Development of Wearable Finger Prosthesis with Pneumatic Actuator for Patients with Partial Amputations
Abstract
:1. Introduction
2. Design of Wearable Finger Prosthesis
2.1. Structural Design
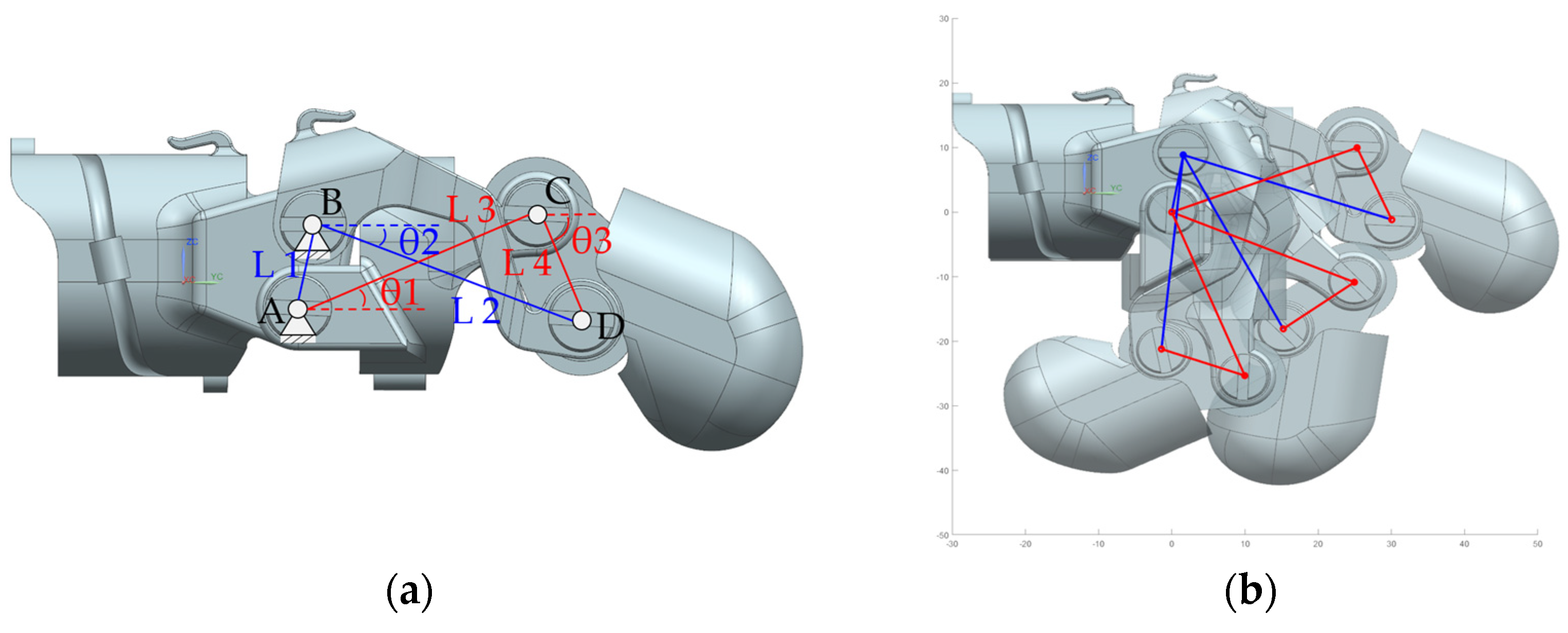
2.2. Kinematic of Linkage Structure
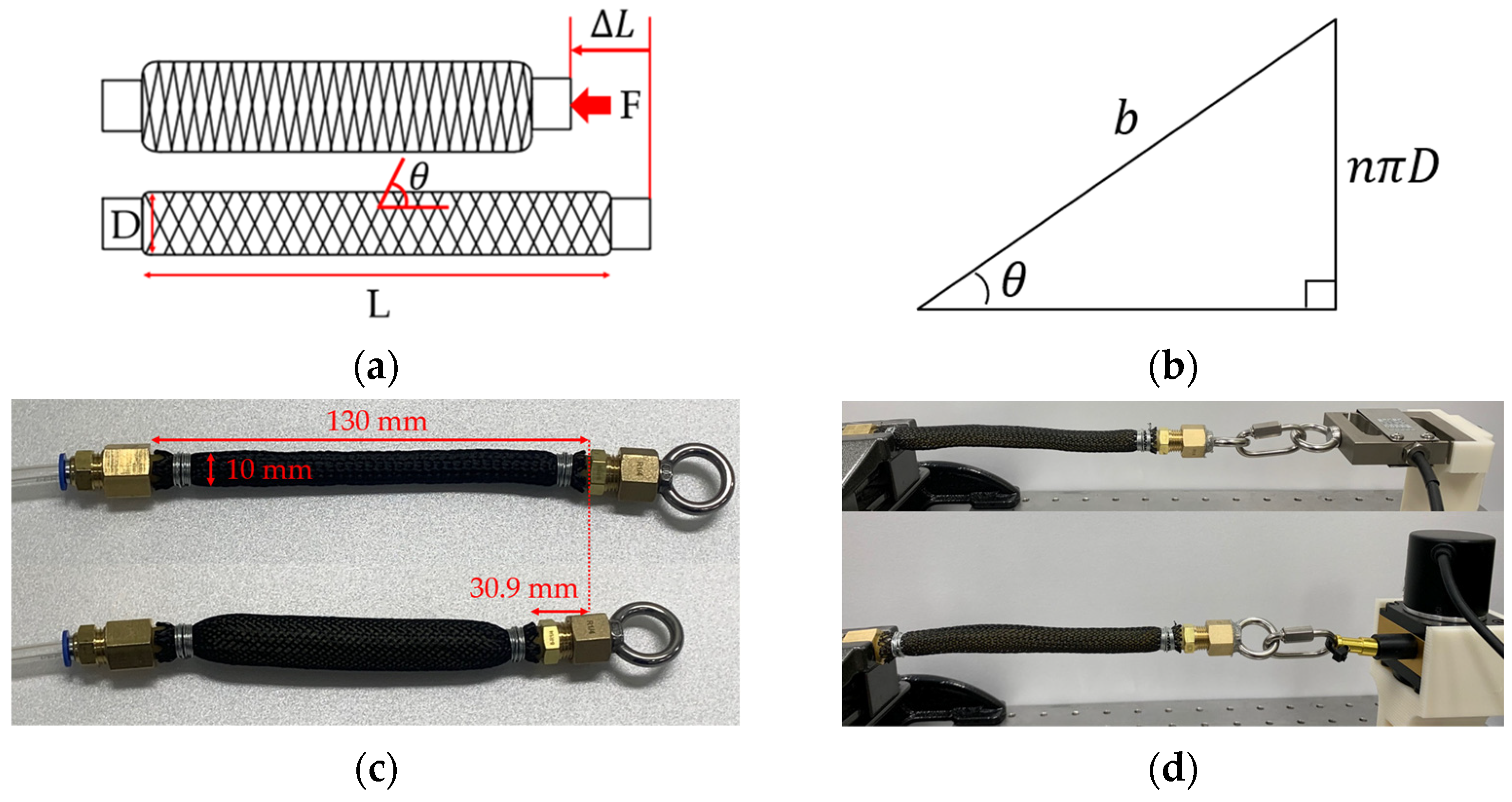
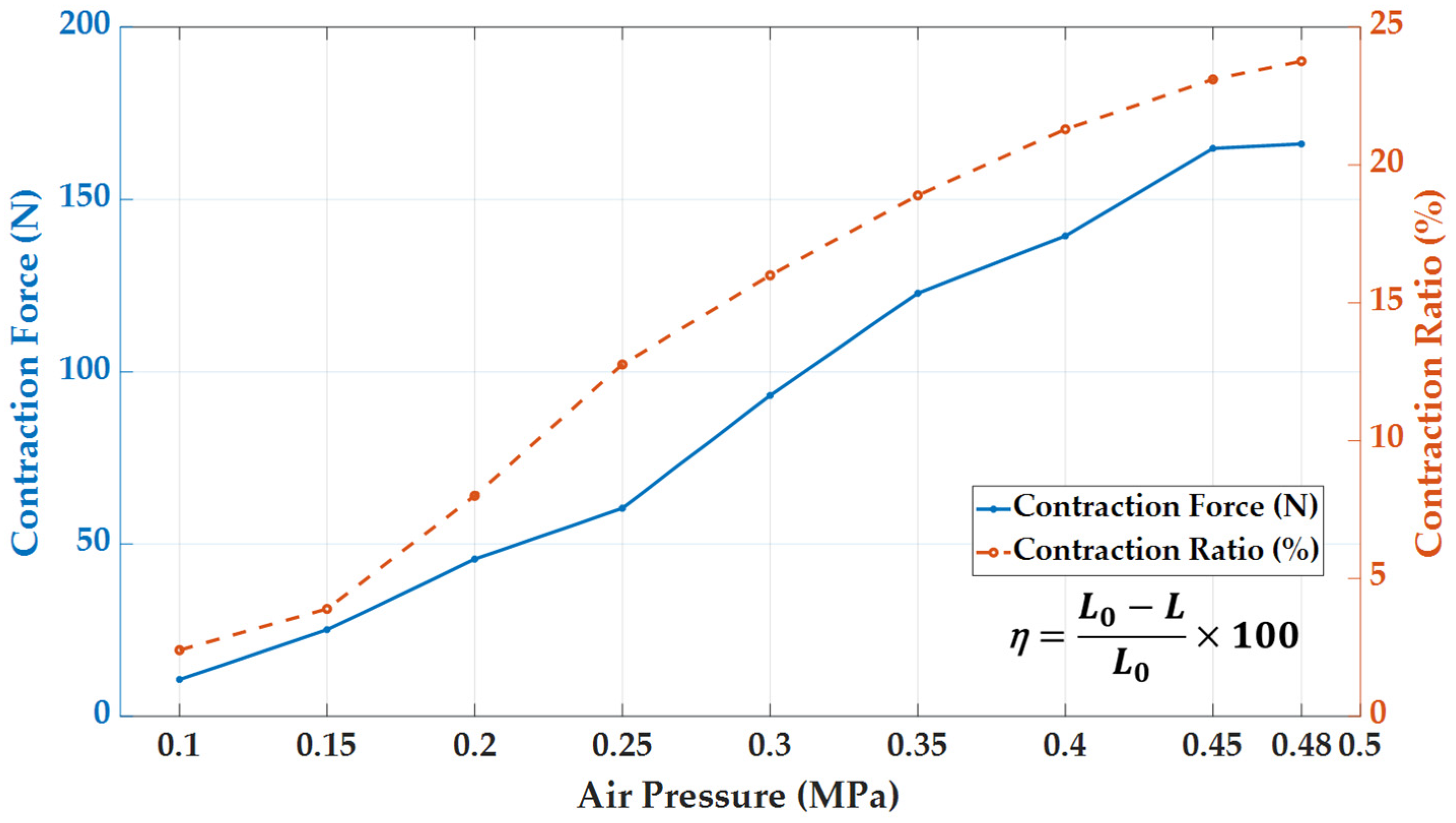
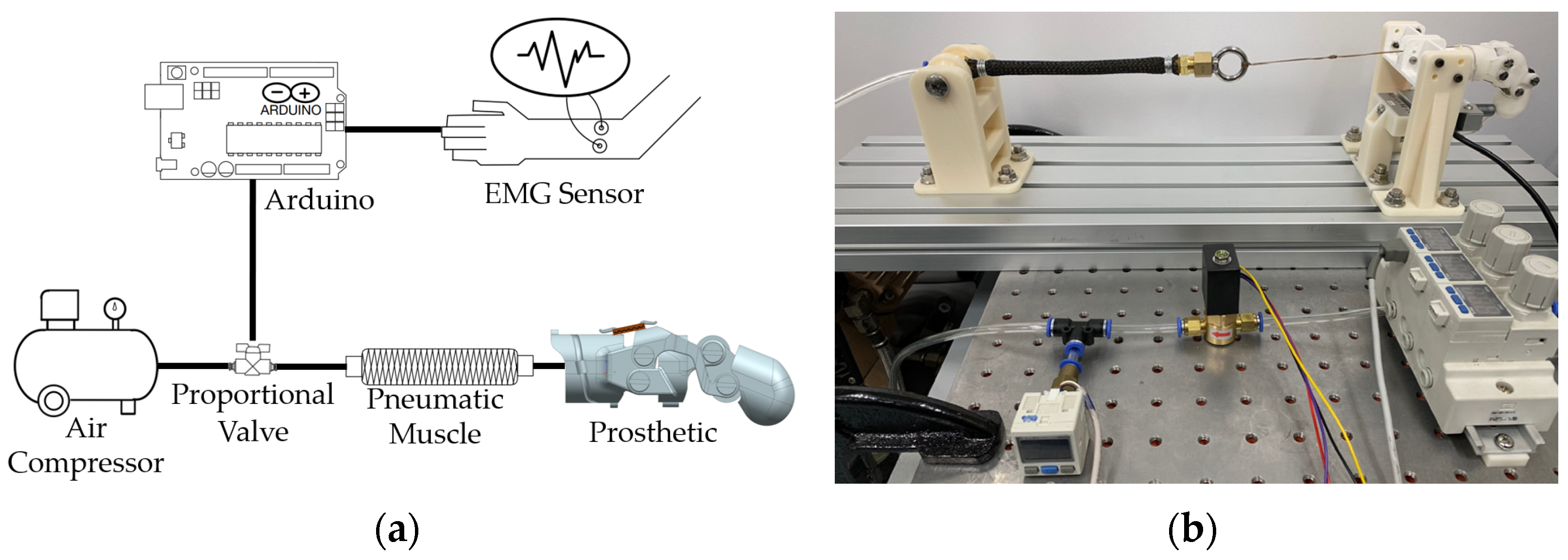
2.3. Pneumatic Actuator
3. EMG Signal According to Grip Force
4. Grip Force Experiment
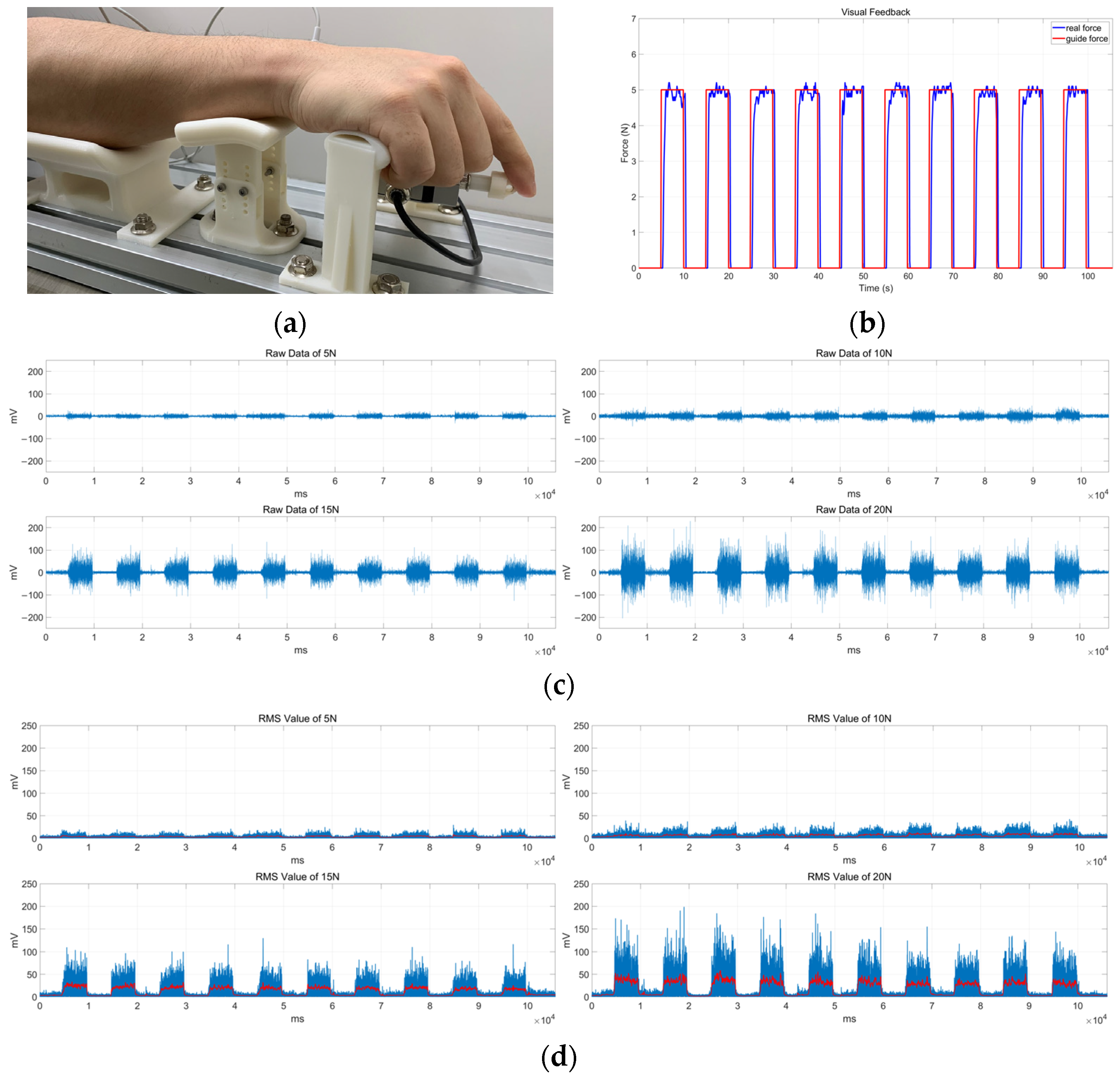
4.1. Experimental Setup
4.2. Experimental Results
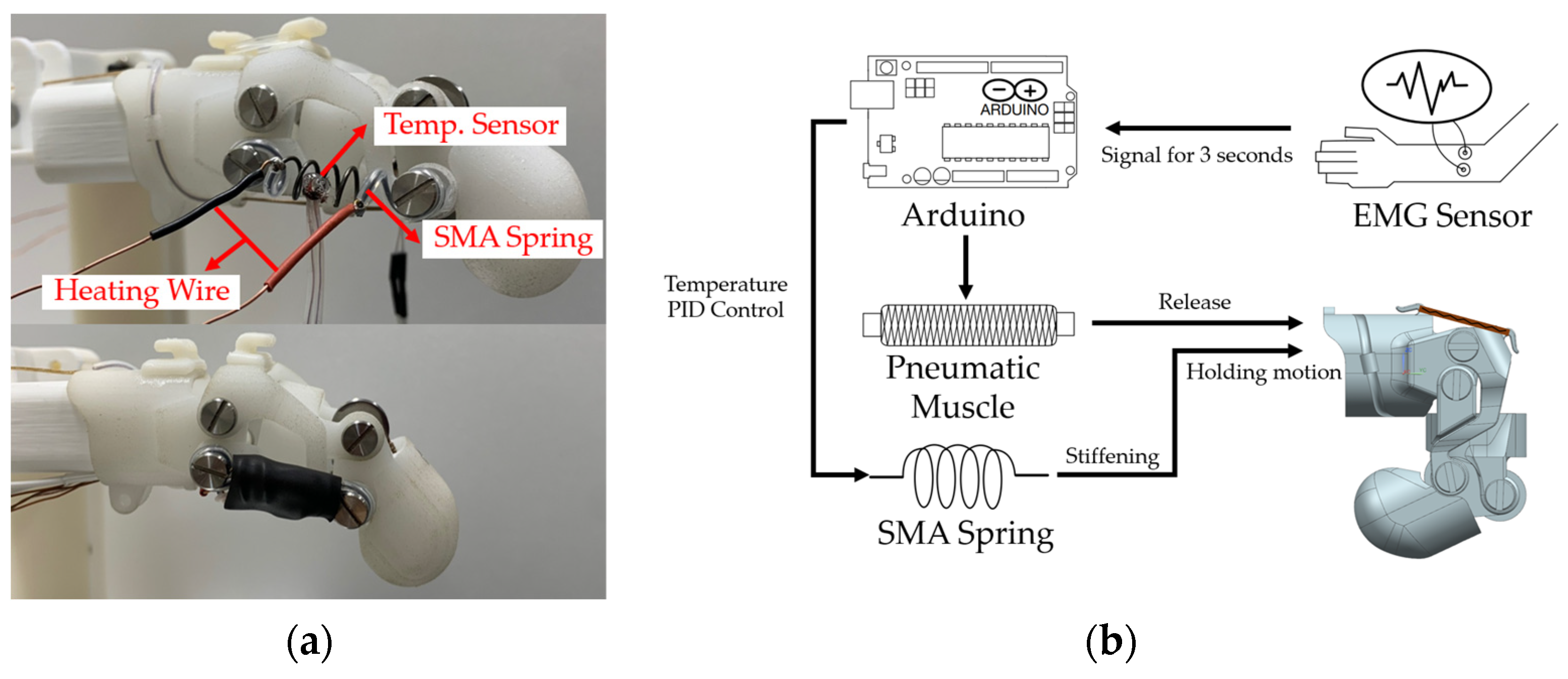
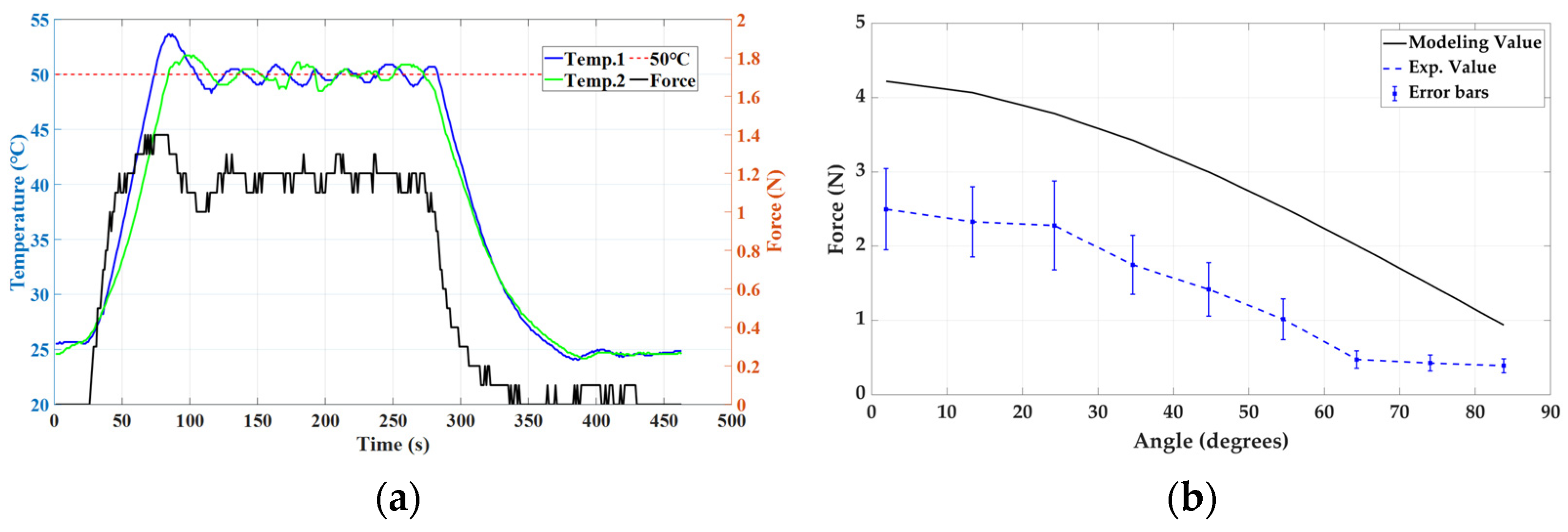
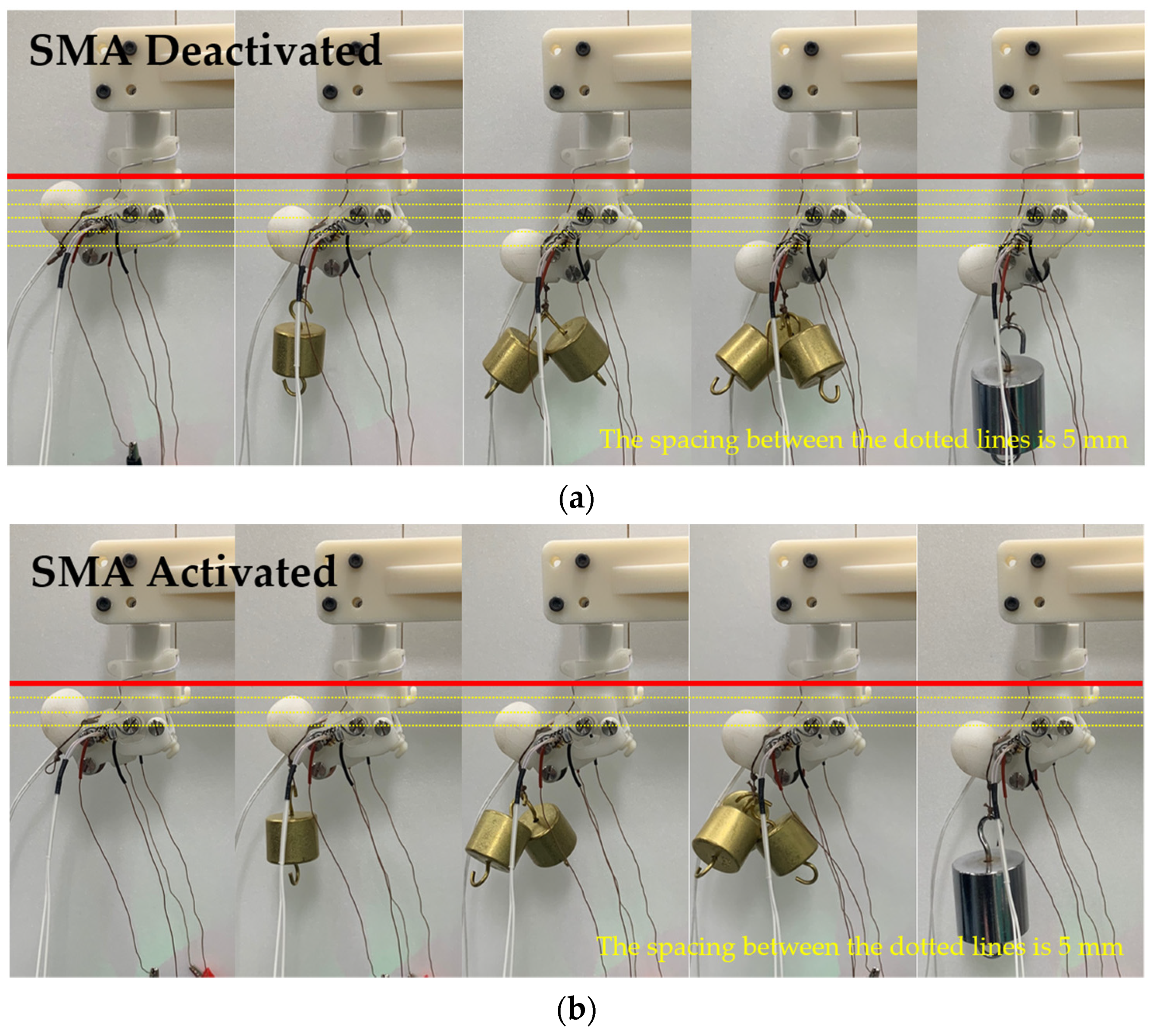
4.3. Stiffness Modulation Using SMA Spring
4.4. Functional Evaluation of Finger Prosthesis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Dunai, L.; Novak, M.; García Espert, C. Human hand anatomy-based prosthetic hand. Sensors 2020, 21, 137. [Google Scholar] [CrossRef] [PubMed]
- Murali, B.; Huddle, S.; Weir, R.F.F. Design and evaluation of a distally actuated powered finger prosthesis with self-contained transmission for individuals with partial hand loss. Adv. Mech. Eng. 2019, 11, 1687814019834114. [Google Scholar] [CrossRef]
- Ryu, W.; Choi, Y.; Choi, Y.J.; Lee, S. Development of a lightweight prosthetic hand for patients with amputated fingers. Appl. Sci. 2020, 10, 3536. [Google Scholar] [CrossRef]
- Sandoval-Gonzalez, O.; Jacinto-Villegas, J.; Herrera-Aguilar, I.; Portillo-Rodiguez, O.; Tripicchio, P.; Hernandez-Ramos, M.; Flores-Cuautle, A.; Avizzano, C. Design and development of a hand exoskeleton robot for active and passive rehabilitation. Int. J. Adv. Robot. Syst. 2016, 13, 66. [Google Scholar] [CrossRef]
- Burns, M.K.; Van Orden, K.; Patel, V.; Vinjamuri, R. Towards a wearable hand exoskeleton with embedded synergies. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju, Republic of Korea, 11–15 July 2017; pp. 213–216. [Google Scholar]
- Ceccarelli, M.; Morales-Cruz, C. A prototype characterization of ExoFinger, a finger exoskeleton. Int. J. Adv. Robot. Syst. 2021, 18, 17298814211024880. [Google Scholar] [CrossRef]
- Li, H.; Cheng, L.; Sun, N.; Cao, R. Design and control of an underactuated finger exoskeleton for assisting activities of daily living. IEEE/ASME Trans. Mechatron. 2021, 27, 2699–2709. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, S.; Song, Z.; Pang, J.; Li, J. A coupling dynamic model for studying the physical interaction between a finger exoskeleton and a human finger. IEEE Access 2020, 8, 125412–125422. [Google Scholar] [CrossRef]
- Jiang, S.; Chen, B.; Qi, F.; Cao, Y.; Ju, F.; Bai, D.; Wang, Y. A variable-stiffness continuum manipulators by an SMA-based sheath in minimally invasive surgery. Int. J. Med. Robot. Comput. Assist. Surg. 2020, 16, e2081. [Google Scholar] [CrossRef]
- Kim, Y.; Cheng, S.S.; Desai, J.P. Active stiffness tuning of a spring-based continuum robot for MRI-guided neurosurgery. IEEE Trans. Robot. 2017, 34, 18–28. [Google Scholar] [CrossRef]
- Jeon, H.; Le, Q.N.; Jeong, S.; Jang, S.; Jung, H.; Chang, H.; Pandya, H.J.; Kim, Y. Towards a snake-like flexible robot with variable stiffness using an SMA spring-based friction change mechanism. IEEE Robot. Autom. Lett. 2022, 7, 6582–6589. [Google Scholar] [CrossRef]
- Thomas, T.L.; Bos, J.; Huaroto, J.J.; Kalpathy Venkiteswaran, V.; Misra, S. A Magnetically Actuated Variable Stiffness Manipulator Based on Deployable Shape Memory Polymer Springs. Adv. Intell. Syst. 2023, 2023, 2200465. [Google Scholar] [CrossRef]
- Kim, J.; Choi, W.-Y.; Kang, S.; Kim, C.; Cho, K.-J. Continuously variable stiffness mechanism using nonuniform patterns on coaxial tubes for continuum microsurgical robot. IEEE Trans. Robot. 2019, 35, 1475–1487. [Google Scholar] [CrossRef]
- Le, Q.N.; Kim, H.; Jeong, S.; Chang, H.; Pandya, H.J.; Kim, Y. Development of a Variable Stiffness Modulating Mechanism Based on Phase-Change Material and a Temperature Control System. Int. J. Precis. Eng. Manuf. 2022, 23, 517–531. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Z.; Zuo, S. Flexible manipulator with low-melting-point alloy actuation and variable stiffness. Soft Robot. 2022, 9, 577–590. [Google Scholar] [CrossRef]
- Kim, H.; Kim, S.J.; Park, J.; Chang, H.; Kim, N.; Kim, Y. Development of Particle Flow-Based Inflatable Robot Body for Shape Rigidity Modulation. Int. J. Precis. Eng. Manuf. 2020, 21, 1857–1864. [Google Scholar] [CrossRef]
- Koh, J. Design of Shape Memory Alloy Coil Spring Acuator for Improving Performance in Cyclic Actuation. Materials 2018, 11, 2324. [Google Scholar] [CrossRef]
- An, S.-M.; Ryu, J.; Cho, M.; Cho, K.-J. Engineering design framework for a shape memory alloy coil spring actuator using a static two-state model. Smart Mater. Struct. 2012, 21, 055009. [Google Scholar] [CrossRef]
- Andrianesis, K.; Tzes, A. Development and control of a multifunctional prosthetic hand with shape memory alloy actuators. J. Intell. Robot. Syst. 2015, 78, 257–289. [Google Scholar] [CrossRef]
- Chou, C.-P.; Hannaford, B. Measurement and modeling of McKibben pneumatic artificial muscles. IEEE Trans. Robot. Autom. 1996, 12, 90–102. [Google Scholar] [CrossRef]
- Kurumaya, S.; Nabae, H.; Endo, G.; Suzumori, K. Design of thin McKibben muscle and multifilament structure. Sens. Actuators A Phys. 2017, 261, 66–74. [Google Scholar] [CrossRef]
- Neumann, D.A. Kinesiology of the Musculoskeletal System: Foundations for Rehabilitation, 3rd ed.; Elsevier Health Sciences: Philadelphia, PA, USA, 2016; pp. 277–336. [Google Scholar]
- Van Boxtel, A. Optimal signal bandwidth for the recording of surface EMG activity of facial, jaw, oral, and neck muscles. Psychophysiology 2001, 38, 22–34. [Google Scholar] [CrossRef] [PubMed]
- Do, P.T.; Le, Q.N.; Luong, Q.V.; Kim, H.-H.; Park, H.-M.; Kim, Y.-J. Tendon-Driven Gripper with Variable Stiffness Joint and Water-Cooled SMA Springs. Actuators 2023, 12, 160. [Google Scholar] [CrossRef]

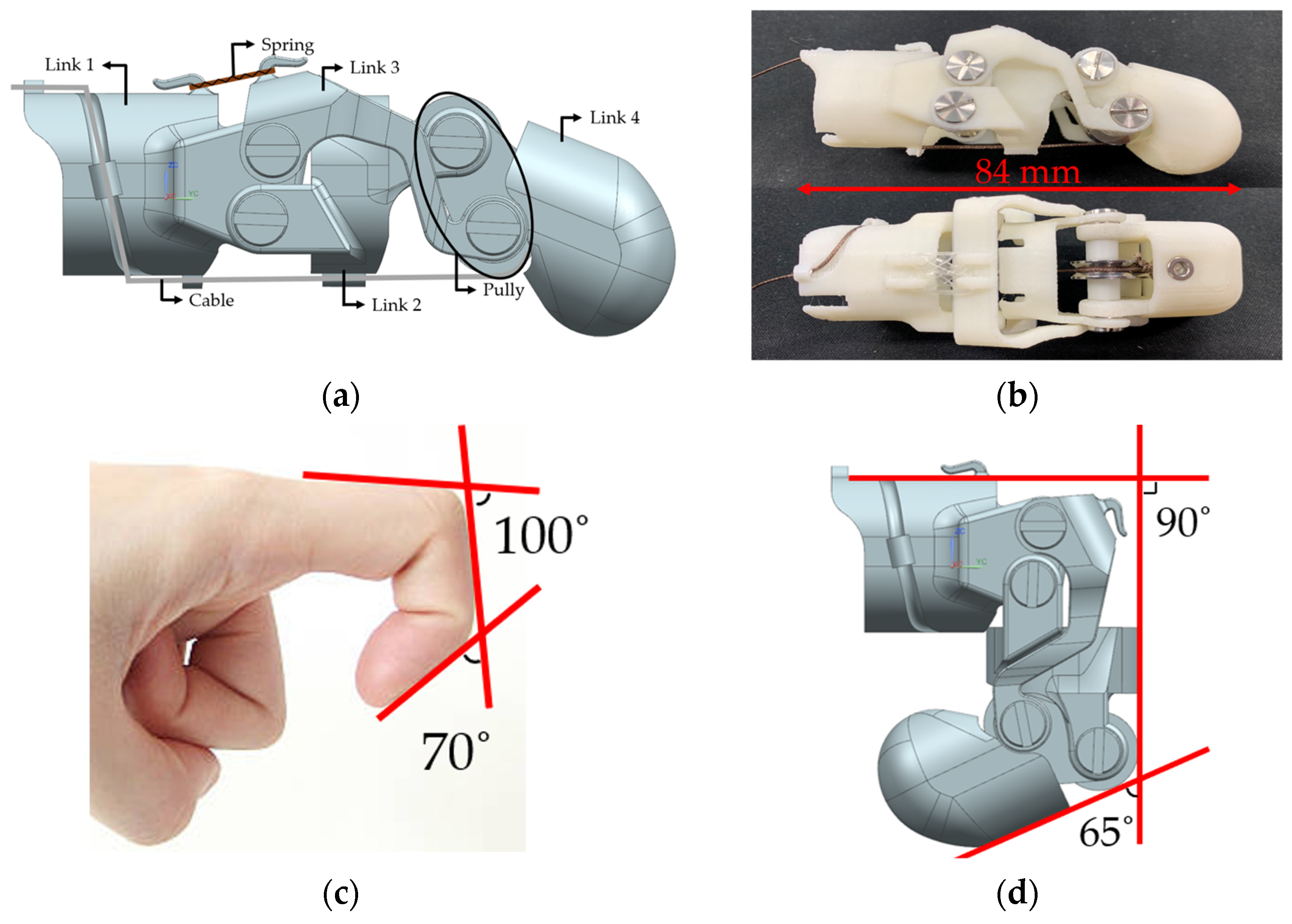



| Developer [Ref.] | Actuator Type | Maximum Angle | Fingertip Maximum Force | Weight |
|---|---|---|---|---|
| Mural [2] | DC Motor | MCP 70° PIP 90° DIP 60° | 6.056 ± 0.396 N | 43.5 g |
| Ryu [3] | DC Motor | MCP 90° PIP 90° DIP 90° | 6.460 ~ 7.487 N | 152.32 g (Except Thumb) |
| Gonzalez [4] | DC Motor | - | - | 731 g |
| Burns [5] | Two Motors | MCP 44 ± 5.3° PIP 36.7 ± 5° | - | 1134 g |
| Ceccarelli [6] | Servo Motors | MCP 37.6° DIP 115.99° | - | - |
| Li [7] | DC Motors | MCP 87.37° PIP 84.16° DIP 82.08° | 12.3 N | 127 g |
| Wang [8] | SMA Wire | MCP 37.5° PIP 42° DIP 29.5° | - | - |
| This Study | Pneumatic | PIP 90° DIP 65° | 6.5 ± 0.3 N | 25 g (80 g including actuator) |
| SMA Spring | 1.2 N |
| Air Pressure, P (MPa) | Diameter, D (mm) | The Angle between the Center Line and Textile, (degree) | Error between Experimental and Model Values (%) |
|---|---|---|---|
| 0.10 | 11.8 | 22 | 7.57 |
| 0.15 | 13.1 | 25 | 13.07 |
| 0.20 | 14.7 | 32 | 5.74 |
| 0.25 | 16.4 | 38 | 7.66 |
| 0.30 | 18.8 | 42 | 3.82 |
| 0.35 | 20.2 | 46 | 3.53 |
| 0.40 | 21.1 | 49 | 3.15 |
| No. | Age | Sex |
|---|---|---|
| Sub 1 | 24 | Male |
| Sub 2 | 26 | Male |
| Sub 3 | 30 | Male |
| Sub 4 | 31 | Male |
| Sub 5 | 26 | Female |
| RMS (mV) | Air Pressure (kPa) | Grip Force of Prosthesis (N) |
|---|---|---|
| 4 ± 1 | 100 | 0.7 ± 0.2 |
| 7 ± 2 | 118 ± 12 | 2.2 ± 0.3 |
| 16 ± 3 | 172 ± 18 | 4.3 ± 0.2 |
| 26 ± 2 | 232 ± 12 | 6.5 ± 0.3 |
| Parameter | Symbol | Value |
|---|---|---|
| Material | NiTi-45 | Nitinol |
| Spring diameter | D | 6.5 mm |
| Wire diameter | d | 0.75 mm |
| Original length | 3 mm | |
| Maximum length | 30.1 mm | |
| Number of coils | n | 4 |
| Shear modulus | G | 16.5 GPa |
| Activation temperature | 50 °C |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.; Jang, S.; Do, P.T.; Lee, C.K.; Ahn, B.; Kwon, S.; Chang, H.; Kim, Y. Development of Wearable Finger Prosthesis with Pneumatic Actuator for Patients with Partial Amputations. Actuators 2023, 12, 434. https://doi.org/10.3390/act12120434
Kim H, Jang S, Do PT, Lee CK, Ahn B, Kwon S, Chang H, Kim Y. Development of Wearable Finger Prosthesis with Pneumatic Actuator for Patients with Partial Amputations. Actuators. 2023; 12(12):434. https://doi.org/10.3390/act12120434
Chicago/Turabian StyleKim, Hyunho, Sujin Jang, Phuoc Thien Do, Chang Kee Lee, Bummo Ahn, Suncheol Kwon, Handdeut Chang, and Yeongjin Kim. 2023. "Development of Wearable Finger Prosthesis with Pneumatic Actuator for Patients with Partial Amputations" Actuators 12, no. 12: 434. https://doi.org/10.3390/act12120434






