-serum-bacitracin-vancomycin) agar [53] is commonly used for culture. If Enterobacterales are present in significant amounts in the samples, a modified version of TSBV is recommended [54]. Detection of A. actinomycetemcomitans in clinical samples renders limited information on prediction, progression, and treatment planning of periodontal disease. For these purposes, the proportion of the bacterium at diseased sites is more relevant. This is in line with the ecological plaque hypothesis [55]. The detection level of A. actinomycetemcomitans is around 100 viable bacteria (colony-forming units) per mL. Fusobacterium nucleatum and other strict anaerobes will grow on TSBV in the absence of oxygen. The total concentration of viable bacteria is estimated by parallel cultivation on 5% blood agar plates, and the proportion of A. actinomycetemcomitans in the sample can be calculated.
A. actinomycetemcomitans is suspected when rough-textured, tenacious colonies appear on selective agar after one or two days (Figure 2). The species is distinguished from closely related bacteria by a positive catalase reaction and negative β-galactosidase reaction. Salient biochemical characters of A. actinomycetemcomitans have been published [56]. In addition, the bacterium is readily identified by MALDI-TOF mass spectrometry [57]; however, the current version of the Bruker database (v3.1) only includes mass spectra from a limited number of strains, and modest log-scores are not unusual when clinical strains are examined.
Leukotoxicity, i.e., the capacity of the bacterium to kill or inactivate immune cells, is properly determined in biological assays involving human cell lines [58], but a semi-quantitative method based on hemolysis on blood agar plates has been reported [42,59]. Quantification of the leukotoxin by enzyme-linked immunosorbent assay (ELISA) is also used; most studies have assessed the leukotoxin released from the surface of the bacterium, either during growth in broth [60], or by treatment of bacteria cultured in media that inhibit leukotoxin release with a hypertonic salt solution [42]. Leukotoxicity may also be estimated by determination of the total amount of leukotoxin produced by the strain. Bacterial suspensions are solubilized by SDS, and the leukotoxin is subsequently quantitated by Western blot–based methodology [60]. It is anticipated that the amount of leukotoxin released from the bacterial cell surface reflects the total amount of leukotoxin produced, but this relationship remains to be corroborated.
Polymerase chain reaction (PCR) is frequently used for identification and characterisation of A. actinomycetemcomitans in clinical samples. The leukotoxin promoter was an early focal point [59]. PCR amplification of the ltx promoter region and visualization on gel can discriminate the JP2 genotype from other strains of the species [61], but preferential amplification of smaller products characterised by a 530-bp deletion will overestimate the prevalence of the JP2 genotype. Recent improvements in PCR offer more precise quantification of periodontal pathogens in a complex plaque biofilm [62]. By real-time or quantitative PCR (qPCR), the instrument reports the cycling threshold (CT)-value, which can estimate the concentration of the target in the sample. qPCR has been used to separately quantitate JP2 and non-JP2 genotypes [63]. To approximate the total number of bacteria by qPCR, the 16S rRNA gene is generally targeted. The method can only provide a rough estimate, as primers and probes may preferentially bind to certain bacterial phyla, and because the number of copies of the gene varies substantially between different bacterial species [64].
Serotypes a through f can be identified by PCR as described [65,66]. A method for detecting St(g) has not yet been described.
3.2. Aggregative Properties and the Leukotoxin Gene Operon
A. actinomycetemcomitans expresses three potential toxins, fimbriae and a number of adhesins, plus a number of other gene products that may have significance for microbial interplay, persistence, transformation to planktonic state, and pathogenicity (Table 2).

Table 2.
Genomic characteristics and putative virulence determinants of A. actinomycetemcomitans.
The distinct growth in broth as small granules adhering to the walls of the test tube was included in the initial description of [Bacterium actinomycetem comitans] [1]. Fresh isolates of A. actinomycetemcomitans invariably form colonies that are rough-textured with an opaque, star-shaped internal structure (Figure 2B). Subculture in broth yields clumps of autoaggregated cells that attach tightly to the glass, leaving a clear broth. A. actinomycetemcomitans possesses fimbriae, and these appendages can be irreversibly lost after prolonged subculture in the laboratory [77]. Antibodies to synthetic fimbrial peptide significantly reduce the binding of A. actinomycetemcomitans to saliva-coated hydroxyapatite beads, buccal epithelial cells and a fibroblast cell line, indicating a decisive role of these structures for adherence to multiple surfaces [78]. Moreover, autoaggregation (spontaneous formation of aggregates with rapid settling in un-agitated suspensions) was completely lost by a smooth-colony, isogenic variant [79]. Fimbriae are assembled as bundles of 5- to 7-nm-diameter pili composed of a 6.5 kDa protein designated Flp (fimbrial low-molecular-weight protein) [80,81]. The RcpA/B (rough colony proteins) were the first outer membrane proteins identified that were associated with rough colony variants [82], and they are encoded by a 14-gene locus designated the tad locus. The Tad (tight adherence) macromolecular transport system is a subtype of type II secretion. The tad locus is composed of nine tad, three rcp and two flp genes [67]. Mutation analysis of the naturally competent strain D7S revealed flp-1, rcpA, rcpB, tadB, tadD, tadE and tadF to be indispensable for expression of fimbriae, while mutants of five other genes expressed reduced levels of fimbriae, or fimbriae that had different gross appearance [83,84]. In a rat model, the tad locus was critical for colonizing the oral cavity and for pathogenesis, measured as maxillae bone loss and A. actinomycetemcomitans-specific IgG levels [85].
Many pathogenic bacteria can undergo phase variation, but smooth-to-rough conversion has not been substantiated for A. actinomycetemcomitans. Rather, the rough-to-smooth conversion is typically caused by mutations in the flp promoter region, and replacement with wild-type promoter can restore the rough phenotype [86]. However, one study indicated that smooth strains could re-express the fimbriae in low humidity environments [87].
In addition to expression of fimbriae decisive for autoaggregation and adherence to a wide range of solid surfaces (biofilm formation), A. actinomycetemcomitans encodes a spectrum of autotransporter adhesins, proteins that promote their own transport from the periplasm to the exterior surface, where they may be decisive for adhesion to specific human cellular epitopes. A homologue with similarity to the monomeric H. influenzae autotransporter, Hap, was designated Aae. Inactivation of aae in two naturally transformable strains caused a 70% reduction in adhesion to cultured epithelial cells [68]. Aae exhibits specificity for buccal epithelial cells from humans and Old World primates, and does not bind to human pharyngeal or cervical epithelial cells [88]. Two trimeric autotransporters with homology to the YadA adhesin/invasin family were identified. Omp100 has also been designated Api (Aggregatibacter putative invasin). Escherichia coli expressing ApiA bound to various types of human collagen plus fibronectin. Adhesion to human cells was specific to buccal epithelial cells from humans and Old-World primates, although the specificity was not as prominent as observed for AaE [70,89]. Screening of a large number of insertion transposon mutants identified the extracellular matrix adhesin A encoded by emaA, which is involved in collagen adhesion [90]. Collagen prevail in the supporting tissue of cardiac valves, and EmaA (extracellular matrix adhesin) may play a role in the pathogenesis of infective endocarditis [91].
Iron is an essential transition metal for nearly all forms of life. The host limits the availability of iron through a process termed nutritional immunity [92]. Haemolysis can be an initial step for release of iron from heme by Gram-negative bacteria. The RTX (repeats in toxin) family is an important group of toxins, whose name refers to glycine- and apartate-rich, calcium-binding repeats in the carboxy terminus of the toxin proteins [93]. RTX toxins are produced by many Gram-negative bacteria including members of family Pasteurellaceae – it has, indeed, been proposed that these toxins may originate in Pasteurellaceae [94].
In 1977, it was shown that polymorphonuclear leukocytes exposed to gingival bacterial plaque in vitro released lysosomal constituents [95], and the leukotoxin (Ltx) of A. actinomycetemcomitans was extracted and partially characterised in 1979 [6]. Ltx is a RTX cytolysin. By 1989, the gene was cloned and analysed [96,97], and the 530-bp deletion in the ltx promoter associated with enhanced expression of Ltx characterising the JP2 genotype was subsequently described [10]. The difference between minimally toxic and highly toxic strains were convincingly illustrated in clinical studies from Northern Africa [12]. The significance of the 530-bp deletion may reside in a potential transcriptional terminator spanning 100 bp [60]. The leukotoxin of A. actinomycetemcomitans is highly specific for human and primate white blood cells and is capable of neutralising local mucosal immune responses. However, purified leukotoxin can lyse sheep and human erythrocytes in vitro, and beta-haemolysis can be demonstrated on certain media [98].
In addition to the JP2 genotype characterised by the 530-bp promoter deletion, two other leukotoxin promoter variants have been reported. One genotype is characterised by a slightly enlarged (640-bp) deletion [99], while the other promoter variant carries an 886-bp insertion sequence [100]. Both these variants produce levels of leukotoxin similar to the JP2 genotype of A. actinomycetemcomitans.
3.3. Geographic Dissemination of Specific Genotypes
The JP2 clone of A. actinomycetemcomitans is suggested to have arisen 2400 years ago in the northern Mediterranean part of Africa [37]. The bacterial clone is endemically present in Moroccan and Ghanaian populations [12,101] and almost exclusively detected among individuals of African origin [37,102]. However, among 17 JP2 clone carriers, living in Sweden and identified during 2000–2014, ten were of Scandinavian heritage [31]. Among six of the identified JP2 clone carriers, three were of Swedish origin. Detection of the JP2 clone of A. actinomycetemcomitans has not been reported in Asian populations [30,100,103,104]. The occurrence of the JP2 clone of A. actinomycetemcomitans in Caucasians may be caused by horizontal transmission, and may weaken the theory of racial tropism of the clone [59]. More data and research are needed to explain the dissemination of the leukotoxic JP2 clone of A. actinomycetemcomitans.
Other genotypes characterised by an increased leukotoxic potential comprise a 640-bp deletion cultured from a host of Ethiopian origin [99], an 886-bp insertion sequence from a host of Japanese origin [100], and two strains of serotype c, originating from Thailand with a JP2-like deletion in the promoter region of ltx, and with virulence of similar magnitude to the JP2 genotype strains [105]. All these genotypes were collected from individuals with severe periodontitis.
4. Prevalence and Clinical Significance
Cultivable A. actinomycetemcomitans is present in at least 10% of periodontally healthy children with primary dentition [106]. An influential publication found carrier rates of 20% for normal juveniles, 36% for normal adults, 50% for adult periodontitis patients, and 90% for young periodontitis patients [107]. Early studies failed to culture the species from edentulous infants [108,109], but molecular studies using PCR on unstimulated saliva samples have challenged this association: 37 of 59 completely edentulous infants were positive for A. actinomycetemcomitans, reaching 100% at 12 months of age [110]. Vertical transmission is common. Two studies reported detection rates by culture of 30–60% in children of adult periodontitis patient, and the genotypes of the strains were always identical [111,112]. A smaller study from Brazil of women with severe chronic periodontitis did not corroborate this finding, as the two culture-positive children carried genotypes that were different from those of their mothers [113]. Horizontal transmission of A. actinomycetemcomitans can occur, and transmission rates between 14% and 60% between spouses have been estimated [18,114]. However, members of most families with aggressive periodontitis also harbour additional clonal types of A. actinomycetemcomitans [115].
Once colonized, A. actinomycetemcomitans remains detectable in patients with periodontitis. Irrespective of periodontal treatment, colonisation by the same strain is remarkably stable within subjects for periods of 5 to 12 years, as revealed by restriction fragment length polymorphism [40], serotyping combined with arbitrarily primed PCR [116], or JP2 clone-specific PCR [117]. Genomic stability during persistent oral infections has been demonstrated by genome sequencing of strains cultured from the same individual 10 years later [118].
The natural habitat of A. actinomycetemcomitans is the oral cavity, but A. actinomycetemcomitans can be isolated from a variety of oral as well as non-oral infectious diseases, including arthritis, bacteraemia, endocarditis, osteomyelitis, skin infections, urinary tract infections and various types of abscesses [119]. Characterisation of 52 non-oral strains showed similarity to oral strains [120], and the portal of entry for systemic infections is usually the oral cavity [121].
4.1. Infective Endocarditis
The oral cavity is the only known habitat of A. actinomycetemcomitans, but only a few layers of crevicular epithelial cells separate the gingival location from the parenteral space of the host. Entry into the blood stream has not been quantitated, but incidental introductions may occur during tooth brushing, injuries, chewing of granular matters etc., and this may be accelerated by the presence of periodontitis. A. actinomycetemcomitans was originally co-isolated with Actinomyces from actinomycotic lesions [1], and the association with Actinomyces has been confirmed by case reports of infections in a variety of anatomical localizations. Among Actinomyces species, co-isolation of A. actinomycetemcomitans appears restricted to Actinomyces israelii [122,123].
Infective endocarditis is an infection of the endocardium, the lining of the interior surfaces of the chambers of the heart. It usually affects the heart valves (Figure 3A), where corrosion and incidental exposure of sub-endothelium tissue during the extensive motion of the valves may serve as a starting point for bacterial adhesion.
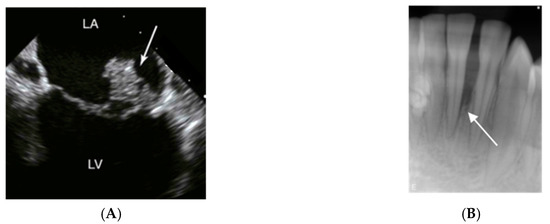
Figure 3.
Imaging signs of infections and inflammation that may be associated with A. actinomycetemcomitans. (A) Transesophageal echocardiography of a heart with mitral valve infective endocarditis. The arrow marks a large vegetation on the posterior leaflet between left atrium (LA) and left ventricle (LV); usually, vegetations caused by A. actinomycetemcomitans are of smaller size. (B) 14-year old girl of African ethnicity. The radiograph shows an extensive and apparently rapid loss of the periodontal support of the lower incisor 31. Pictures by courtesy of close clinical collaborators of the authors.
A. actinomycetemcomitans is part of the Haemophilus, Aggregatibacter, Cardiobacterium, Eikenella, and Kingella (HACEK) group of fastidious Gram-negative bacteria that is responsible for 1.4–3% of cases infective endocarditis [124,125]. The group originally included Haemophilus species, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, and Kingella kingae [7]. The HACEK acronym is still valid, but currently denotes non-influenzae Haemophilus sp., Aggregatibacter sp., Cardiobacterium sp., E. corrodens, and Kingella sp. [126]. A. actinomycetemcomitans is the HACEK organism most strongly associated with infective endocarditis [121,125], and bacteraemia with A. actinomycetemcomitans necessitates clarification of this putative focus of infection. In a retrospective study of 87 cases of HACEK bacteraemia from New Zealand, the association between HACEK bacteraemia and infective endocarditis varied among bacterial species ranging from 0% (E. corrodens) to 100% (A. actinomycetemcomitans) [127]. Specific features of infective endocarditis caused by A. actinomycetemcomitans have been reviewed [121].
4.2. The Complex Interplay with Periodontitis
Periodontitis is an inflammatory disease associated with loss of connective tissue and bone around teeth (Figure 3B). The bacterial tooth biofilm initiates the gingival inflammation, and further progression of the periodontal lesion depends on dysbiotic ecological changes within the gingival sulcus area. Unfavourable lifestyles and hygiene contribute to the development and progression of periodontitis, which has been designated as one of mankind’s most common chronic inflammatory diseases [48].
The complexity of the periodontal microbiota and the variety of clinical symptoms delayed the identification of specific microbial aetiological agents. In 1996, A. actinomycetemcomitans, Porphyromonas gingivalis, and Tannerella forsythia were officially designated as aetiological agents of periodontitis [128]. A. actinomycetemcomitans was targeted based on prevalence studies in health and disease, serum antibody levels, and presence of virulence determinants (Table 1 and Table 2). More recently, attention has been directed to the complex interplay between other cultivable and other non-cultivable bacteria in the oral microbiota, as well as to the interplay with the host [16,48,129,130]. Indeed, it has been suggested that A. actinomycetemcomitans conducts its business by concealing itself from the scrutiny of the host immune system, or even being a community activist that suppresses host responses to allow overgrowth of its collaborators [19].
The earlier classification of aggressive periodontitis was based mainly on the clinical presentation and the rapid loss of periodontal tissue [131]. A new classification scheme has been adopted, in which chronic and aggressive forms of the disease are now merged into a single category, which is characterised by a multi-dimensional staging and grading system [132,133]. Staging assesses severity and extent of disease at presentation, and attempts to include the complexity of disease management. The grading provides an evaluation of the risk of progression, and attempts to predict response to standard periodontal therapy [132].
5. Therapy
Treatment of periodontitis aims to stop the progression of the periodontal lesion and to maximise periodontal health [134]. Mechanical debridement of biofilm is considered the most effective therapy, but must be combined with a detailed oral hygiene. If periodontal lesions persist after 3–6 months, a second phase of therapy is planned. A favourable healing potential has been documented for lesions associated with the rapidly progressive, localised periodontitis that affects adolescents [135]. Systemic antibiotics should only be administered as adjunctive therapy in selected cases.
Access surgery with regenerative techniques have been used for periodontitis stages III–IV [132,134]. Notable risk factors are non-compliance, smoking, elevated gingival bleeding index, and inadequate plaque control [136].
Very different amoxicillin resistance rates have been reported, ranging from 0% in Switzerland [137], over 33% in Spain [138] to 84% in the United Kingdom [139]. The mechanisms of resistance were not reported. Production of β-lactamase is the most common cause of β-lactam resistance in Gram-negative bacteria, but these enzymes have not been detected in A. actinomycetemcomitans. The fastidious nature of the bacterium is a challenge for antimicrobial susceptibility testing, and methodology as well as interpretative criteria must be addressed when reports of resistance are evaluated. A recent investigation using different methods could not confirm the emergence of resistance to β-lactams in A. actinomycetemcomitans; the study included strains that had previously been reported as resistant [140]. Thus, there is currently no convincing evidence for replacement of oral amoxicillin when antimicrobial agents are indicated for treatment of A. actinomycetemcomitans-associated periodontitis.
Gram-negative bacteria are generally more susceptible to the cephalosporin-class than the penicillin-class of β-lactams. For infective endocarditis, an intravenous course of at least four weeks with a third-generation cephalosporin, or a combination of ampicillin and an aminoglycoside, is recommended [121]. Recently, a well-designed randomised study reported favourable outcomes for oral antimicrobial follow up regimens given to patients with infective endocarditis deemed clinically stable and without complications [141]. A. actinomycetemcomitans could be a candidate microorganism for use of partial oral antimicrobial treatment of infective endocarditis, but the relative rare occurrence of HACEK bacteraemia poses difficulties for additional clinical studies.
6. Conclusions
A. actinomycetemcomitans is part of the human microbiota. It can be cultured from one-third of healthy adults, while PCR-based methods suggest a more ubiquitous presence. The bacterium’s tenacious, aggregative character is instrumental for the remarkable genotype stability in colonised hosts, and for progression to persistent, distant infections after incidental entry into the parental space. A. actinomycetemcomitans are commonly detected if adolescents present with periodontitis. Chronically inflamed gingival crevices may spark the repeated, intermittent entry into the blood stream. Its participation in the disease process of periodontitis is beyond reasonable doubt, but its orchestration of severe periodontitis continues to be fascinating and disputed. Adhesion and leukotoxic features are well-described, but interplay with other members of the oral microbiota is more difficult to elucidate, as is the interchangeable position of eliciting antibody response and “staying under the radar”. The recent division into three subspecies or lineages has not been investigated by clinical studies linking disease and lineage. Disease-specific treatment options are currently widely accepted.
Author Contributions
All authors made a substantial, direct, and intellectual contribution to the work. N.N.-L. compiled the contributions and made the first draft of the manuscript. All authors approved it for publication.
Funding
This research received no external funding.
Acknowledgments
Mogens Kilian is thanked for profound inspiration. Furthermore, we thank the members of the European Network for Aggregatibacter actinomycetemcomitans Research (ENAaR; https://projects.au.dk/aggregatibacter/) for valuable discussions.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
aae, Aggregatibacter autotransporter adhesin; Api, Aggregatibacter putative invasion; CT, cycling threshold; emaA, extracellular matrix adhesin A; HACEK, Haemophilus, Aggregatibacter, Cardiobacterium, Eikenella, and Kingella; JP2 clone, a specific juvenile periodontitis-related bacterial clone; Ltx, leukotoxin; MLST, multilocus sequence type; Omp, outer membrane protein; PCR, polymerase chain reaction; RTX, repeats in toxin; St, serotype; SNP, single nucleotide polymorphism; YadA, Yersinia adhesin A.
References
- Klinger, R. Untersuchungen über menschliche Aktinomykose. Zentralbl. Bakteriol. 1912, 62, 191–200. (In German) [Google Scholar]
- Topley, W.W.C.; Wilson, G.S. The Principles of Bacteriology and Immunity; Edward Arnold: London, UK, 1929; pp. 1–587. [Google Scholar]
- King, E.O.; Tatum, H.W. Actinobacillus actinomycetemcomitans and Hemophilus aphrophilus. J. Infect. Dis. 1962, 111, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Slots, J. The predominant cultivable organisms in juvenile periodontitis. Scand. J. Dent. Res. 1976, 84, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Newman, M.G.; Socransky, S.S.; Savitt, E.D.; Propas, D.A.; Crawford, A. Studies of the microbiology of periodontosis. J. Periodontol. 1976, 47, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; McArthur, W.P.; Baehni, P.C.; Hammond, B.F.; Taichman, N.S. Extraction and partial characterization of a leukotoxin from a plaque-derived Gram-negative microorganism. Infect. Immun. 1979, 25, 427–439. [Google Scholar] [PubMed]
- Geraci, J.E.; Wilson, W.R. Symposium on infective endocarditis. III. Endocarditis due to gram-negative bacteria. Report of 56 cases. Mayo Clin. Proc. 1982, 57, 145–148. [Google Scholar]
- Ebersole, J.L.; Taubman, M.A.; Smith, D.J.; Genco, R.J.; Frey, D.E. Human immune responses to oral micro-organisms. I. Association of localized juvenile periodontitis (LJP) with serum antibody responses to Actinobacillus actinomycetemcomitans. Clin. Exp. Immunol. 1982, 47, 43–52. [Google Scholar]
- Zambon, J.J.; Slots, J.; Genco, R.J. Serology of oral Actinobacillus actinomycetemcomitans and serotype distribution in human periodontal disease. Infect. Immun. 1983, 41, 19–27. [Google Scholar]
- Brogan, J.M.; Lally, E.T.; Poulsen, K.; Kilian, M.; Demuth, D.R. Regulation of Actinobacillus actinomycetemcomitans leukotoxin expression: Analysis of the promoter regions of leukotoxic and minimally leukotoxic strains. Infect. Immun. 1994, 62, 501–508. [Google Scholar]
- Nørskov-Lauritsen, N.; Kilian, M. Reclassification of Actinobacillus actinomycetemcomitans, Haemophilus aphrophilus, Haemophilus paraphrophilus and Haemophilus segnis as Aggregatibacter actinomycetemcomitans gen. nov., comb. nov., Aggregatibacter aphrophilus comb. nov. and Aggregatibacter segnis comb. nov., and emended description of Aggregatibacter aphrophilus to include V factor-dependent and V factor-independent isolates. Int. J. Syst. Evol. Microbiol. 2006, 56, 2135–2146. [Google Scholar]
- Haubek, D.; Ennibi, O.K.; Poulsen, K.; Vaeth, M.; Poulsen, S.; Kilian, M. Risk of aggressive periodontitis in adolescent carriers of the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans in Morocco: A prospective longitudinal cohort study. Lancet 2008, 371, 237–242. [Google Scholar] [CrossRef]
- Moore, W.E.; Moore, L.V. The bacteria of periodontal diseases. Periodontology 2000 1994, 5, 66–77. [Google Scholar] [CrossRef] [PubMed]
- Socransky, S.S.; Haffajee, A.D. Evidence of bacterial etiology: A historical perspective. Periodontology 2000 1994, 5, 7–25. [Google Scholar] [CrossRef] [PubMed]
- Wilson, M.J.; Weightman, A.J.; Wade, W.G. Applications of molecular ecology in the characterisation of uncultured microorganisms associated with human disease. Rev. Med. Microbiol. 1997, 8, 91–101. [Google Scholar] [CrossRef]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.H.; Lakshmanan, A.; Wade, W.G. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar]
- Bisgaard, M.; Christensen, H.; Clermont, D.; Dijkshoorn, L.; Janda, J.M.; Moore, E.R.B.; Nemec, A.; Nørskov-Lauritsen, N.; Overmann, J.; Reubsaet, F.A.G. The use of genomic DNA sequences as type material for valid publication of bacterial species names will have severe implications for clinical microbiology and related disciplines. Diagn. Microbiol. Infect. Dis. 2019, 95, 102–103. [Google Scholar] [CrossRef] [PubMed]
- Asikainen, S.; Chen, C. Oral ecology and person-to-person transmission of Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis. Periodontol 2000 1999, 20, 65–81. [Google Scholar]
- Fine, D.H.; Pati, A.G.; Velusamy, S.K. Aggregatibacter actinomycetemcomitans (Aa) Under the Radar: Myths and Misunderstandings of Aa and Its Role in Aggressive Periodontitis. Front. Immunol. 2019, 10, 728. [Google Scholar] [CrossRef]
- Hornef, M. Pathogens, commensal symbionts, and pathobionts: Discovery and functional effects on the host. ILAR J. 2015, 56, 159–162. [Google Scholar] [CrossRef]
- Cowan, S.T. Cowan and Steel’s Manual for the Identification of Medical Bacteria, 2nd ed.; Cambridge University Press: Cambridge, UK, 1974; p. 95. [Google Scholar]
- Potts, T.V.; Mitra, T.; O’Keefe, T.; Zambon, J.J.; Genco, R.J. Relationships among isolates of oral haemophili as determined by DNA-DNA hybridization. Arch. Microbiol. 1986, 145, 136–141. [Google Scholar] [CrossRef]
- Munson, E.; Carroll, K.C. What’s in a name? New bacterial species and changes to taxonomic status from 2012 through 2015. J. Clin. Microbiol. 2017, 55, 24–42. [Google Scholar] [PubMed]
- Murra, M.; Lützen, L.; Barut, A.; Zbinden, R.; Lund, M.; Villesen, P.; Nørskov-Lauritsen, N. Whole-genome sequencing of aggregatibacter species isolated from human clinical specimens and description of aggregatibacter kilianii sp. nov. J. Clin. Microbiol. 2018, 56, e00053-18. [Google Scholar] [PubMed]
- Takada, K.; Saito, M.; Tsuzukibashi, O.; Kawashima, Y.; Ishida, S.; Hirasawa, M. Characterization of a new serotype g isolate of Aggregatibacter actinomycetemcomitans. Mol. Oral Microbiol. 2010, 25, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Brígido, J.A.; da Silveira, V.R.; Rego, R.O.; Nogueira, N.A. Serotypes of Aggregatibacter actinomycetemcomitans in relation to periodontal status and geographic origin of individuals—A review of the literature. Med. Oral Patol. Oral Cir. Bucal 2014, 19, e184–e191. [Google Scholar] [CrossRef]
- Saarela, M.; Asikainen, S.; Alaluusua, S.; Pyhälä, L.; Lai, C.H.; Jousimies-Somer, H. Frequency and stability of mono-or poly-infection by Actinobacillus actinomycetemcomitans serotypes a, b, c, d or e. Oral Microbiol. Immunol. 1992, 7, 277–279. [Google Scholar] [CrossRef]
- Thiha, K.; Takeuchi, Y.; Umeda, M.; Huang, Y.; Ohnishi, M.; Ishikawa, I. Identification of periodontopathic bacteria in gingival tissue of Japanese periodontitis patients. Oral Microbiol. Immunol. 2007, 22, 201–207. [Google Scholar] [CrossRef]
- Rylev, M.; Kilian, M. Prevalence and distribution of principal periodontal pathogens worldwide. J. Clin. Periodontol. 2011, 3, 346–361. [Google Scholar] [CrossRef]
- Bandhaya, P.; Saraithong, P.; Likittanasombat, K.; Hengprasith, B.; Torrungruang, K. Aggregatibacter actinomycetemcomitans serotypes, the JP2 clone and cytolethal distending toxin genes in a Thai population. J. Clin. Periodontol. 2012, 39, 519–525. [Google Scholar]
- Claesson, R.; Höglund Åberg, C.; Haubek, D.; Johansson, A. Age-related prevalence and characteristics of Aggregatibacter actinomycetemcomitans in periodontitis patients living in Sweden. J. Oral Microbiol. 2017, 9, 1334504. [Google Scholar] [CrossRef]
- Yamamoto, M.; Nishihara, T.; Koseki, T.; He, T.; Yamato, K.; Zhang, Y.J.; Nakashima, K.; Oda, S.; Ishikawa, I. Prevalence of Actinobacillus actinomycetemcomitans serotypes in Japanese patients with periodontitis. J. Periodontal Res. 1997, 32, 676–681. [Google Scholar] [CrossRef]
- Celenligil, H.; Ebersole, J.L. Analysis of serum antibody responses to periodontopathogens in early-onset periodontitis patients from different geographical locations. J. Clin. Periodontol. 1998, 25, 994–1002. [Google Scholar] [CrossRef] [PubMed]
- Saraiva, L.; Rebeis, E.S.; Martins Ede, S.; Sekiguchi, R.T.; Ando-Suguimoto, E.S.; Mafra, C.E.; Holzhausen, M.; Romito, G.A.; Mayer, M.P. IgG sera levels against a subset of periodontopathogens and severity of disease in aggressive periodontitis patients: A cross-sectional study of selected pocket sites. J. Clin. Periodontol. 2014, 41, 943–951. [Google Scholar] [CrossRef] [PubMed]
- Poulsen, K.; Theilade, E.; Lally, E.T.; Demuth, D.R.; Kilian, M. Population structure of Actinobacillus actinomycetemcomitans: A framework for studies of disease-associated properties. Microbiology 1994, 140, 2049–2060. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kaplan, J.B.; Schreiner, H.C.; Furgang, D.; Fine, D.H. Population structure and genetic diversity of Actinobacillus actinomycetemcomitans strains isolated from localized juvenile periodontitis patients. J. Clin. Microbiol. 2002, 40, 1181–1187. [Google Scholar] [CrossRef]
- Haubek, D.; Poulsen, K.; Kilian, M. Microevolution and patterns of dissemination of the JP2 clone of Aggregatibacter (Actinobacillus) Actinomycetemcomitans. Infect. Immun. 2007, 75, 3080–3088. [Google Scholar] [CrossRef]
- DiRienzo, J.M.; Slots, J. Genetic approach to the study of epidemiology and pathogenesis of Actinobacillus actinomycetemcomitans in localized juvenile periodontitis. Arch. Oral Biol. 1990, 35, S79–S84. [Google Scholar] [CrossRef]
- Zambon, J.J.; Sunday, G.J.; Smutko, J.S. Molecular genetic analysis of Actinobacillus actinomycetemcomitans epidemiology. J. Periodontol. 1990, 61, 75–80. [Google Scholar] [CrossRef]
- DiRienzo, J.M.; Slots, J.; Sixou, M.; Sol, M.A.; Harmon, R.; McKay, T.L. Specific genetic variants of Actinobacillus actinomycetemcomitans correlate with disease and health in a regional population of families with localized juvenile periodontitis. Infect. Immun. 1994, 62, 3058–3065. [Google Scholar]
- Eriksen, K.T.; Haubek, D.; Poulsen, K. Intragenomic recombination in the highly leukotoxic JP2 clone of Actinobacillus actinomycetemcomitans. Microbiology 2005, 151, 3371–3379. [Google Scholar] [CrossRef][Green Version]
- Höglund Åberg, C.; Haubek, D.; Kwamin, F.; Johansson, A.; Claesson, R. Leukotoxic activity of Aggregatibacter actinomycetemcomitans and periodontal attachment loss. PLoS ONE 2014, 9, e104095. [Google Scholar] [CrossRef]
- Kittichotirat, W.; Bumgarner, R.E.; Asikainen, S.; Chen, C. Identification of the pangenome and its components in 14 distinct Aggregatibacter actinomycetemcomitans strains by comparative genomic analysis. PLoS ONE 2011, 6, e22420. [Google Scholar] [CrossRef]
- Jorth, P.; Whiteley, M. An evolutionary link between natural transformation and CRISPR adaptive immunity. MBio 2012, 3, e00309-12. [Google Scholar] [CrossRef] [PubMed]
- Kittichotirat, W.; Bumgarner, R.E.; Chen, C. Evolutionary divergence of Aggregatibacter actinomycetemcomitans. J. Dent. Res. 2016, 95, 94–101. [Google Scholar] [CrossRef]
- Nedergaard, S.; Kobel, C.M.; Nielsen, M.B.; Møller, R.T.; Jensen, A.B.; Nørskov-Lauritsen, N.; The Danish HACEK Study Group. Whole genome sequencing of Aggregatibacter actinomycetemcomitans cultured from blood stream infections reveals three major phylogenetic groups including a novel lineage expressing serotype a membrane O polysaccharide. Pathogens. submitted.
- Holm, P. The influence of carbon dioxide on the growth of Actinobacillus actinomycetemcomitans (Bacterium actinomycetem comitans Klinger 1912). Acta. Pathol. Microbiol. Scand. 1954, 34, 235–248. [Google Scholar] [CrossRef] [PubMed]
- Henderson, B.; Ward, J.M.; Ready, D. Aggregatibacter (Actinobacillus) actinomycetemcomitans: A triple A* periodontopathogen? Periodontology 2000 2010, 54, 78–105. [Google Scholar] [CrossRef] [PubMed]
- Möller, Å.J.R. Microbiological examination of root canals and periapical tissues of human teeth. Scand. Dent. J. 1966, 74, 5–6. [Google Scholar]
- Johansson, E.; Claesson, R.; van Dijken, J.W.V. Antibacterial effect of ozone on cariogenic bacterial species. J. Dent. 2009, 37, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Zohaib, S.; Najeeb, S.; Zafar, M.S.; Slowey, P.D.; Almas, K. Human saliva collection devices for proteomics: An update. Int. J. Mol. Sci. 2016, 17, 846. [Google Scholar] [CrossRef] [PubMed]
- Khurshid, Z.; Mali, M.; Naseem, M.; Najeeb, S.; Zafar, M.S. Human gingival crevicular fluids (GCF) proteomics: An overview. Dent. J. 2017, 5, 12. [Google Scholar] [CrossRef]
- Slots, J. Selective medium for isolation of Actinobacillus actinomycetemcomitans. J. Clin. Microbiol. 1982, 15, 606–609. [Google Scholar] [PubMed]
- Höglund Åberg, C.; Kwamin, F.; Claesson, R.; Johansson, A.; Haubek, D. Presence of JP2 and non-JP2 genotypes of Aggregatibacter actinomycetemcomitans and periodontal attachment loss in adolescents in Ghana. J. Periodontol. 2012, 83, 1520–1528. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P. Microbial ecology of dental plaque and its significance in health and disease. Adv. Dent. Res. 1994, 8, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Slots, J. Salient Biochemical Characters of Actinobacillus actinomycetemcomitans. Arch. Microbiol. 1982, 131, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Couturier, M.R.; Mehinovic, E.; Croft, A.C.; Mark, A.; Fisher, M.A. Identification of HACEK clinical isolates by matrix-assisted laser desorption ionization—Time of flight mass spectrometry. J. Clin. Microbiol. 2011, 49, 1104–1106. [Google Scholar] [CrossRef] [PubMed]
- Zambon, J.J.; DeLuca, C.; Slots, J.; Genco, R.J. Studies of leukotoxin from Actinobacillus actinomycetemcomitans using the promyelocytic HL-60 cell line. Infect. Immun. 1983, 40, 205–212. [Google Scholar]
- Haubek, D.; DiRienzo, J.J.; Tinoco, E.M.; Westergaard, J.; Lopez, N.J.; Chung, C.P.; Poulsen, K.; Kilian, M. Racial tropism of a highly toxic clone of Actinbacillus actinomycetemcomitans associated with juvenile periodontitis. J. Clin. Microbiol. 1997, 35, 3037–3042. [Google Scholar]
- Sampathkumar, V.; Velusamy, S.K.; Godboley, D.; Fine, D.H. Increased leukotoxin production: Characterization of 100 base pairs within the 530 base pair leukotoxin promoter region of Aggregatibacter actinomycetemcomitans. Sci. Rep. 2017, 7, 1887. [Google Scholar] [CrossRef]
- Poulsen, K.; Ennibi, O.K.; Haubek, D. Improved PCR for detection of the highly leukotoxic JP2 clone of Actinobacillus actinomycetemcomitans in subgingival plaque samples. J. Clin. Microbiol. 2003, 41, 4829–4832. [Google Scholar] [CrossRef]
- Kirakodu, S.S.; Govindaswami, M.; Novak, M.J.; Ebersole, J.L.; Novak, K.F. Optimizing qPCR for the quantification of periodontal pathogens in a complex plaque biofilm. Open Dent. J. 2008, 2, 49–55. [Google Scholar] [CrossRef]
- Yoshida, A.; Ennibi, O.K.; Miyazaki, H.; Hoshino, T.; Hayashida, H.; Nishihara, T.; Awano, S.; Ansai, T. Quantitative discrimination of Aggregatibacter actinomycetemcomitans highly leukotoxic JP2 clone from non-JP2 clones in diagnosis of aggressive periodontitis. BMC Inf. Dis. 2012, 12, 253. [Google Scholar] [CrossRef] [PubMed]
- Větrovský, T.; Baldrian, P. The variability of the 16S rRNA gene in bacterial genomes and its consequences for bacterial community analyses. PLoS ONE 2013, 8, e57923. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, N.; Nakano, Y.; Yoshida, Y.; Ikeda, D.; Koga, T. Identification of Actinobacillus actinomycetemcomitans serotypes by multiplex PCR. J. Clin. Microbiol. 2001, 9, 2002–2005. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, J.B.; Perry, M.B.; MacLean, L.L.; Furgang, D.; Wilson, M.E.; Fine, D.H. Structural and genetic analyses of O polysaccharide from Actinobacillus actinomycetemcomitans serotype f. Infect. Immun. 2001, 69, 5375–5384. [Google Scholar] [CrossRef]
- Tomich, M.; Planet, P.J.; Figurski, D.H. The tad locus: Postcards from the widespread colonization island. Nat. Rev. Microbiol. 2007, 5, 363–375. [Google Scholar] [CrossRef]
- Rose, J.E.; Meyer, D.H.; Fives-Taylor, P.M. Aae, an autotransporter involved in adhesion of Actinobacillus actinomycetemcomitans to epithelial cells. Infect. Immun. 2003, 71, 2384–2393. [Google Scholar] [CrossRef]
- Danforth, D.R.; Tang-Siegel, G.; Ruiz, T.; Mintz, K.P. A nonfimbrial adhesin of Aggregatibacter actinomycetemcomitans mediates biofilm biogenesis. Infect. Immun. 2018, 87, e00704-18. [Google Scholar] [CrossRef]
- Yue, G.; Kaplan, J.B.; Furgang, D.; Mansfield, K.G.; Fine, D.H. A second Aggregatibacter actinomycetemcomitans autotransporter adhesin exhibits specificity for buccal epithelial cells in humans and Old World primates. Infect. Immun. 2007, 75, 4440–4448. [Google Scholar] [CrossRef]
- Vega, B.A.; Belinka, B.A.; Kachlany, S.C. Aggregatibacter actinomycetemcomitans Leukotoxin (LtxA; Leukothera®): Mechanisms of Action and Therapeutic Applications. Toxins 2019, 11, 489. [Google Scholar] [CrossRef]
- Sugai, M.; Kawamoto, T.; Pérès, S.Y.; Ueno, Y.; Komatsuzawa, H.; Fujiwara, T.; Kurihara, H.; Suginaka, H.; Oswald, E. The cell cycle-specific growth-inhibitory factor produced by Actinobacillus actinomycetemcomitans is a cytolethal distending toxin. Infect. Immun. 1998, 66, 5008–5019. [Google Scholar]
- Boesze-Battaglia, K.; Alexander, D.; Dlakić, M.; Shenker, B.J. A Journey of cytolethal distending toxins through cell membranes. Front. Cell. Infect. Microbiol. 2016, 6, 81. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.T.; Hu, W. Expression cloning of a periodontitis-associated apoptotic effector, cagE homologue, in Actinobacillus actinomycetemcomitans. Biochem. Biophys. Res. Commun. 2003, 303, 1086–1094. [Google Scholar] [CrossRef]
- Ramasubbu, N.; Thomas, L.M.; Ragunath, C.; Kaplan, J.B. Structural analysis of dispersin B, a biofilm-releasing glycoside hydrolase from the periodontopathogen Actinobacillus actinomycetemcomitans. J. Mol. Biol. 2005, 349, 475–486. [Google Scholar] [CrossRef] [PubMed]
- Karched, M.; Ihalin, R.; Eneslätt, K.; Zhong, D.; Oscarsson, J.; Wai, S.N.; Chen, C.; Asikainen, S.E. Vesicle-independent extracellular release of a proinflammatory outer membrane lipoprotein in free-soluble form. BMC Microbiol. 2008, 8, 18. [Google Scholar] [CrossRef] [PubMed]
- Rosan, B.; Slots, J.; Lamont, R.J.; Listgarten, M.A.; Nelson, G.M. Actinobacillus actinomycetemcomitans fimbriae. Oral. Microbiol. Immunol. 1988, 3, 58–63. [Google Scholar] [CrossRef]
- Harano, K.; Yamanaka, A.; Okuda, K. An antiserum to a synthetic fimbrial peptide of Actinobacillus actinomycetemcomitans blocked adhesion of the microorganism. FEMS Microbiol. Lett. 1995, 130, 279–285. [Google Scholar] [CrossRef]
- Fine, D.H.; Furgang, D.; Schreiner, H.C.; Goncharoff, P.; Charlesworth, J.; Ghazwan, G.; Fitzgerald-Bocarsly, P.; Figurski, D.H. Phenotypic variation in Actinobacillus actinomycetemcomitans during laboratory growth: Implications for virulence. Microbiology 1999, 145, 1335–1347. [Google Scholar] [CrossRef][Green Version]
- Inoue, T.; Tanimoto, I.; Ohta, H.; Kato, K.; Murayama, Y.; Fukui, K. Molecular characterization of low-molecular-weight component protein, Flp, in Actinobacillus actinomycetemcomitans fimbriae. Microbiol. Immunol. 1998, 42, 253–258. [Google Scholar] [CrossRef]
- Kachlany, S.C.; Planet, P.J.; Desalle, R.; Fine, D.H.; Figurski, D.H.; Kaplan, J.B. flp-1, the first representative of a new pilin gene subfamily, is required for non-specific adherence of Actinobacillus actinomycetemcomitans. Mol. Microbiol. 2001, 40, 542–554. [Google Scholar] [CrossRef]
- Haase, E.M.; Zmuda, J.L.; Scannapieco, F.A. Identification and molecular analysis of rough-colony-specific outer membrane proteins of Actinobacillus actinomycetemcomitans. Infect. Immun. 1999, 67, 2901–2908. [Google Scholar]
- Wang, Y.; Chen, C. Mutation analysis of the flp operon in Actinobacillus actinomycetemcomitans. Gene 2005, 351, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Perez, B.A.; Planet, P.J.; Kachlany, S.C.; Tomich, M.; Fine, D.H.; Figurski, D.H. Genetic analysis of the requirement for flp-2, tadV, and rcpB in Actinobacillus actinomycetemcomitans biofilm formation. J. Bacteriol. 2006, 188, 6361–6375. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, H.C.; Sinatra, K.; Kaplan, J.B.; Furgang, D.; Kachlany, S.C.; Planet, P.J.; Perez, B.A.; Figurski, D.H.; Fine, D.H. Tight-adherence genes of Actinobacillus actinomycetemcomitans are required for virulence in a rat model. Proc. Natl. Acad. Sci. USA 2003, 100, 7295–7300. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, A.; Chen, C. Genetic basis for conversion of rough-to-smooth colony morphology in Actinobacillus actinomycetemcomitans. Infect. Immun. 2005, 73, 3749–3753. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Pei, Z.; Niu, Z.; Shi, S.; Shi, L.; Tang, C. Phenotypic changes in nonfimbriated smooth strains of Aggregatibacter actinomycetemcomitans grown in low-humidity solid medium. Ultrastruct. Pathol. 2013, 37, 121–126. [Google Scholar] [CrossRef]
- Fine, D.H.; Velliyagounder, K.; Furgang, D.; Kaplan, J.B. The Actinobacillus actinomycetemcomitans autotransporter adhesin Aae exhibits specificity for buccal epithelial cells from humans and old world primates. Infect. Immun. 2005, 73, 1947–1953. [Google Scholar] [CrossRef]
- Li, L.; Matevski, D.; Aspiras, M.; Ellen, R.P.; Lépine, G. Two epithelial cell invasion-related loci of the oral pathogen Actinobacillus actinomycetemcomitans. Oral. Microbiol. Immunol. 2004, 19, 16–25. [Google Scholar] [CrossRef]
- Mintz, K.P. Identification of an extracellular matrix protein adhesin, EmaA, which mediates the adhesion of Actinobacillus actinomycetemcomitans to collagen. Microbiology 2004, 150, 2677–2688. [Google Scholar] [CrossRef]
- Tang, G.; Kitten, T.; Munro, C.L.; Wellman, G.C.; Mintz, K.P. EmaA, a potential virulence determinant of Aggregatibacter actinomycetemcomitans in infective endocarditis. Infect. Immun. 2008, 76, 2316–2324. [Google Scholar] [CrossRef]
- Cornelissen, C.N. Subversion of nutritional immunity by the pathogenic Neisseriae. Pathog. Dis. 2018, 76, ftx112. [Google Scholar] [CrossRef]
- Benz, R. Channel formation by RTX-toxins of pathogenic bacteria: Basis of their biological activity. Biochim. Biophys. Acta 2016, 1858, 526–537. [Google Scholar] [CrossRef] [PubMed]
- Frey, J.; Kuhnert, P. RTX toxins in Pasteurellaceae. Int. J. Med. Microbiol. 2002, 292, 149–158. [Google Scholar] [CrossRef] [PubMed]
- Taichman, N.S.; Tsai, C.C.; Baehni, P.C.; Stoller, N.; McArthur, W.P. Interaction of inflammatory cells and oral microorganisms. IV. In vitro release of lysosomal constituents from polymorphonuclear leukocytes exposed to supragingival and subgingival bacterial plaque. Infect. Immun. 1977, 16, 1013–1023. [Google Scholar] [PubMed]
- Lally, E.T.; Golub, E.E.; Kieba, I.R.; Taichman, N.S.; Rosenbloom, J.; Rosenbloom, J.C.; Gibson, C.W.; Demuth, D.R. Analysis of the Actinobacillus actinomycetemcomitans leukotoxin gene. Delineation of unique features and comparison to homologous toxins. J. Biol. Chem. 1989, 264, 15451–15456. [Google Scholar] [PubMed]
- Kolodrubetz, D.; Dailey, T.; Ebersole, J.; Kraig, E. Cloning and expression of the leukotoxin gene from Actinobacillus actinomycetemcomitans. Infect. Immun. 1989, 57, 1465–1469. [Google Scholar] [PubMed]
- Balashova, N.V.; Crosby, J.A.; Al Ghofaily, L.; Kachlany, S.C. Leukotoxin confers beta-hemolytic activity to Actinobacillus actinomycetemcomitans. Infect. Immun. 2006, 74, 2015–2021. [Google Scholar] [CrossRef] [PubMed]
- Claesson, R.; Gudmundson, J.; Åberg, C.H.; Haubek, D.; Johansson, A. Detection of 640-bp deletion in Aggregatibacter actinomycetemcomitans leukotoxin promoter region in isolates from an adolescent of Ethiopian origin. J. Oral Microbiol. 2015, 7, 26974. [Google Scholar] [CrossRef]
- He, T.; Nishihara, T.; Demuth, D.R.; Ishikawa, I. A novel insertion sequence increases the expression of leukotoxicity in Actinobacillus actinomycetemcomitans clinical isolates. J. Periodontol. 1999, 70, 1261–1268. [Google Scholar] [CrossRef]
- Höglund Åberg, C.; Kwamin, F.; Claesson, R.; Dáhlen, G.; Johansson, A.; Haubek, D. Progression of attachment loss is strongly associated with presence of the JP2 genotype of Aggregatibacter actinomycetemcomitans: A prospective cohort study of a young adolescent population. J. Clin. Periodontol. 2014, 41, 232–241. [Google Scholar] [CrossRef]
- Burgess, D.; Huang, H.; Harrison, P.; Aukhil, I.; Shaddox, L. Aggregatibacter actinomycetemcomitans in African Americans with localized aggressive periodontitis. JDR Clin. Trans. Res. 2017, 2, 249–257. [Google Scholar] [CrossRef]
- Leung, W.K.; Ngai, V.K.; Yau, J.Y.; Cheung, B.P.; Tsang, P.W.; Corbet, E.F. Characterization of Actinobacillus actinomycetemcomitans isolated from young Chinese aggressive periodontitis patients. J. Periodontal Res. 2005, 40, 258–268. [Google Scholar] [CrossRef] [PubMed][Green Version]
- van der Reijden, W.A.; Bosch-Tijhof, C.J.; van der Velden, U.; van Winkelhoff, A.J. Java project on periodontal diseases: Serotype distribution of Aggregatibacter actinomycetemcomitans and serotype dynamics over an 8-year period. J. Clin. Periodontol. 2008, 35, 407–492. [Google Scholar] [CrossRef] [PubMed]
- Pahumunto, N.; Ruangsri, P.; Wongsuwanlert, M.; Piwat, S.; Dahlen, G.; Teanpaisan, R. Aggregatibacter actinomycetemcomitans serotypes and DGGE subtypes in Thai adults with chronic periodontitis. Arch. Oral Biol. 2015, 60, 1789–1796. [Google Scholar] [CrossRef] [PubMed]
- Alaluusua, S.; Asikainen, S. Detection and distribution of Actinobacillus actinomycetemcomitans in the primary dentition. J. Periodontol. 1988, 59, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Slots, J.; Reynolds, H.S.; Genco, R.J. Actinobacillus actinomycetemcomitans in human periodontal disease: A cross-sectional microbiological investigation. Infect. Immun. 1980, 29, 1013–1020. [Google Scholar] [PubMed]
- Frisken, W.; Higgins, T.; Palmer, J.M. The incidence of periodontopathic microorganisms in young children. Oral Microbiol. Immunol. 1990, 5, 43–45. [Google Scholar] [CrossRef]
- Kononen, E.; Asikainen, S.; Jousimies-Somer, H. The early colonization of gram-negative anaerobic bacteria in edentulous infants. Oral Microbiol. Immunol. 1992, 7, 28–31. [Google Scholar] [CrossRef]
- Merglova, V.; Polenik, P. Early colonization of the oral cavity in 6-and 12-month-old infants by cariogenic and periodontal pathogens: A case-control study. Folia Microbiol. 2016, 61, 423–429. [Google Scholar] [CrossRef]
- Preus, H.R.; Zambon, J.J.; Dunford, R.G.; Genco, R.J. The distribution and transmission of Actinobacillus actinomycetemcomitans in families with established adult periodontitis. J. Periodontol. 1994, 65, 2–7. [Google Scholar] [CrossRef]
- Asikainen, S.; Chen, C.; Slots, J. Likelihood of transmitting Actinobacillus actinomycetemcomitans and Porplyromonna gingivalis in families with periodontitis. Oral Microbiol. Immunol. 1996, 11, 387–394. [Google Scholar] [CrossRef]
- Rêgo, R.O.; Spolidorio, D.M.; Salvador, S.L.; Cirelli, J.A. Transmission of Aggregatibacter actinomycetemcomitans between Brazilian women with severe chronic periodontitis and their children. Braz. Dent. J. 2007, 18, 220–224. [Google Scholar] [CrossRef]
- Van Winkelhoff, A.J.; Boutaga, K. Transmission of periodontal bacteria and models of infection. J. Clin. Periodontol. 2005, 32, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Doğan, B.; Kipalev, A.S.; Okte, E.; Sultan, N.; Asikainen, S.E. Consistent intrafamilial transmission of Actinobacillus actinomycetemcomitans despite clonal diversity. J. Periodontol. 2008, 79, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Saarela, M.H.; Doğan, B.; Alaluusua, S.; Asikainen, S. Persistence of oral colonization by the same Actinobacillus actinomycetemcomitans strain(s). J. Periodontol. 1999, 70, 504–509. [Google Scholar] [CrossRef] [PubMed]
- Haubek, D.; Ennibi, O.K.; Vaeth, M.; Poulsen, S.; Poulsen, K. Stability of the JP2 clone of Aggregatibacter actinomycetemcomitans. J. Dent. Res. 2009, 88, 856–860. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Kittichotirat, W.; Wang, J.; Jan, M.; Chen, W.; Asikainen, S.; Bumgarner, R.; Chen, C. Genomic stability of Aggregatibacter actinomycetemcomitans during persistent oral infection in human. PLoS ONE 2013, 8, e66472. [Google Scholar] [CrossRef]
- van Winkelhoff, A.J.; Slots, J. Actinobacillus actinomycetemcomitans and Porphyromonas gingivalis in nonoral infections. Periodontology 2000 1999, 20, 122–135. [Google Scholar] [CrossRef]
- Paju, S.; Carlson, P.; Jousimies-Somer, H.; Asikainen, S. Heterogeneity of Actinobacillus actinomycetemcomitans strains in various human infections and relationships between serotype, genotype, and antimicrobial susceptibility. J. Clin. Microbiol. 2000, 38, 79–84. [Google Scholar]
- Paturel, L.; Casalta, J.P.; Habib, G.; Nezri, M.; Raoult, D. Actinobacillus actinomycetemcomitans endocarditis. Clin. Microbiol. Infect. 2004, 10, 98–118. [Google Scholar] [CrossRef]
- Clarridge, J.E.; Zhang, Q. Genotypic diversity of clinical Actinomyces species: Phenotype, source, and disease correlation among genospecies. J. Clin. Microbiol. 2002, 40, 3442–3448. [Google Scholar] [CrossRef]
- Kaplan, A.H.; Weber, D.J.; Oddone, E.Z.; Perfect, J.R. Infection due to Actinobacillus actinomycetemcomitans: 15 cases and review. Rev. Infect. Dis. 1989, 11, 46–63. [Google Scholar] [CrossRef] [PubMed]
- Brouqui, P.; Raoult, D. Endocarditis due to rare and fastidious bacteria. Clin. Microbiol. Rev. 2001, 14, 177–207. [Google Scholar] [CrossRef] [PubMed]
- Nørskov-Lauritsen, N. Classification, identification, and clinical significance of Haemophilus and Aggregatibacter species with host specificity for humans. Clin. Microbiol. Rev. 2014, 27, 214–240. [Google Scholar] [CrossRef] [PubMed]
- Lützen, L.; Olesen, B.; Voldstedlund, M.; Christensen, J.J.; Moser, C.; Knudsen, J.D.; Fuursted, K.; Hartmeyer, G.N.; Chen, M.; Søndergaard, T.S.; et al. Incidence of HACEK bacteraemia in Denmark: A 6-year population-based study. Int. J. Infect. Dis. 2018, 68, 83–87. [Google Scholar] [CrossRef] [PubMed]
- Yew, H.S.; Chambers, S.T.; Roberts, S.A.; Holland, D.J.; Julian, K.A.; Raymond, N.J.; Beardsley, J.; Read, K.M.; Murdoch, D.R. Association between HACEK bacteraemia and endocarditis. J. Med. Microbiol. 2014, 63, 892–895. [Google Scholar] [PubMed]
- Armitage, G.C.; Offenbacher, S. Consensus Report. Periodontal diseases: Epidemiology and diagnosis. Ann. Periodontol. 1996, 1, 16–22. [Google Scholar] [CrossRef]
- Fine, D.H.; Markowitz, K.; Fairlie, K.; Tischio-Bereski, D.; Ferrendiz, J.; Furgang, D.; Paster, B.J.; Dewhirst, F.E. A consortium of Aggregatibacter actinomycetemcomitans, Streptococcus parasanguinis, and Filifactor alocis is present in sites prior to bone loss in a longitudinal study of localized aggressive periodontitis. J. Clin. Microbiol. 2013, 51, 2850–2861. [Google Scholar] [CrossRef]
- Ebbers, M.; Lübcke, P.M.; Volzke, J.; Kriebel, K.; Hieke, C.; Engelmann, R.; Lang, H.; Kreikemeyer, B.; Müller-Hilke, B. Interplay between P. gingivalis, F. nucleatum and A. actinomycetemcomitans in murine alveolar bone loss, arthritis onset and progression. Sci. Rep. 2018, 8, 15129. [Google Scholar] [CrossRef]
- Armitage, G.C. Development of a classification system for periodontal diseases and conditions. Northwest Dent. 2000, 79, 31–35. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Clin. Periodontal. 2018, 45, S149–S161. [Google Scholar] [CrossRef]
- Fine, D.H.; Patil, A.G.; Loos, B.G. Classification and diagnosis of aggressive periodontitis. J. Periodontol. 2018, 89, s103–s119. [Google Scholar] [CrossRef] [PubMed]
- Teughels, W.; Dhondt, R.; Dekeyser, D.; Quirynen, M. Treatment of aggressive periodontitis. Periodontology 2000 2014, 65, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Hamad, C.; Haller, B.; Hoffmann, T.; Lorenz, K. Five-year results of nonsurgical generalized aggressive periodontitis. Quintessence Int. 2019, 50, 104–113. [Google Scholar] [PubMed]
- Dopico, J.; Nibali, L.; Donos, N. Disease progression in aggressive periodontitis patients. A restrspective study. J. Clin. Periodontol. 2016, 43, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Kulik, E.M.; Lenkeit, K.; Chenaux, S.; Meyer, J. Antimicrobial susceptibility of periodontopathogenic bacteria. J. Antimicrob. Chemother. 2008, 61, 1087–1091. [Google Scholar] [CrossRef] [PubMed]
- van Winkelhoff, A.J.; Herrera, D.; Oteo, A.; Sanz, M. Antimicrobial profiles of periodontal pathogens isolated from periodontitis patients in the Netherlands and Spain. J. Clin. Periodontol. 2005, 32, 893–898. [Google Scholar] [CrossRef] [PubMed]
- Akrivopoulou, C.; Green, I.M.; Donos, N.; Nair, S.P.; Ready, D. Aggregatibacter actinomycetemcomitans serotype prevalence and antibiotic resistance in a UK population with periodontitis. J. Glob. Antimicrob. Resist. 2017, 10, 54–58. [Google Scholar] [CrossRef]
- Jensen, A.B.; Haubek, D.; Claesson, R.; Johansson, A.; Nørskov-Lauritsen, N. Comprehensive antimicrobial susceptibility testing of a large collection of clinical strains of Aggregatibacter actinomycetemcomitans does not identify resistance to amoxicillin. J. Clin. Periodontol. 2019, 46, 846–854. [Google Scholar] [CrossRef]
- Iversen, K.; Ihlemann, N.; Gill, S.U.; Madsen, T.; Elming, H.; Jensen, K.T.; Bruun, N.E.; Høfsten, D.E.; Fursted, K.; Christensen, J.J.; et al. Partial oral versus intravenous antibiotic treatment of endocarditis. N. Engl. J. Med. 2019, 380, 415–424. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).