Lactobacillus acidophilus LA-5 Ameliorates Inflammation and Alveolar Bone Loss Promoted by A. actinomycetemcomitans and S. gordonii in Mice and Impacts Oral and Gut Microbiomes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Group Allocation
2.2. Blinding
2.3. Exclusion Criteria
2.4. Sample Size
2.5. Bacteria Strains and Culture Conditions
2.6. Experimental Treatments
2.7. Euthanasia and Samples Collection
2.8. Alveolar Bone Loss Analysis
2.9. Gene Expression in Gingiva
2.10. Oral and Gut Microbiomes
2.11. Statistical Analysis
3. Results
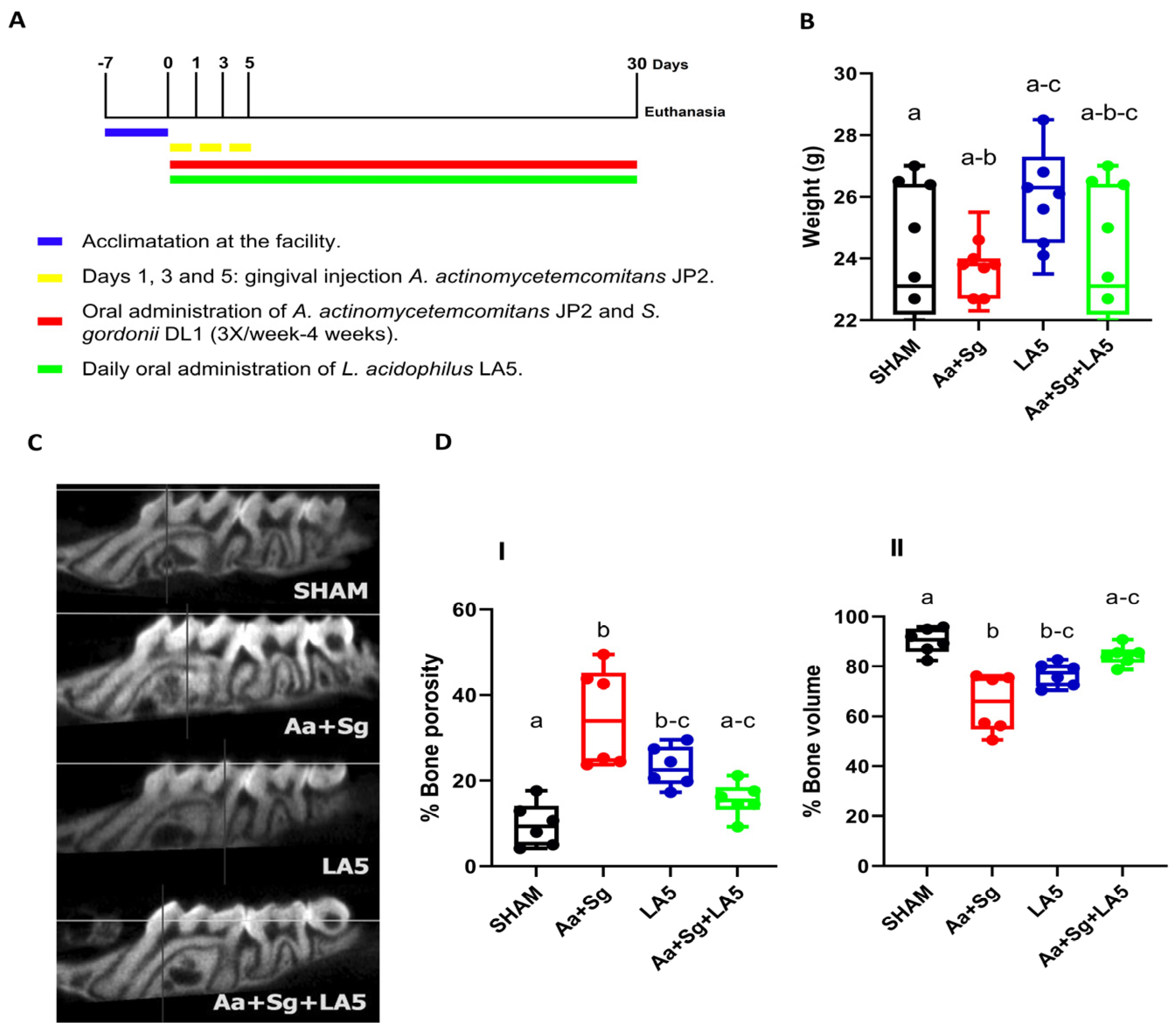
3.1. Animals Changes
3.2. Alveolar Bone Loss
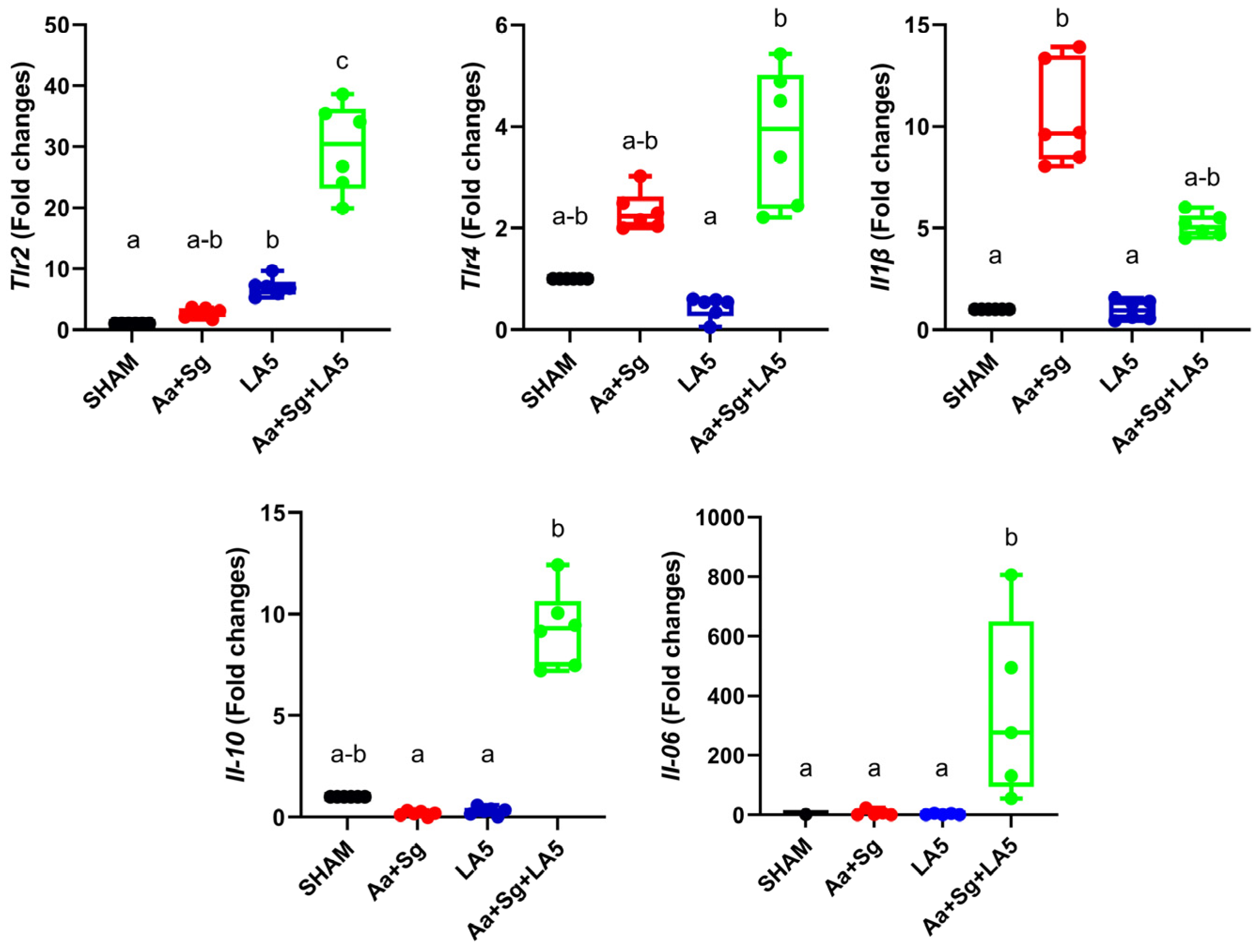
3.3. Transcription Analysis in Gingival Tissue
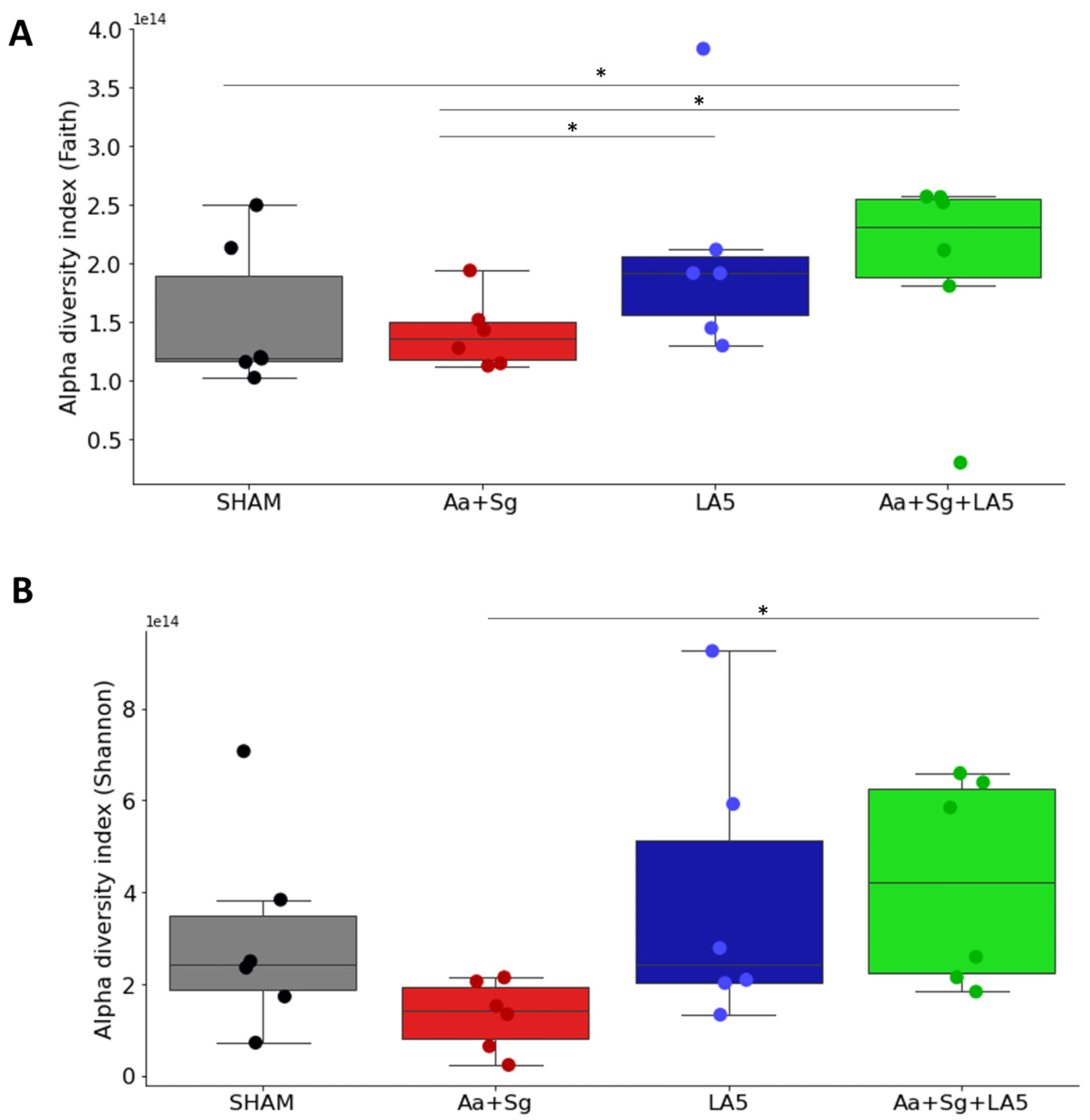
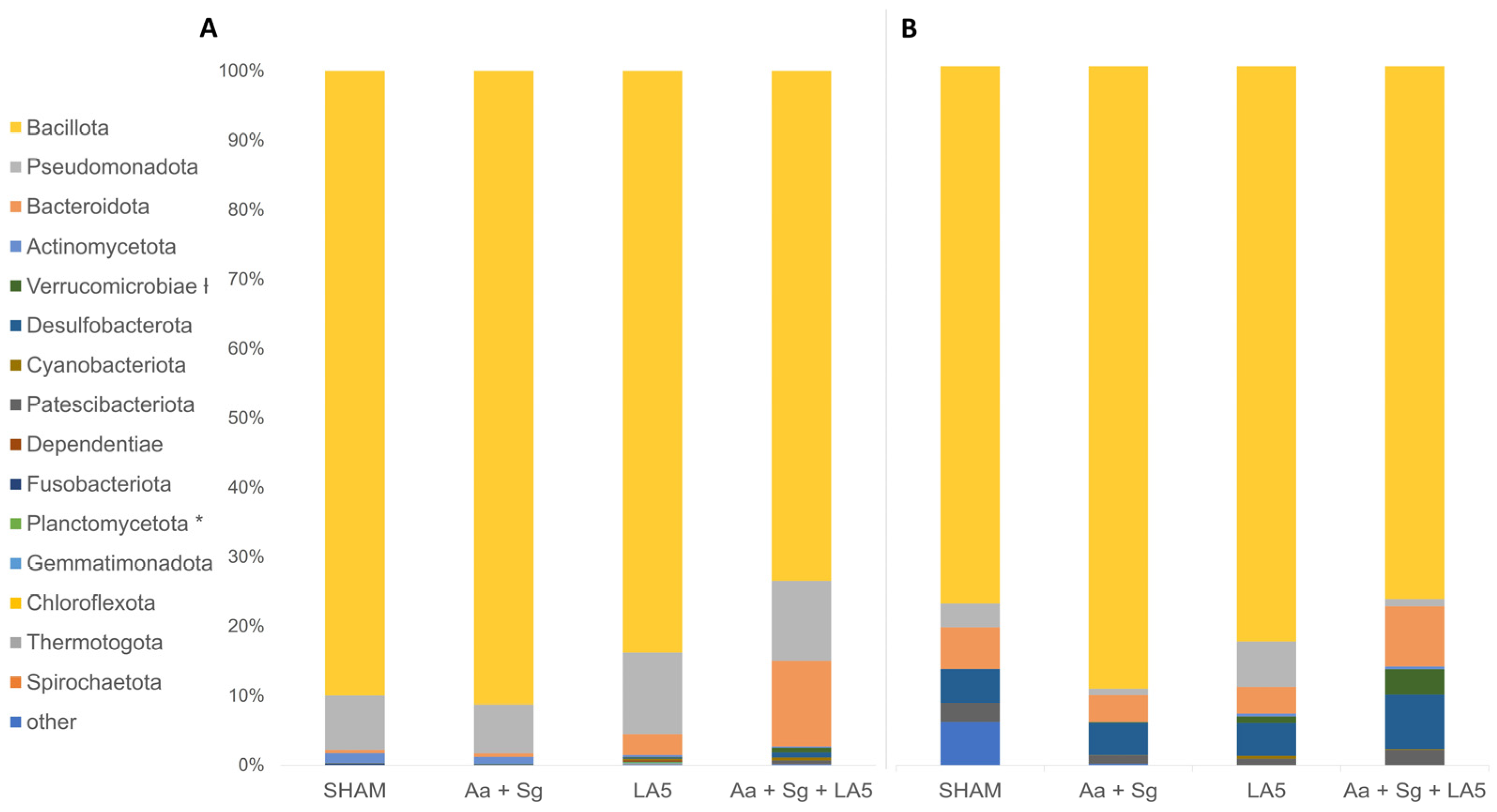
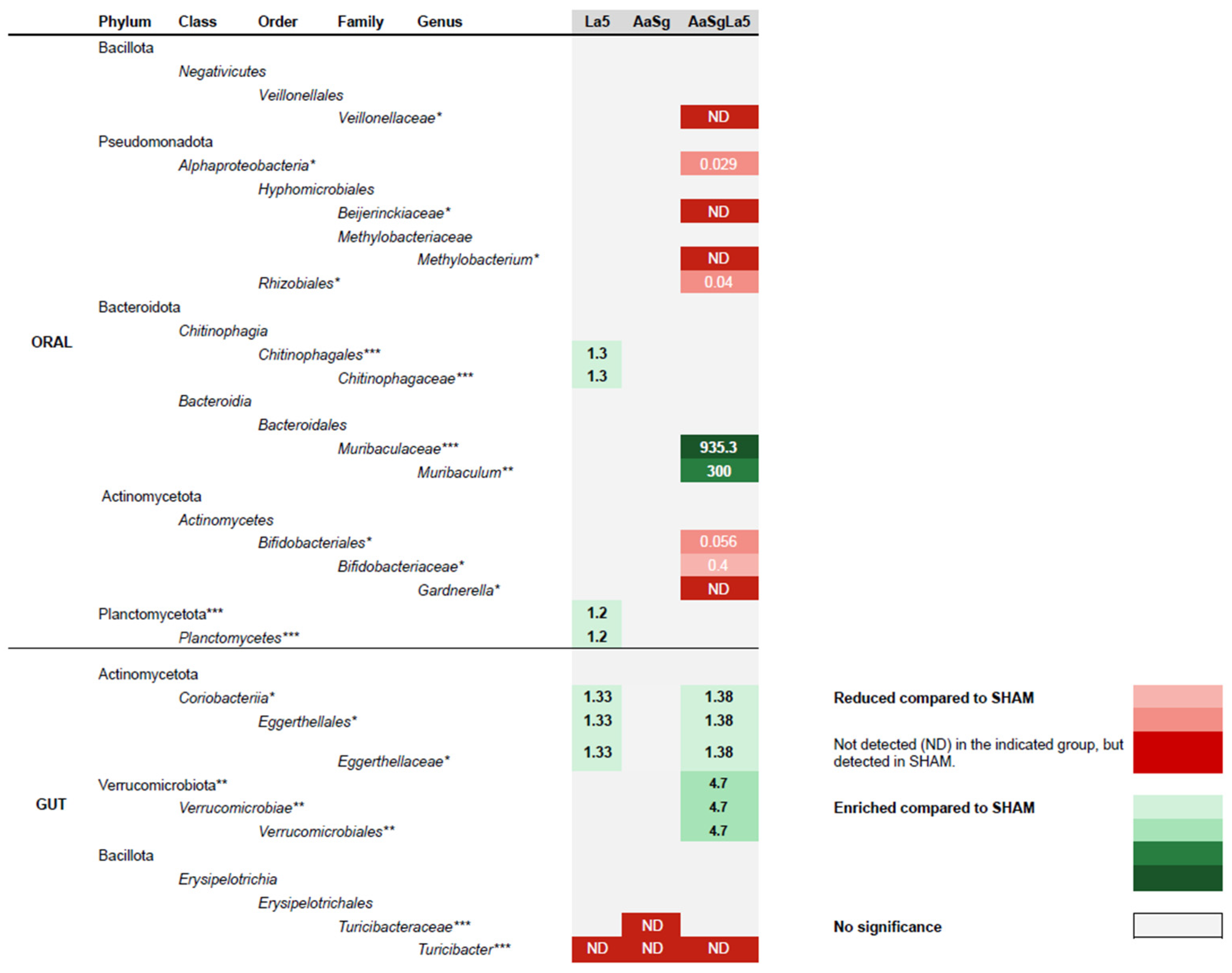
3.4. Oral and Gut Microbiomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Haubek, D.; Johansson, A. Pathogenicity of the highly leukotoxic JP2 clone of Aggregatibacter actinomycetemcomitans and its geographic dissemination and role in aggressive periodontitis. J. Oral Microbiol. 2014, 6, 23980. [Google Scholar] [CrossRef] [PubMed]
- Amado, P.P.P.; Kawamoto, D.; Albuquerque-Souza, E.; Franco, D.C.; Saraiva, L.; Casarin, R.C.V.; Horliana, A.; Mayer, M.P.A. Oral and Fecal Microbiome in Molar-Incisor Pattern Periodontitis. Front. Cell Infect. Microbiol. 2020, 10, 583761. [Google Scholar] [CrossRef] [PubMed]
- Feres, M.; Figueiredo, L.C.; Soares, G.M.; Faveri, M. Systemic antibiotics in the treatment of periodontitis. Periodontol. 2000 2015, 67, 131–186. [Google Scholar] [CrossRef] [PubMed]
- Sanz, M.; Herrera, D.; Kebschull, M.; Chapple, I.; Jepsen, S.; Beglundh, T.; Sculean, A.; Tonetti, M.S.; Participants, E.F.P.W.; Methodological, C. Treatment of stage I-III periodontitis-The EFP S3 level clinical practice guideline. J. Clin. Periodontol. 2020, 47 (Suppl. S22), 4–60. [Google Scholar] [CrossRef] [PubMed]
- Myneni, S.R.; Brocavich, K.; Wang, H.H. Biological strategies for the prevention of periodontal disease: Probiotics and vaccines. Periodontol. 2000 2020, 84, 161–175. [Google Scholar] [CrossRef] [PubMed]
- Gheisary, Z.; Mahmood, R.; Harri Shivanantham, A.; Liu, J.; Lieffers, J.R.L.; Papagerakis, P.; Papagerakis, S. The Clinical, Microbiological, and Immunological Effects of Probiotic Supplementation on Prevention and Treatment of Periodontal Diseases: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 1036. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, G.; Zhang, H.M.; Zhu, F.F. Probiotic adjuvant treatment in combination with scaling and root planing in chronic periodontitis: A systematic review and meta-analysis. Benef. Microbes 2023, 14, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Vincenzi, A.; Goettert, M.I.; Volken de Souza, C.F. An evaluation of the effects of probiotics on tumoral necrosis factor (TNF-α) signaling and gene expression. Cytokine Growth Factor. Rev. 2021, 57, 27–38. [Google Scholar] [CrossRef]
- Kleerebezem, M.; Binda, S.; Bron, P.A.; Gross, G.; Hill, C.; van Hylckama Vlieg, J.E.; Lebeer, S.; Satokari, R.; Ouwehand, A.C. Understanding mode of action can drive the translational pipeline towards more reliable health benefits for probiotics. Curr. Opin. Biotechnol. 2019, 56, 55–60. [Google Scholar] [CrossRef]
- Bueno, M.R.; Dudu-Silva, G.; Macedo, T.T.; Gomes, A.P.d.A.P.; Rodrigues Oliveira Braga, A.; Aguiar Silva, L.D.; Bueno-Silva, B. Lactobacillus acidophilus impairs the establishment of pathogens in a subgingival multispecies biofilm. Front. Dent. Med. 2023, 4, 1212773. [Google Scholar] [CrossRef]
- Bueno, M.R.; Ishikawa, K.H.; Almeida-Santos, G.; Ando-Suguimoto, E.S.; Shimabukuro, N.; Kawamoto, D.; Mayer, M.P.A. Lactobacilli Attenuate the Effect of Aggregatibacter actinomycetemcomitans Infection in Gingival Epithelial Cells. Front. Microbiol. 2022, 13, 846192. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, K.H.; Bueno, M.R.; Kawamoto, D.; Simionato, M.R.L.; Mayer, M.P.A. Lactobacilli postbiotics reduce biofilm formation and alter transcription of virulence genes of Aggregatibacter actinomycetemcomitans. Mol. Oral Microbiol. 2021, 36, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque-Souza, E.; Balzarini, D.; Ando-Suguimoto, E.S.; Ishikawa, K.H.; Simionato, M.R.L.; Holzhausen, M.; Mayer, M.P.A. Probiotics alter the immune response of gingival epithelial cells challenged by Porphyromonas gingivalis. J. Periodontal Res. 2019, 54, 115–127. [Google Scholar] [CrossRef] [PubMed]
- Salgaço, M.K.; de Oliveira, F.L.; Sartoratto, A.; Mesa, V.; Mayer, M.P.A.; Sivieri, K. Impact of Lactobacillus acidophilus—La5 on Composition and Metabolism of the Intestinal Microbiota of Type 2 Diabetics (T2D) and Healthy Individuals Using a Microbiome Model. Fermentation 2023, 9, 740. [Google Scholar] [CrossRef]
- Vale, G.C.; Mota, B.I.S.; Ando-Suguimoto, E.S.; Mayer, M.P.A. Lactobacilli Probiotics Modulate Antibacterial Response Gene Transcription of Dendritic Cells Challenged with LPS. Probiotics Antimicrob. Proteins 2024, 16, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Vale, G.C.; Mota, B.I.S.; Ando-Suguimoto, E.S.; Mayer, M.P.A. Effect of Probiotics Lactobacillus acidophilus and Lacticaseibacillus rhamnosus on Antibacterial Response Gene Transcription of Human Peripheral Monocytes. Probiotics Antimicrob. Proteins 2023, 15, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Catarucci, A.C.S.K.D.; Shimabukuro, N.; Ishikawa, K.H.; Ando-Suguimoto, E.S.; Ribeiro, R.A.; Nicastro, G.G.; Albuquerque-Souza, E.; Robson Souza, R.F.; Mayer, M.P.A. Oral Administration of Lactobacillus Acidophilus LA5 Prevents Alveolar Bone Loss and Alters Oral and Gut Microbiomes in a Murine Periodontitis Experimental Model; University of São Paulo: São Paulo, Brazil, 2024; to be submitted. [Google Scholar]
- Charan, J.; Kantharia, N.D. How to calculate sample size in animal studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, N.; Cataruci, A.C.S.; Ishikawa, K.H.; de Oliveira, B.E.; Kawamoto, D.; Ando-Suguimoto, E.S.; Albuquerque-Souza, E.; Nicoli, J.R.; Ferreira, C.M.; de Lima, J.; et al. Bifidobacterium Strains Present Distinct Effects on the Control of Alveolar Bone Loss in a Periodontitis Experimental Model. Front. Pharmacol. 2021, 12, 713595. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.C.; Shenker, B.J.; DiRienzo, J.M.; Malamud, D.; Taichman, N.S. Extraction and isolation of a leukotoxin from Actinobacillus actinomycetemcomitans with polymyxin B. Infect. Immun. 1984, 43, 700–705. [Google Scholar] [CrossRef]
- Hsu, S.D.; Cisar, J.O.; Sandberg, A.L.; Kilian, M. Adhesive Properties of Viridans Streptoccocal Species. Microb. Ecol. Health Dis. 2009, 7, 125–137. [Google Scholar]
- Repeke, C.E.; Ferreira, S.B., Jr.; Claudino, M.; Silveira, E.M.; de Assis, G.F.; Avila-Campos, M.J.; Silva, J.S.; Garlet, G.P. Evidences of the cooperative role of the chemokines CCL3, CCL4 and CCL5 and its receptors CCR1+ and CCR5+ in RANKL+ cell migration throughout experimental periodontitis in mice. Bone 2010, 46, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Madeira, M.F.; Queiroz-Junior, C.M.; Costa, G.M.; Werneck, S.M.; Cisalpino, D.; Garlet, G.P.; Teixeira, M.M.; Silva, T.A.; Souza, D.G. Platelet-activating factor receptor blockade ameliorates Aggregatibacter actinomycetemcomitans-induced periodontal disease in mice. Infect. Immun. 2013, 81, 4244–4251. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Herlemann, D.P.; Labrenz, M.; Jurgens, K.; Bertilsson, S.; Waniek, J.J.; Andersson, A.F. Transitions in bacterial communities along the 2000 km salinity gradient of the Baltic Sea. ISME J. 2011, 5, 1571–1579. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Quast, C.; Pruesse, E.; Yilmaz, P.; Gerken, J.; Schweer, T.; Yarza, P.; Peplies, J.; Glockner, F.O. The SILVA ribosomal RNA gene database project: Improved data processing and web-based tools. Nucleic Acids Res. 2013, 41, D590–D596. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, P.; Parfrey, L.W.; Yarza, P.; Gerken, J.; Pruesse, E.; Quast, C.; Schweer, T.; Peplies, J.; Ludwig, W.; Glockner, F.O. The SILVA and “All-species Living Tree Project (LTP)” taxonomic frameworks. Nucleic Acids Res. 2014, 42, D643–D648. [Google Scholar] [CrossRef] [PubMed]
- Lozupone, C.A.; Stombaugh, J.I.; Gordon, J.I.; Jansson, J.K.; Knight, R. Diversity, stability and resilience of the human gut microbiota. Nature 2012, 489, 220–230. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Peddada, S.D. Analysis of compositions of microbiomes with bias correction. Nat. Commun. 2020, 11, 3514. [Google Scholar] [CrossRef]
- Nobbs, A.; Kreth, J. Genetics of sanguinis-Group Streptococci in Health and Disease. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Stacy, A.; Everett, J.; Jorth, P.; Trivedi, U.; Rumbaugh, K.P.; Whiteley, M. Bacterial fight-and-flight responses enhance virulence in a polymicrobial infection. Proc. Natl. Acad. Sci. USA 2014, 111, 7819–7824. [Google Scholar] [CrossRef] [PubMed]
- Stacy, A.; Fleming, D.; Lamont, R.J.; Rumbaugh, K.P.; Whiteley, M. A Commensal Bacterium Promotes Virulence of an Opportunistic Pathogen via Cross-Respiration. mBio 2016, 7, e00782-16. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.A.; Whiteley, M. A novel exclusion mechanism for carbon resource partitioning in Aggregatibacter actinomycetemcomitans. J. Bacteriol. 2007, 189, 6407–6414. [Google Scholar] [CrossRef] [PubMed]
- Ramsey, M.M.; Rumbaugh, K.P.; Whiteley, M. Metabolite cross-feeding enhances virulence in a model polymicrobial infection. PLoS Pathog. 2011, 7, e1002012. [Google Scholar] [CrossRef] [PubMed]
- Kanmani, P.; Kim, H. Functional capabilities of probiotic strains on attenuation of intestinal epithelial cell inflammatory response induced by TLR4 stimuli. Biofactors 2019, 45, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Vizoso Pinto, M.G.; Rodriguez Gomez, M.; Seifert, S.; Watzl, B.; Holzapfel, W.H.; Franz, C.M. Lactobacilli stimulate the innate immune response and modulate the TLR expression of HT29 intestinal epithelial cells in vitro. Int. J. Food Microbiol. 2009, 133, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, R.; Altermann, E.; Anderson, R.C.; McNabb, W.C.; Moughan, P.J.; Roy, N.C. The role of cell surface architecture of lactobacilli in host-microbe interactions in the gastrointestinal tract. Mediators Inflamm. 2013, 2013, 237921. [Google Scholar] [CrossRef]
- Sun, K.Y.; Xu, D.H.; Xie, C.; Plummer, S.; Tang, J.; Yang, X.F.; Ji, X.H. Lactobacillus paracasei modulates LPS-induced inflammatory cytokine release by monocyte-macrophages via the up-regulation of negative regulators of NF-kappaB signaling in a TLR2-dependent manner. Cytokine 2017, 92, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Vale, G.C.; Mayer, M.P.A. Effect of probiotic Lactobacillus rhamnosus by-products on gingival epithelial cells challenged with Porphyromonas gingivalis. Arch. Oral Biol. 2021, 128, 105174. [Google Scholar] [CrossRef]
- Taiete, T.; Monteiro, M.F.; Casati, M.Z.; do Vale, H.F.; Ambosano, G.M.B.; Nociti, F.H.; Sallum, E.A.; Casarin, R.C.V. Local IL-10 level as a predictive factor in generalized aggressive periodontitis treatment response. Scand. J. Immunol. 2019, 90, e12816. [Google Scholar] [CrossRef]
- Rabelo, M.S.; Gomes, G.H.; Foz, A.M.; Stadler, A.F.; Cutler, C.W.; Susin, C.; Romito, G.A. Short-term effect of non-surgical periodontal treatment on local and systemic cytokine levels: Role of hyperglycemia. Cytokine 2021, 138, 155360. [Google Scholar] [CrossRef] [PubMed]
- Balta, M.G.; Papathanasiou, E.; Blix, I.J.; Van Dyke, T.E. Host Modulation and Treatment of Periodontal Disease. J. Dent. Res. 2021, 100, 798–809. [Google Scholar] [CrossRef]
- Jain, S.; Yadav, H.; Sinha, P.R.; Naito, Y.; Marotta, F. Dahi containing probiotic Lactobacillus acidophilus and Lactobacillus casei has a protective effect against Salmonella enteritidis infection in mice. Int. J. Immunopathol. Pharmacol. 2008, 21, 1021–1029. [Google Scholar] [CrossRef]
- Yamasaki-Yashiki, S.; Miyoshi, Y.; Nakayama, T.; Kunisawa, J.; Katakura, Y. IgA-enhancing effects of membrane vesicles derived from Lactobacillus sakei subsp. sakei NBRC15893. Biosci. Microbiota Food Health 2019, 38, 23–29. [Google Scholar] [CrossRef]
- Verma, D.; Garg, P.K.; Dubey, A.K. Insights into the human oral microbiome. Arch. Microbiol. 2018, 200, 525–540. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.J.; Miller, R.A.; Ericsson, A.C.; Harrison, D.C.; Strong, R.; Schmidt, T.M. Changes in the gut microbiome and fermentation products concurrent with enhanced longevity in acarbose-treated mice. BMC Microbiol. 2019, 19, 130. [Google Scholar] [CrossRef]
- Ma, F.; Sun, M.; Song, Y.; Wang, A.; Jiang, S.; Qian, F.; Mu, G.; Tuo, Y. Lactiplantibacillus plantarum-12 Alleviates Inflammation and Colon Cancer Symptoms in AOM/DSS-Treated Mice through Modulating the Intestinal Microbiome and Metabolome. Nutrients 2022, 14, 1916. [Google Scholar] [CrossRef] [PubMed]
- Rooks, M.G.; Veiga, P.; Wardwell-Scott, L.H.; Tickle, T.; Segata, N.; Michaud, M.; Gallini, C.A.; Beal, C.; van Hylckama-Vlieg, J.E.; Ballal, S.A.; et al. Gut microbiome composition and function in experimental colitis during active disease and treatment-induced remission. ISME J. 2014, 8, 1403–1417. [Google Scholar] [CrossRef] [PubMed]
- Cui, C.; Han, Y.; Li, H.; Yu, H.; Zhang, B.; Li, G. Curcumin-driven reprogramming of the gut microbiota and metabolome ameliorates motor deficits and neuroinflammation in a mouse model of Parkinson’s disease. Front. Cell Infect. Microbiol. 2022, 12, 887407. [Google Scholar] [CrossRef]
- Krych, Ł.; Nielsen, D.S.; Hansen, A.K.; Hansen, C.H. Gut microbial markers are associated with diabetes onset, regulatory imbalance, and IFN-γ level in NOD mice. Gut Microbes 2015, 6, 101–109. [Google Scholar] [CrossRef]
- Xie, Z.; Li, M.; Qian, M.; Yang, Z.; Han, X. Co-Cultures of Lactobacillus acidophilus and Bacillus subtilis Enhance Mucosal Barrier by Modulating Gut Microbiota-Derived Short-Chain Fatty Acids. Nutrients 2022, 14, 4475. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Arimatsu, K.; Kato, T.; Matsuda, Y.; Minagawa, T.; Takahashi, N.; Ohno, H.; Yamazaki, K. Oral Administration of P. gingivalis Induces Dysbiosis of Gut Microbiota and Impaired Barrier Function Leading to Dissemination of Enterobacteria to the Liver. PLoS ONE 2015, 10, e0134234. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, D.; Borges, R.; Ribeiro, R.A.; de Souza, R.F.; Amado, P.P.P.; Saraiva, L.; Horliana, A.; Faveri, M.; Mayer, M.P.A. Oral Dysbiosis in Severe Forms of Periodontitis Is Associated With Gut Dysbiosis and Correlated with Salivary Inflammatory Mediators: A Preliminary Study. Front. Oral Health 2021, 2, 722495. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, T.G.B.; de Oliveira, A.M.; Tsute Chen, G.; Colombo, A.P.V. Oral-gut bacterial profiles discriminate between periodontal health and diseases. J. Periodontal Res. 2022, 57, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Yu, J.; Hao, Y.; Zhou, H.; Hu, Y.; Zhang, C.; Zheng, H.; Wang, X.; Zeng, F.; Hu, J.; et al. Akkermansia muciniphila plays critical roles in host health. Crit. Rev. Microbiol. 2023, 49, 82–100. [Google Scholar] [CrossRef]
- Ondee, T.; Pongpirul, K.; Visitchanakun, P.; Saisorn, W.; Kanacharoen, S.; Wongsaroj, L.; Kullapanich, C.; Ngamwongsatit, N.; Settachaimongkon, S.; Somboonna, N.; et al. Lactobacillus acidophilus LA5 improves saturated fat-induced obesity mouse model through the enhanced intestinal Akkermansia muciniphila. Sci. Rep. 2021, 11, 6367. [Google Scholar] [CrossRef] [PubMed]
- Fine, D.H.; Velliyagounder, K.; Furgang, D.; Kaplan, J.B. The Actinobacillus actinomycetemcomitans autotransporter adhesin Aae exhibits specificity for buccal epithelial cells from humans and old world primates. Infect. Immun. 2005, 73, 1947–1953. [Google Scholar] [CrossRef]
- Tsai, C.C.; Ho, Y.P.; Chou, Y.S.; Ho, K.Y.; Wu, Y.M.; Lin, Y.C. Aggregatibacter (Actinobacillus) actimycetemcomitans leukotoxin and human periodontitis—A historic review with emphasis on JP2. Kaohsiung J. Med. Sci. 2018, 34, 186–193. [Google Scholar] [CrossRef]
- Yue, G.; Kaplan, J.B.; Furgang, D.; Mansfield, K.G.; Fine, D.H. A second Aggregatibacter actinomycetemcomitans autotransporter adhesin exhibits specificity for buccal epithelial cells in humans and Old World primates. Infect. Immun. 2007, 75, 4440–4448. [Google Scholar] [CrossRef]
- Hajifaraji, M.; Jahanjou, F.; Abbasalizadeh, F.; Aghamohammadzadeh, N.; Abbasi, M.M.; Dolatkhah, N. Effect of probiotic supplements in women with gestational diabetes mellitus on inflammation and oxidative stress biomarkers: A randomized clinical trial. Asia Pac. J. Clin. Nutr. 2018, 27, 581–591. [Google Scholar]
- Goldstein, E.J.C.; Johnson, S.J.; Maziade, P.J.; Evans, C.T.; Sniffen, J.C.; Millette, M.; McFarland, L.V. Probiotics and prevention of Clostridium difficile infection. Anaerobe 2017, 45, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bueno, M.R.; Martins, F.H.; Rocha, C.M.; Kawamoto, D.; Ishikawa, K.H.; Ando-Suguimoto, E.S.; Carlucci, A.R.; Arroteia, L.S.; Casarin, R.V.; Mayer, M.P.A. Lactobacillus acidophilus LA-5 Ameliorates Inflammation and Alveolar Bone Loss Promoted by A. actinomycetemcomitans and S. gordonii in Mice and Impacts Oral and Gut Microbiomes. Microorganisms 2024, 12, 836. https://doi.org/10.3390/microorganisms12040836
Bueno MR, Martins FH, Rocha CM, Kawamoto D, Ishikawa KH, Ando-Suguimoto ES, Carlucci AR, Arroteia LS, Casarin RV, Mayer MPA. Lactobacillus acidophilus LA-5 Ameliorates Inflammation and Alveolar Bone Loss Promoted by A. actinomycetemcomitans and S. gordonii in Mice and Impacts Oral and Gut Microbiomes. Microorganisms. 2024; 12(4):836. https://doi.org/10.3390/microorganisms12040836
Chicago/Turabian StyleBueno, Manuela R., Fernando H. Martins, Catarina M. Rocha, Dione Kawamoto, Karin H. Ishikawa, Ellen S. Ando-Suguimoto, Aline R. Carlucci, Leticia S. Arroteia, Renato V. Casarin, and Marcia P. A. Mayer. 2024. "Lactobacillus acidophilus LA-5 Ameliorates Inflammation and Alveolar Bone Loss Promoted by A. actinomycetemcomitans and S. gordonii in Mice and Impacts Oral and Gut Microbiomes" Microorganisms 12, no. 4: 836. https://doi.org/10.3390/microorganisms12040836





