Investigation of Effects of Novel Bifidobacterium longum ssp. longum on Gastrointestinal Microbiota and Blood Serum Parameters in a Conventional Mouse Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Origin of the Strain
2.2. Animal Trial
2.2.1. Experimental Design
2.2.2. Sample Collection
2.2.3. Sample Analysis
Translocation Analysis
DNA Extraction from Faecal Samples
2.2.4. Analysis of Short-Chain Fatty Acids from Faecal Samples
2.2.5. Analysis of Short-Chain Fatty Acids from Blood Serum
2.2.6. Targeted Metabolic Profiling of Blood Serum
2.2.7. Lipid Level Measurement in Mouse Blood Serum
2.2.8. Statistical Analyses
3. Results
3.1. Safety Assessment
3.2. Body Weight
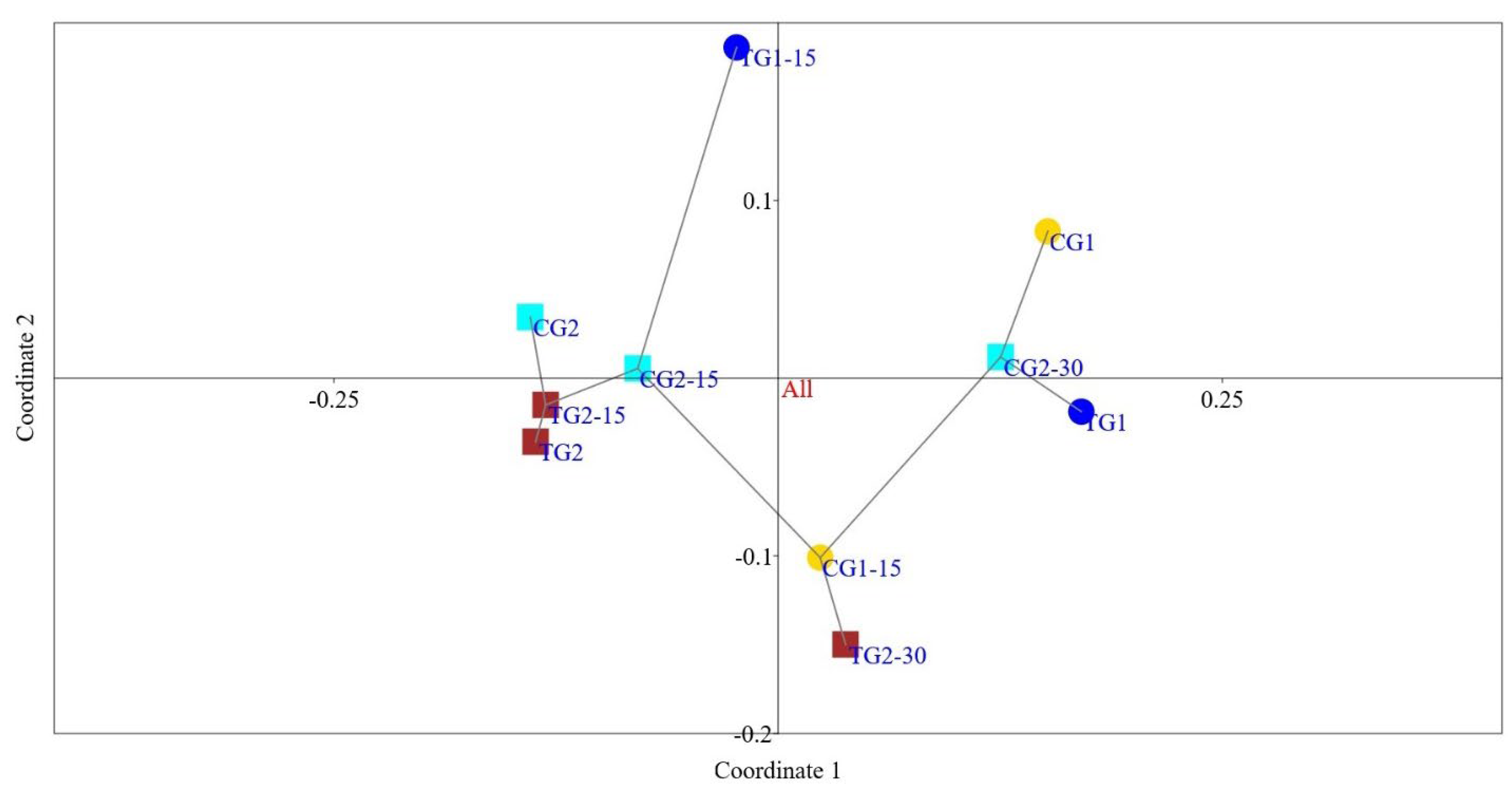
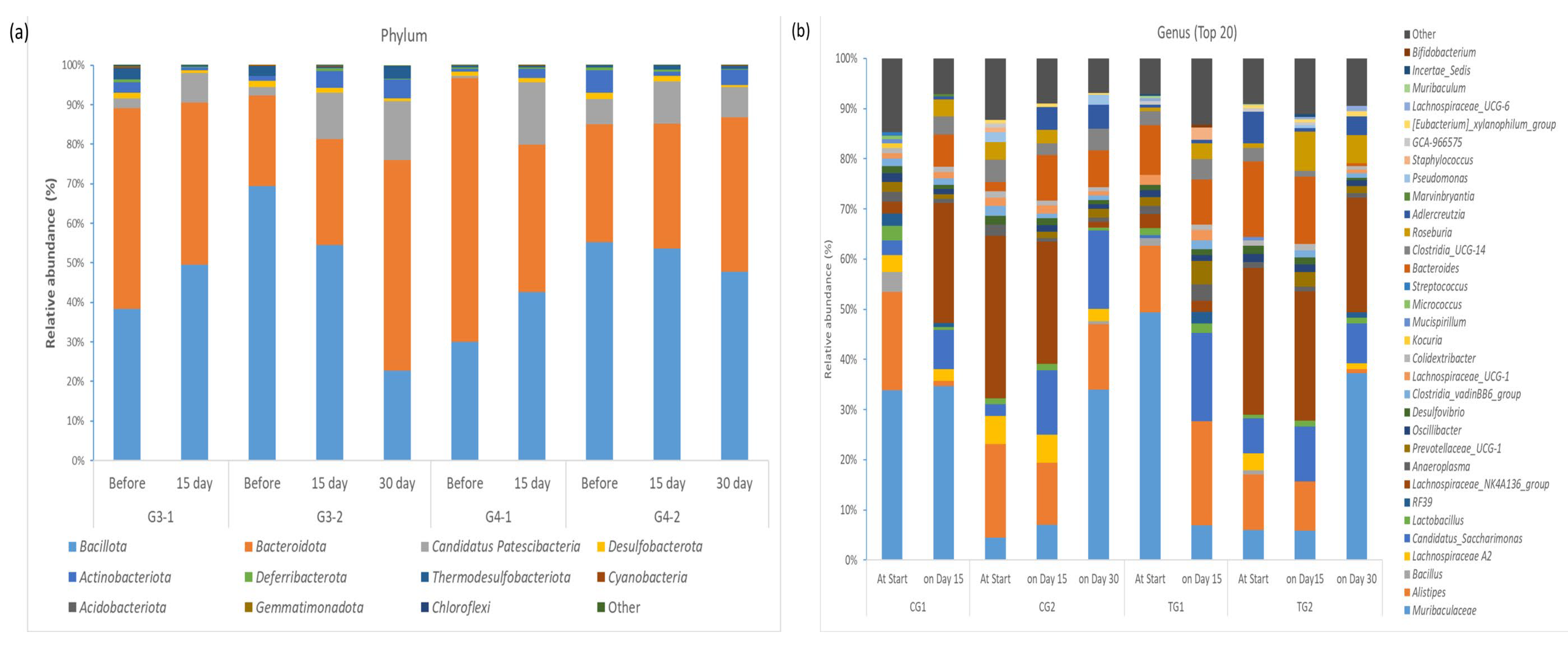
3.3. Faecal Microbiota
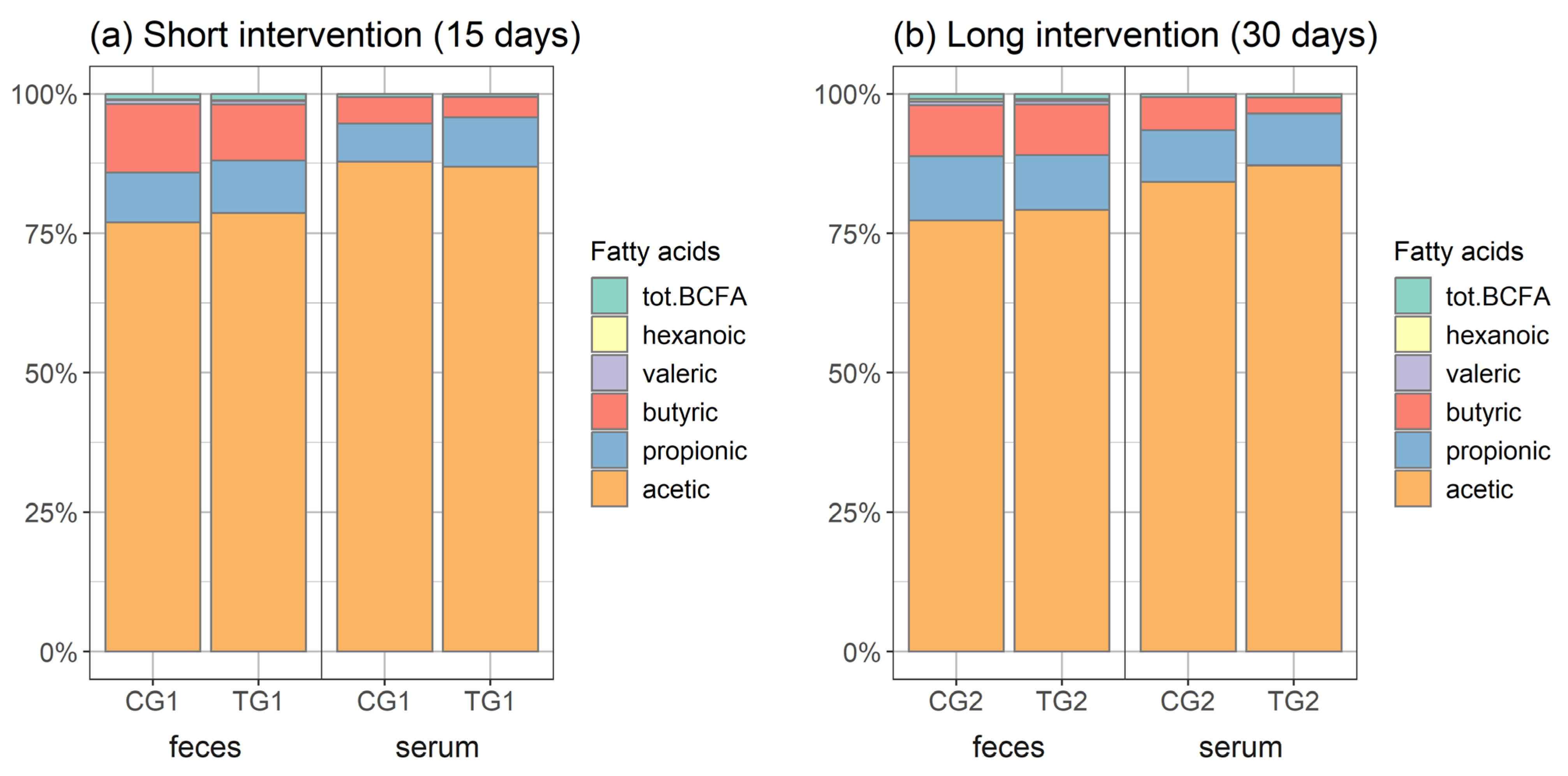
3.4. Short-Chain Organic Acids in Faeces and Serum
3.5. Targeted Metabolic Profiling of Blood Serum
3.6. Lipid Level in Blood Serum
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Tojo, R.; Suarez, A.; Clemente, M.G.; de los Reyes-Gavilan, C.G.; Margolles, A.; Gueimonde, M.; Ruas-Madiedo, P. Intestinal microbiota in health and disease: Role of bifidobacteria in gut homeostasis. World J. Gastroenterol. 2014, 20, 15163–15176. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Huang, X.; Tan, H.; Chen, X.; Chen, C.; Nie, S. Interaction between dietary fiber and bifidobacteria in promoting intestinal health. Food Chem. 2022, 393, 133407. [Google Scholar] [CrossRef] [PubMed]
- Barber, T.M.; Kabisch, S.; Pfeiffer, A.F.H.; Weickert, M.O. The Health Benefits of Dietary Fibre. Nutrients 2020, 12, 3209. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, X.; Ho, C.L. Recent Development of Probiotic. Front. Bioeng. Biotechnol. 2021, 9, 770248. [Google Scholar] [CrossRef]
- Wong, C.B.; Odamaki, T.; Xiao, J.-Z. Beneficial effects of Bifidobacterium longum subsp. longum BB536 on human health: Modulation of gut microbiome as the principal action. J. Funct. Foods 2019, 54, 506–519. [Google Scholar] [CrossRef]
- Araya, M.; Gopal, P.; Lindgren, S.E.; Lodi, R.; Oliver, G.; Saxelin, M.-L.; Servin, A.L.; Stanton, C. Health and Nutritional Properties of Probiotics in Food including Powder Milk with Live Lactic Acid Bacteria; Food and Agriculture Organization of the United Nations and World Health Organization: Cordoba, Argentina, 2001. [Google Scholar]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert consensus document. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef]
- Kothari, D.; Patel, S.; Kim, S.K. Probiotic supplements might not be universally-effective and safe: A review. Biomed. Pharmacother. 2019, 111, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Mandal, S. Bifidobacteria-Insight into clinical outcomes and mechanisms of its probiotic action. Microbiol. Res. 2016, 192, 159–171. [Google Scholar] [CrossRef]
- Weber, E.; Reynaud, Q.; Suy, F.; Gagneux-Brunon, A.; Carricajo, A.; Guillot, A.; Botelho-Nevers, E. Bifidobacterium species bacteremia: Risk factors in adults and infants. Clin. Infect. Dis. 2015, 61, 482–484. [Google Scholar] [CrossRef]
- Ratsep, M.; Kilk, K.; Zilmer, M.; Kuus, L.; Songisepp, E. A Novel Bifidobacterium longum ssp. longum Strain with Pleiotropic Effects. Microorganisms 2024, 12, 174. [Google Scholar] [CrossRef]
- Yu, Y.; Lee, C.H.; Kim, J.; Hwang, S. Group-specific primer and probe sets to detect methanogenic communities using quantitative real-time polymerase chain reaction. Biotechnol. Bioeng. 2005, 89, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef] [PubMed]
- Zhao, G.; Nyman, M.; Jonsson, J.A. Rapid determination of short-chain fatty acids in colonic contents and faeces of humans and rats by acidified water-extraction and direct-injection gas chromatography. Biomed. Chromatogr. 2006, 20, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological Statistics Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Rivière, A.; Selak, M.; Lantin, D.; Leroy, F.; De Vuyst, L. Bifidobacteria and Butyrate-Producing Colon Bacteria: Importance and Strategies for Their Stimulation in the Human Gut. Front. Microbiol. 2016, 7, 979. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Zhang, K.; Ma, X.; He, P. Clostridium species as probiotics: Potentials and challenges. J. Anim. Sci. Biotechnol. 2020, 11, 24. [Google Scholar] [CrossRef]
- Onyszkiewicz, M.; Gawrys-Kopczynska, M.; Konopelski, P.; Aleksandrowicz, M.; Sawicka, A.; Kozniewska, E.; Samborowska, E.; Ufnal, M. Butyric acid, a gut bacteria metabolite, lowers arterial blood pressure via colon-vagus nerve signaling and GPR41/43 receptors. Pflug. Arch. 2019, 471, 1441–1453. [Google Scholar] [CrossRef]
- Wang, S.P.; Rubio, L.A.; Duncan, S.H.; Donachie, G.E.; Holtrop, G.; Lo, G.; Farquharson, F.M.; Wagner, J.; Parkhill, J.; Louis, P.; et al. Pivotal Roles for pH, Lactate, and Lactate-Utilizing Bacteria in the Stability of a Human Colonic Microbial Ecosystem. mSystems 2020, 5, e00645-20. [Google Scholar] [CrossRef] [PubMed]
- Xiong, R.G.; Zhou, D.D.; Wu, S.X.; Huang, S.Y.; Saimaiti, A.; Yang, Z.J.; Shang, A.; Zhao, C.N.; Gan, R.Y.; Li, H.B. Health Benefits and Side Effects of Short-Chain Fatty Acids. Foods 2022, 11, 2863. [Google Scholar] [CrossRef]
- Larabi, A.B.; Masson, H.L.P.; Bäumler, A.J. Bile acids as modulators of gut microbiota composition and function. Gut Microbes 2023, 15, 2172671. [Google Scholar] [CrossRef]
- Jones, B.V.; Begley, M.; Hill, C.; Gahan, C.G.; Marchesi, J.R. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc. Natl. Acad. Sci. USA 2008, 105, 13580–13585. [Google Scholar] [CrossRef] [PubMed]
- Jain, U.; Lai, C.W.; Xiong, S.; Goodwin, V.M.; Lu, Q.; Muegge, B.D.; Christophi, G.P.; VanDussen, K.L.; Cummings, B.P.; Young, E.; et al. Temporal Regulation of the Bacterial Metabolite Deoxycholate during Colonic Repair Is Critical for Crypt Regeneration. Cell Host Microbe 2018, 24, 353–363.e5. [Google Scholar] [CrossRef] [PubMed]
- Larque, E.; Sabater-Molina, M.; Zamora, S. Biological significance of dietary polyamines. Nutrition 2007, 23, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Moinard, C.; Cynober, L.; de Bandt, J.P. Polyamines: Metabolism and implications in human diseases. Clin. Nutr. 2005, 24, 184–197. [Google Scholar] [CrossRef] [PubMed]
- Al Hinai, E.A.; Kullamethee, P.; Rowland, I.R.; Swann, J.; Walton, G.E.; Commane, D.M. Modelling the role of microbial p-cresol in colorectal genotoxicity. Gut Microbes 2019, 10, 398–411. [Google Scholar] [CrossRef] [PubMed]
- Candeliere, F.; Simone, M.; Leonardi, A.; Rossi, M.; Amaretti, A.; Raimondi, S. Indole and p-cresol in feces of healthy subjects: Concentration, kinetics, and correlation with microbiome. Front. Mol. Med. 2022, 2, 13. [Google Scholar] [CrossRef]
- Summers, S.C.; Quimby, J.M.; Isaiah, A.; Suchodolski, J.S.; Lunghofer, P.J.; Gustafson, D.L. The fecal microbiome and serum concentrations of indoxyl sulfate and p-cresol sulfate in cats with chronic kidney disease. J. Vet. Intern. Med. 2019, 33, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Ranhotra, H.S. Discrete interplay of gut microbiota L-tryptophan metabolites in host biology and disease. Mol. Cell Biochem. 2023. [Google Scholar] [CrossRef]
- Vanholder, R.; Schepers, E.; Pletinck, A.; Nagler, E.V.; Glorieux, G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: A systematic review. J. Am. Soc. Nephrol. 2014, 25, 1897–1907. [Google Scholar] [CrossRef]
- Hwang, I.K.; Yoo, K.-I.; Li, H.; Park, O.K.; Lee, C.H.; Choi, J.H.; Jeong, Y.G.; Lee, Y.L.; Kim, Y.-M.; Kwon, Y.-G.; et al. Indole-3-propionic acid attenuates neuronal damage and oxidative stress in the ischemic hippocampus. J. Neurosci. Res. 2009, 87, 2126–2137. [Google Scholar] [CrossRef]
- Ahn, T.; Bae, C.-S.; Yun, C.-H. Acute stress-induced changes in hormone and lipid levels in mouse plasma. Vet. Med. 2016, 61, 57–64. [Google Scholar] [CrossRef]
- Sinulingga, S.; Putri, H.O.V.; Haryadi, K.; Subandrate. The effect of pindang patin intake on serum cholesterol and LDL levels of male mice (Mus musculus L.). J. Phys. Conf. Ser. 2019, 1246, 012060. [Google Scholar] [CrossRef]

| Study Group | Richness | Shannon Diversity Index H | InvSimpson | |
|---|---|---|---|---|
| CG1 | At start | 62 | 2.65 | 0.16 |
| On day 15 | 54 | 2.28 | 0.19 | |
| CG2 | At start | 56 | 2.61 | 0.15 |
| On day 15 | 50 | 2.68 | 0.11 | |
| On day 30 | 60 | 2.41 | 0.17 | |
| TG1 | At start | 53 | 2.08 | 0.28 |
| On day 15 | 57 | 2.88 | 0.10 | |
| TG2 | At start | 53 | 2.54 | 0.14 |
| On day 15 | 55 | 2.66 | 0.12 | |
| On day 30 | 57 | 2.30 | 0.20 | |
| Group | Total SCFAs | Total Straight-Chain SCFAs | Total BCFAs | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| At Start | On Day 15 | On Day 30 | At Start | On Day 15 | On Day 30 | At Start | On Day 15 | On Day 30 | ||
| Short intervention | CG1 | 79.56 | 79.71 | 78.62 | 78.90 | 0.94 | 0.81 | |||
| TC1 | 67.84 | 86.66 | 67.00 | 85.68 | 0.84 | 0.98 | ||||
| Long intervention | CG2 | 89.72 | 92.32 | 89.68 | 88.60 | 91.48 | 88.82 | 1.12 | 0.84 | 0.87 |
| TC2 | 89.43 | 92.77 | 93.80 | 88.50 | 91.94 | 92.91 | 0.93 | 0.83 | 0.89 | |
| SCFA | CG1 | TG1 | ||
|---|---|---|---|---|
| At Start | On Day 15 | At Start | On Day 15 | |
| Acetic acid | 60.58 | 61.32 | 51.06 | 68.11 |
| Propionic acid | 8.17 | 7.15 | 6.68 | 8.17 |
| Butyric acid | 9.15 | 9.83 | 8.55 | 8.75 |
| Isobutyric acid | 0.40 | 0.36 | 0.37 | 0.45 |
| Valeric acid | 0.53 | 0.49 | 0.53 | 0.52 |
| Isovaleric acid | 0.54 | 0.45 | 0.46 | 0.54 |
| Hexanoic acid | 0.19 | 0.11 | 0.19 | 0.12 |
| SCFA | CG2 | TG2 | ||||
|---|---|---|---|---|---|---|
| At Start | On Day 15 | Day 30 | At Start | On Day 15 | Day 30 | |
| Acetic acid | 68.06 | 66.46 | 69.33 | 67.65 | 73.06 | 74.30 |
| Propionic acid | 8.78 | 9.48 | 10.35 | 8.27 | 8.33 | 9.25 |
| Butyric acid | 10.71 | 14.77 | 8.21 | 11.69 | 9.91 | 8.49 |
| Isobutyric acid | 0.49 | 0.37 | 0.39 | 0.41 | 0.37 | 0.39 |
| Valeric acid | 0.68 | 0.64 | 0.58 | 0.61 | 0.53 | 0.54 |
| Isovaleric acid | 0.63 | 0.47 | 0.48 | 0.52 | 0.47 | 0.50 |
| Hexanoic acid | 0.37 | 0.14 | 0.36 | 0.27 | 0.11 | 0.33 |
| SCFA | CG1 | TG1 | p Value CG1 vs. TG1 | CG2 | TG2 | p Value CG2 vs. TG2 | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| On Day 15 | On Day 30 | |||||||||
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | |||
| Acetic acid | 15.89 | 0.55 | 15.37 | 4.00 | 0.27 | 15.23 | 1.91 | 15.14 | 1.60 | 0.54 |
| Propionic acid | 1.24 | 0.60 | 1.76 | 0.48 | 0.31 | 1.69 | 0.62 | 1.54 | 0.61 | 0.78 |
| Butyric acid | 0.86 | 0.20 | 0.46 | 0.60 | 0.09 | 1.06 | 0.34 | 0.63 | 0.22 | 0.016 |
| Isobutyric acid | 0.06 | 0.01 | 0.09 | 0.03 | 1.0 | 0.07 | 0.02 | 0.06 | 0.01 | 0.06 |
| Isovaleric acid | 0.04 | 0.01 | 0.03 | 0.02 | 0.06 | 0.03 | 0.01 | 0.02 | 0.02 | 0.60 |
| Hexanoic acid | 0.01 | 0.00 | 0.01 | 0.00 | 1.0 | 0.01 | 0.01 | 0.01 | 0.00 | 0.04 |
| Lactic acid | 9084 | 2535 | 9037 | 1637 | 0.965 | 11465 | 5792 | 9084 | 1966 | 0.129 |
| Succinic acid | 42.15 | 23.87 | 39.4 | 7.44 | 0.514 | 39.25 | 9.15 | 38.3 | 5.2 | 0.841 |
| Bio-Chemical Class | Metabolite | CG1 | TG1 | p Value CG1 vs. TG1 | CG2 | TG2 | p Value CG2 vs. TG2 |
|---|---|---|---|---|---|---|---|
| Day 15 | Day 30 | ||||||
| Bile acids | Cholic acid (CA) | 0.00 (0.00) | 0.01 (0.01) | 0.008 | 0.01 (0.02) | 0.02 (0.03) | 0.250 |
| Taurocholic acid (TCA) | 0.20 (0.13) | 0.25 (0.48) | 0.89 | 0.21 (0.25) | 0.17 (0.11) | 0.41 | |
| Deoxycholic acid (DCA) | 0.02 (0.01) | 0.030 (0.01) | 0.015 | 0.020 (0.00) | 0.050 (0.04) | 0.052 | |
| Aminoacids | Tyrosine | 74.45 (8.75) | 93.25 (10.79) | 0.186 | 93.20 (47.10) | 103.00 (31.30) | 0.575 |
| Tryptophan | 116 (18.25) | 114.5 (29.05) | 0.694 | 104.5 (23.45) | 144 (45.00) | 0.320 | |
| Aspartate | 20.85 (4.60) | 28.90 (12.52) | 0.031 | 31.15 (18.18) | 33.6 (14.70) | 0.968 | |
| Arginine | 193.50 (36.00) | 240.00 (29.25) | 0.067 | 255.00 (68.00) | 225.00 (92.00) | 0.841 | |
| Cysteine | 23.95 (3.18) | 26.90 (5.43) | 0.056 | 27.20 (5.41) | 30.2 (4.00) | 0.071 | |
| Glutamate | 72.70 (17.80) | 112.50 (18.50) | 0.026 | 105.50 (30.90) | 118.00 (27.50) | 0.208 | |
| Ornitine | 117.50 (34.05) | 119 (17.75) | 0.769 | 132 (46.40) | 112 (44.00) | 0.904 | |
| Polyamines | Putrescine | 0.85 (3.51) | 0.91 (0.13) | 0.760 | 0.80 (0.25) | 0.97 (0.09) | 0.089 |
| Spermidine | 2.08 (0.59) | 2.65 (0.90) | 0.280 | 2.29 (0.97) | 2.31 (1.3) | 0.748 | |
| Spermine | 0.50 (0.26) | 0.52 (0.37) | 0.631 | 0.46 (0.10) | 0.48 (0.24) | 0.904 | |
| Cresols | p-cresol-SO4 | 1.12 (1.21) | 1.09 (1.69) | 0.460 | 0.49 (0.72) | 0.14 (0.20) | 0.073 |
| Indoles and derivatives | Indoxyl-SO4 | 14.72 (19.01) | 10.14 (18.08) | 0.514 | 9.39 (6.89) | 5.33 (6.20) | 0.186 |
| 3-indolepropionic acid | 0.59 (0.72) | 0.62 (0.91) | 1.00 | 0.46 (0.62) | 0.73 (1.13) | 0.061 | |
| Variable | Day 15 | p Value CG1 vs. TG1 | Day 30 | p Value CG2 vs. TG2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CG1 | TG1 | CG2 | TG2 | |||||||
| Median | IQR | Median | IQR | Median | IQR | Median | IQR | |||
| Total cholesterol | 2.87 | 0.19 | 2.75 | 0.3 | 0.689 | 2.63 | 0.2 | 2.56 | 0.62 | 1 |
| LDL cholesterol | 0.12 | 0.01 | 0.13 | 0.04 | 0.069 | 0.13 | 0.06 | 0.15 | 0.06 | 0.411 |
| HDL cholesterol | 2.41 | 0.18 | 2.36 | 0.19 | 0.476 | 2.25 | 0.18 | 2.02 | 0.51 | 0.902 |
| Triglycerides | 1.96 | 0.31 | 1.39 | 0.8 | 0.198 | 1.58 | 0.37 | 1.95 | 1.09 | 0.438 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rätsep, M.; Kilk, K.; Zilmer, M.; Kuusik, S.; Kuus, L.; Vallas, M.; Gerulis, O.; Štšepetova, J.; Orav, A.; Songisepp, E. Investigation of Effects of Novel Bifidobacterium longum ssp. longum on Gastrointestinal Microbiota and Blood Serum Parameters in a Conventional Mouse Model. Microorganisms 2024, 12, 840. https://doi.org/10.3390/microorganisms12040840
Rätsep M, Kilk K, Zilmer M, Kuusik S, Kuus L, Vallas M, Gerulis O, Štšepetova J, Orav A, Songisepp E. Investigation of Effects of Novel Bifidobacterium longum ssp. longum on Gastrointestinal Microbiota and Blood Serum Parameters in a Conventional Mouse Model. Microorganisms. 2024; 12(4):840. https://doi.org/10.3390/microorganisms12040840
Chicago/Turabian StyleRätsep, Merle, Kalle Kilk, Mihkel Zilmer, Sirje Kuusik, Liina Kuus, Mirjam Vallas, Oksana Gerulis, Jelena Štšepetova, Aivar Orav, and Epp Songisepp. 2024. "Investigation of Effects of Novel Bifidobacterium longum ssp. longum on Gastrointestinal Microbiota and Blood Serum Parameters in a Conventional Mouse Model" Microorganisms 12, no. 4: 840. https://doi.org/10.3390/microorganisms12040840






