Enhancement of Biodegradable Poly(Ethylene Oxide) Ionic–Polymer Metallic Composite Actuators with Nanocrystalline Cellulose Fillers
Abstract
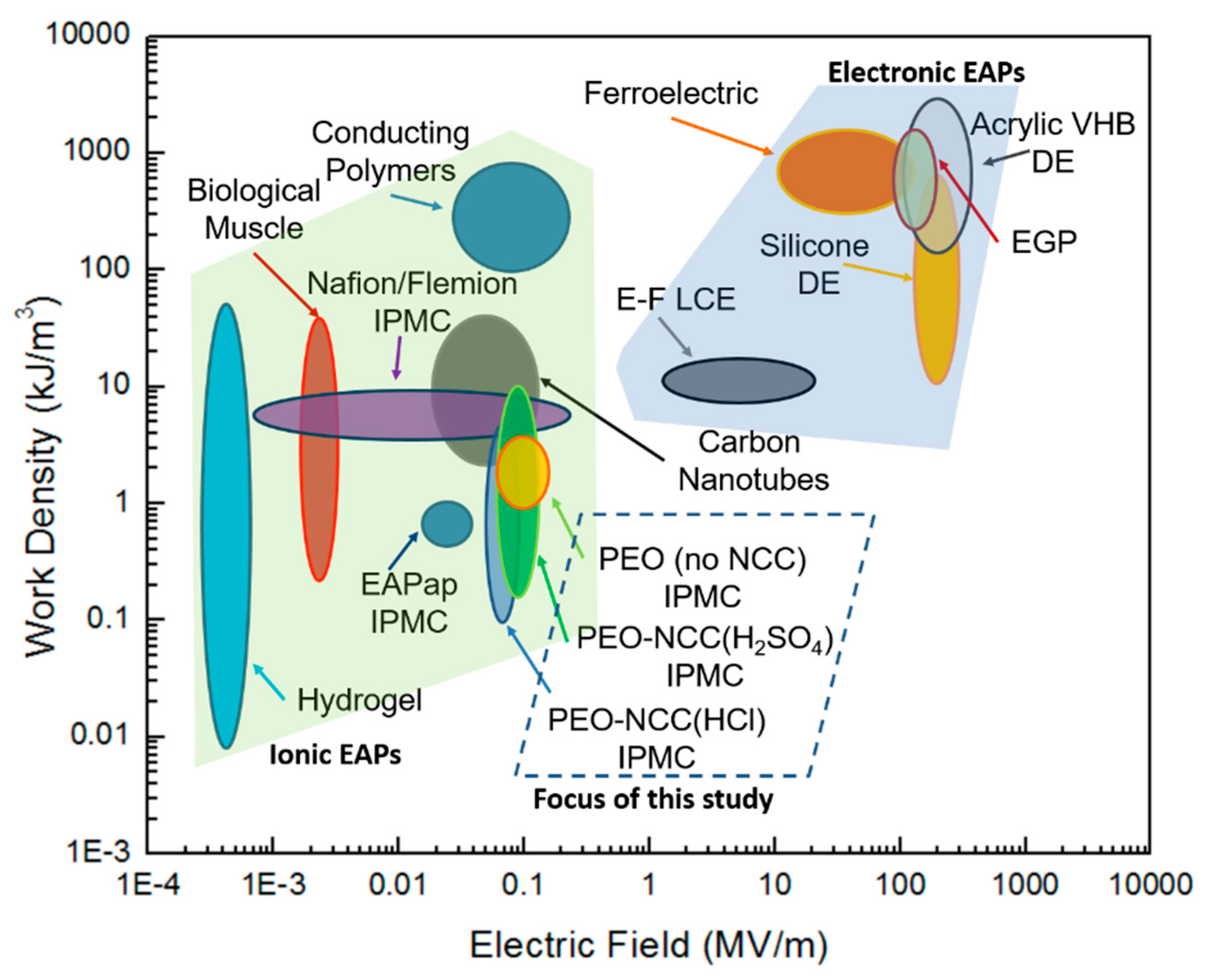
:1. Introduction
2. Materials and Methods
3. Results
3.1. Elastic Modulus Evaluation
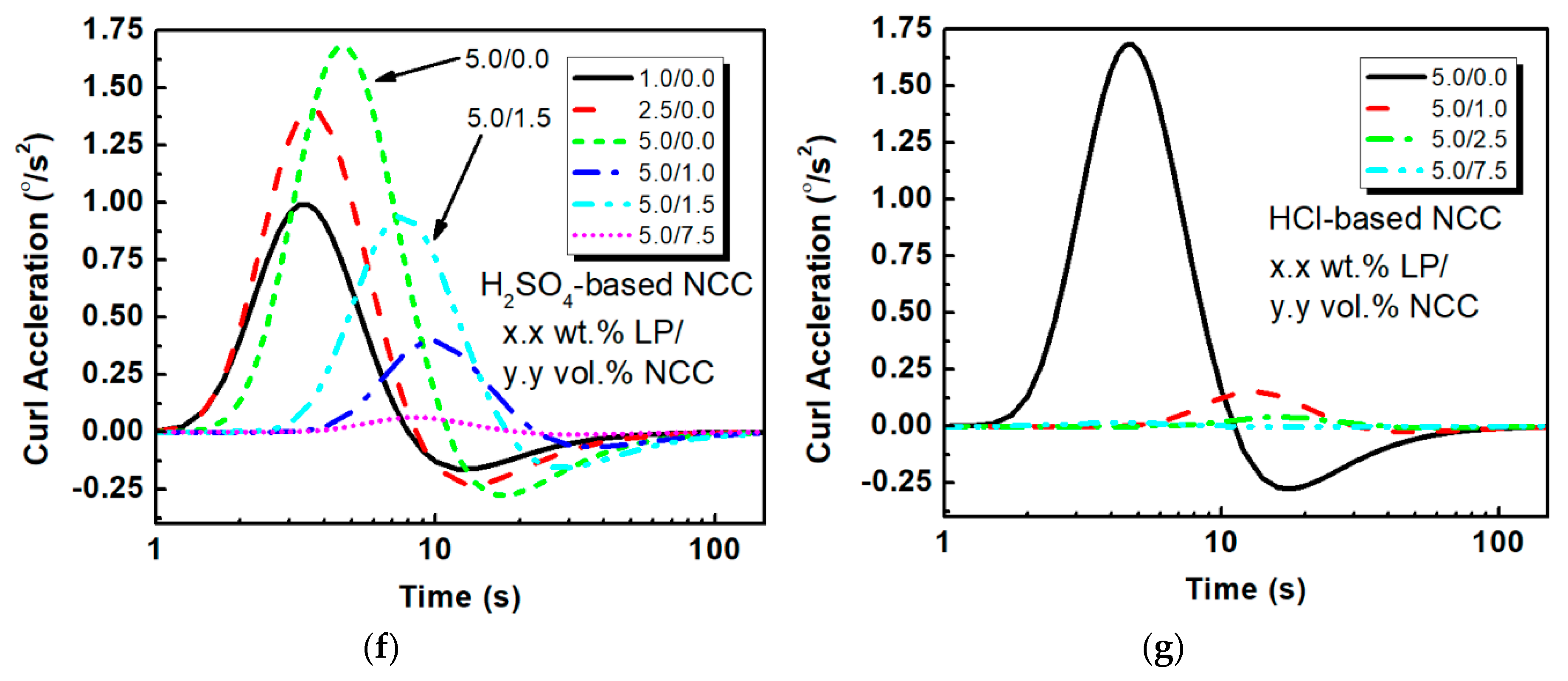
3.2. Electromechanical Actuation Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bar-Cohen, Y.; Cardoso, V.; Ribeiro, C.; Lanceros-Méndez, S. Electroactive polymers as actuators. In Advanced Piezoelectric Materials, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 319–352. [Google Scholar]
- Cheng, Z.; Zhang, Q. Field-activated electroactive polymers. MRS Bull. 2008, 33, 183–187. [Google Scholar] [CrossRef]
- Shahinpoor, M. Ionic polymer-conductor composites as biomimetic sensors, robotic actuators and artificial muscles—A review. Electrochim. Acta 2003, 48, 2343–2353. [Google Scholar] [CrossRef]
- Madden, J.D.; Vandesteeg, N.A.; Anquetil, P.A.; Madden, P.G.; Takshi, A.; Pytel, R.Z.; Lafontaine, S.R.; Wieringa, P.A.; Hunter, I.W. Artificial muscle technology: Physical principles and naval prospects. Ocean. Eng. IEEE J. 2004, 29, 706–728. [Google Scholar] [CrossRef]
- Bahramzadeh, Y.; Shahinpoor, M. A review of ionic polymeric soft actuators and sensors. Soft Robot. 2014, 1, 38–52. [Google Scholar] [CrossRef]
- Safe Handling and Use of Perfluorosulfonic Acid Products; DuPont: Wilmington, DE, USA, 2009.
- Fergus, J.W. Ceramic and polymeric solid electrolytes for lithium-ion batteries. J. Power Sources 2010, 195, 4554–4569. [Google Scholar] [CrossRef]
- Huang, Y.P.; Woo, E.M. Effects of entrapment on spherulite morphology and growth kinetics in poly(ethylene oxide)/epoxy networks. Polymer 2001, 42, 6493–6502. [Google Scholar] [CrossRef]
- Reed, A.M.; Gilding, D.K. Biodegradable polymers for use in surgery—Poly(ethylene oxide)/poly(ethylene terephthalate) (PEO/PET) copolymers: 2. In vitro degradation. Polymer 1981, 22, 499–504. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.C.; Xu, C.; Wu, X.; Wang, X.; Xing, L.; Scott, K. A poly(ethylene oxide)/graphene oxide electrolyte membrane for low temperature polymer fuel cells. J. Power Sources 2011, 196, 8377–8382. [Google Scholar] [CrossRef]
- Benedetti, J.E.; Goncalves, A.D.; Formiga, A.L.B.; De Paoli, M.A.; Li, X.; Durrant, J.R.; Norueira, A.F. A polymer gel electrolyge composed of a poly(ethylene oxide) copolymer and the influence of its composition on the dynamics and performance of dye-sensitized solar cells. J. Power Sources 2010, 195, 1246–1255. [Google Scholar] [CrossRef]
- Lee, S.I.; Schomer, M.; Peng, H.; Page, K.A.; Wilms, D.; Frey, H.; Soles, C.L.; Yoon, D.Y. Correlations between ion conductivity and polymer dynamics in hyperbranched poly(ethylene oxide) electrolytes for lithium-ion batteries. Chem. Mater. 2011, 23, 2685–2688. [Google Scholar] [CrossRef]
- Shahinpoor, M.; Kim, K.J. Solid-state soft actuator exhibiting large electromechanical effect. Appl. Phys. Lett. 2002, 80, 3445–3447. [Google Scholar] [CrossRef]
- Mahadeva, S.K.; Kim, J.; Kang, K.S.; Kim, H.S.; Park, J.M. Effect of poly(ethylene oxide)-poly(ethylene glycol) addition on actuation behavior of cellulose electroactive paper. J. Appl. Polym. Sci. 2009b, 114, 847–852. [Google Scholar] [CrossRef]
- Plesse, C.; Khaldi, A.; Wang, Q.; Cattan, E.; Teyssié, D.; Chevrot, C.; Vidal, F. Polyethylene oxide-polytetrahydrofurane-PEDOT conducting interpenetrating polymer networks for high speed actuators. Smart Mater. Struct. 2011, 20, 124002. [Google Scholar] [CrossRef]
- Bruce, P.; Vincent, C. Structure of an amorphous polymer electrolyte, poly(ethylene oxide) 3: LiCF3SO3. Chem. Commun. 1997, 2, 157–158. [Google Scholar]
- Hayamizu, K.; Akiba, E.; Bando, T.; Aihara, Y.; Price, W.S. NMR studies on poly(ethylene oxide)-based polymer electrolytes with different cross-linking doped with LiN (SO2CF3) 2. Restricted diffusion of the polymer and lithium ion and time-dependent diffusion of the anion. Macromolecules 2003, 36, 2785–2792. [Google Scholar] [CrossRef]
- O’SULLIVAN, A.C. Cellulose: The structure slowly unravels. Cellulose 1997, 4, 173–207. [Google Scholar] [CrossRef]
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 3941–3994. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, S.; Kai, W.; Isogai, A.; Iwata, T. Elastic modulus of single cellulos microfibrils from tunicate measured by atomic force microscopy. Biomacromolecules 2009, 10, 2571–2576. [Google Scholar] [CrossRef] [PubMed]
- Elazzouzi-Hafraoui, S.; Nishiyama, Y.; Putaux, J.L.; Heux, L.; Dubreuil, F.; Rochas, C. The shape and size distribution of crystalline nanoparticles prepared by acid hydrolysis of native cellulose. Biomacromolecules 2008, 9, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Hubbe, M.A.; Rojas, O.J.; Lucia, L.A.; Sain, M. Cellulosic nanocomposites: A review. Bioresources 2008, 55, 929–980. [Google Scholar]
- Habibi, Y.; Chanzy, H.; Vignon, M.R. TEMPO-mediated surface oxidation of cellulose whiskers. Cellulose 2006, 13, 679–687. [Google Scholar] [CrossRef] [Green Version]
- Samir, M.A.S.A.; Alloin, F.; Dufresne, A. Review of recent research into cellulosic whiskers, their properties and their application in nanocomposite field. Biomacromolecules 2005, 6, 612–626. [Google Scholar] [CrossRef] [PubMed]
- Nishiyama, Y.; Langan, P.; Chanzy, H. Crystal structure and hydrogen-bonding system in cellulose Iβ from synchrotron X-ray and neutron fiber diffraction. J. Am. Chem. Soc. 2002, 124, 9074–9082. [Google Scholar] [CrossRef] [PubMed]
- Hammouda, B.; Ho, D.L.; Kline, S. Insight into clustering in poly(ethylene oxide). Marcomolecules 2004, 37, 6932–6937. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Huo, H.; Jiang, S. Effects of lithium perchlorate on poly(ethylene oxide) spherulite morphology and spherulite growth kinetics. J. Appl. Polym. Sci. 2012, 123, 1935–1943. [Google Scholar] [CrossRef]
- Ragavan, V. Materials Science and Engineering—A first Course; Prentice Hall of India: New Delhi, India, 2006. [Google Scholar]
- Bass, P.S.; Zhang, L.; Cheng, Z.Y. Time-dependence of the electromechanical bending actuation observed in ionic-electroactive polymers. J. Adv. Dielectr. 2017, 7, 1720002. [Google Scholar] [CrossRef] [Green Version]




| wt.% LP | vol.% NCC | |||||
|---|---|---|---|---|---|---|
| PEO | 0.0 | -- | 491 | -- | -- | -- |
| 1.0 | -- | 311 | 0.31 | 1.49 | 1.31 | |
| 2.5 | -- | 248 | 0.51 | 3.23 | 2.83 | |
| 5.0 | -- | 92.9 | 0.97 | 4.36 | 3.83 | |
| 7.5 | -- | 20.1 | -- | -- | -- | |
| Sulfuric Acid | 5.0 | 1.0 | 135 | 0.91 | 5.54 | 4.87 |
| Hydrolysis | 5.0 | 1.5 | 112 | 1.47 | 12.1 | 10.6 |
| NCC | 5.0 | 2.5 | 154 | 1.06 | 8.64 | 7.59 |
| 5.0 | 5.0 | 231 | 0.42 | 2.08 | 1.82 | |
| 5.0 | 7.5 | 316 | 0.11 | 0.198 | 0.174 | |
| Hydrochloric | 5.0 | 1.0 | 176 | 0.18 | 0.296 | 0.260 |
| Acid Hydrolysis | 5.0 | 2.5 | 339 | 0.41 | 2.90 | 2.55 |
| NCC | 5.0 | 5.0 | 454 | 0.21 | 1.01 | 0.886 |
| 5.0 | 7.5 | 501 | 0.09 | 0.198 | 0.174 | |
| Bulk NCC–H2SO4 | -- | -- | 7760 | -- | -- |
| PEO–NCC Composites | Figure 4a–c Fittings | Figure 4d–g Analysis | |||||
|---|---|---|---|---|---|---|---|
| x.x/y.y wt.% Salt/vol.% NCC | B (s) | ||||||
| PEO with LP | 1.0/0.0 | 16 | 100 | 0.25 | 3.4 | 0.99 | −0.16 |
| No NCC | 2.5/0.0 | 17 | 160 | 0.40 | 5.1 | 1.4 | −0.23 |
| 5.0/0.0 | 22 | 320 | 0.81 | 7.8 | 1.7 | −0.28 | |
| Sulfuric Acid | 5.0/1.0 | 46 | 330 | 0.83 | 3.9 | 0.39 | −0.065 |
| Hydrolysis | 5.0/1.5 | 36 | 480 | 1.2 | 7.2 | 0.94 | −0.16 |
| NCC | 5.0/7.5 | 40 | 40 | 0.1 | 0.54 | 0.063 | −0.010 |
| Hydrochloric | 5.0/1.0 | 60.9 | 224 | 0.56 | 2.0 | 0.15 | −0.025 |
| Acid Hydrolysis | 5.0/2.5 | 76.3 | 93.1 | 0.23 | 0.66 | 0.041 | -- |
| NCC | 5.0/7.5 | 20.6 | 2.53 | 6.3 × 10−5 | -- | -- | -- |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bass, P.S.; Zhang, L.; Tu, M.; Cheng, Z. Enhancement of Biodegradable Poly(Ethylene Oxide) Ionic–Polymer Metallic Composite Actuators with Nanocrystalline Cellulose Fillers. Actuators 2018, 7, 72. https://doi.org/10.3390/act7040072
Bass PS, Zhang L, Tu M, Cheng Z. Enhancement of Biodegradable Poly(Ethylene Oxide) Ionic–Polymer Metallic Composite Actuators with Nanocrystalline Cellulose Fillers. Actuators. 2018; 7(4):72. https://doi.org/10.3390/act7040072
Chicago/Turabian StyleBass, Patrick S., Lin Zhang, Maobing Tu, and ZhongYang Cheng. 2018. "Enhancement of Biodegradable Poly(Ethylene Oxide) Ionic–Polymer Metallic Composite Actuators with Nanocrystalline Cellulose Fillers" Actuators 7, no. 4: 72. https://doi.org/10.3390/act7040072




