SLC34A2 Targets in Calcium/Phosphorus Homeostasis of Mammary Gland and Involvement in Development of Clinical Mastitis in Dairy Cows
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Collection
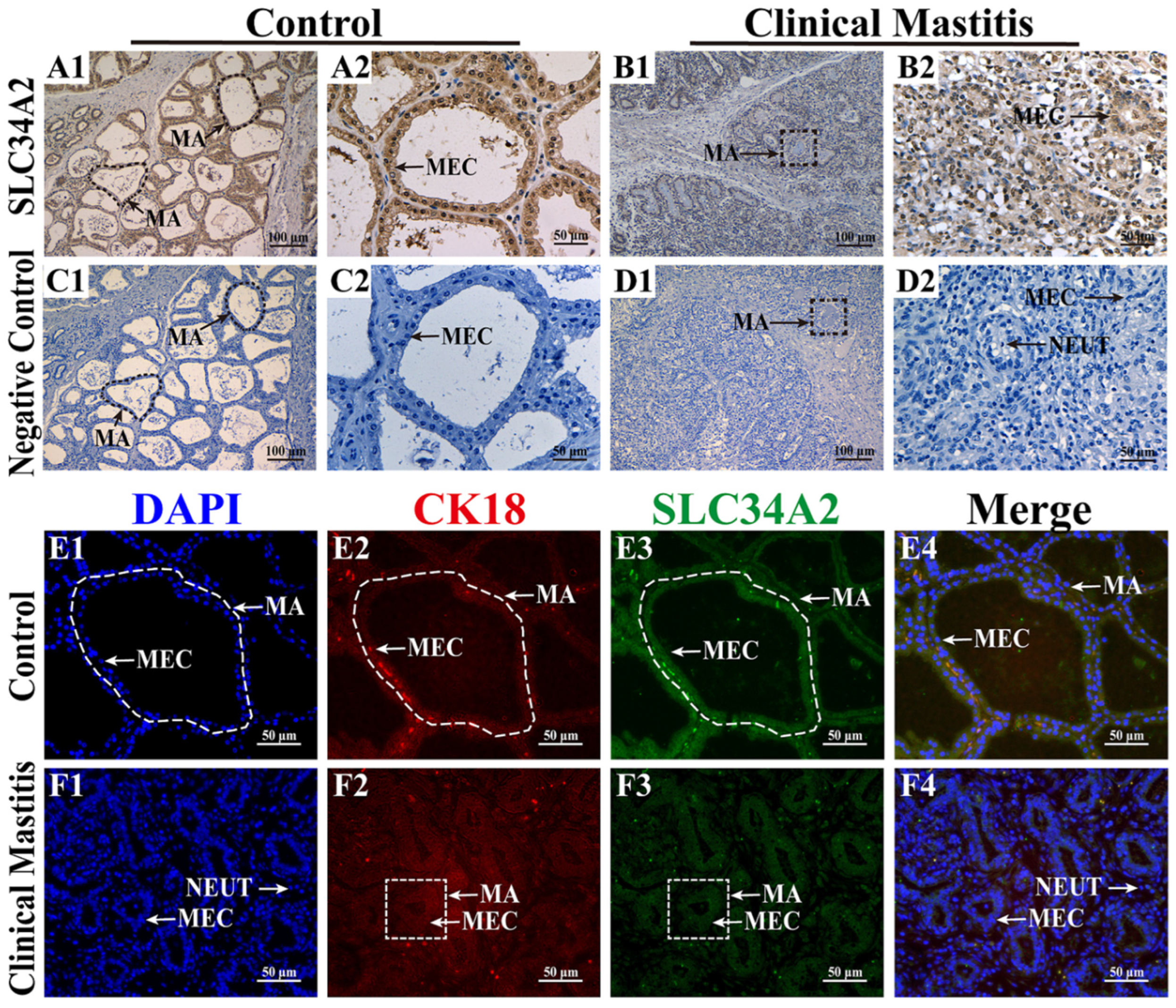
2.2. Immunohistochemistry (IHC) Staining
2.3. Immunofluorescence (IF) Staining
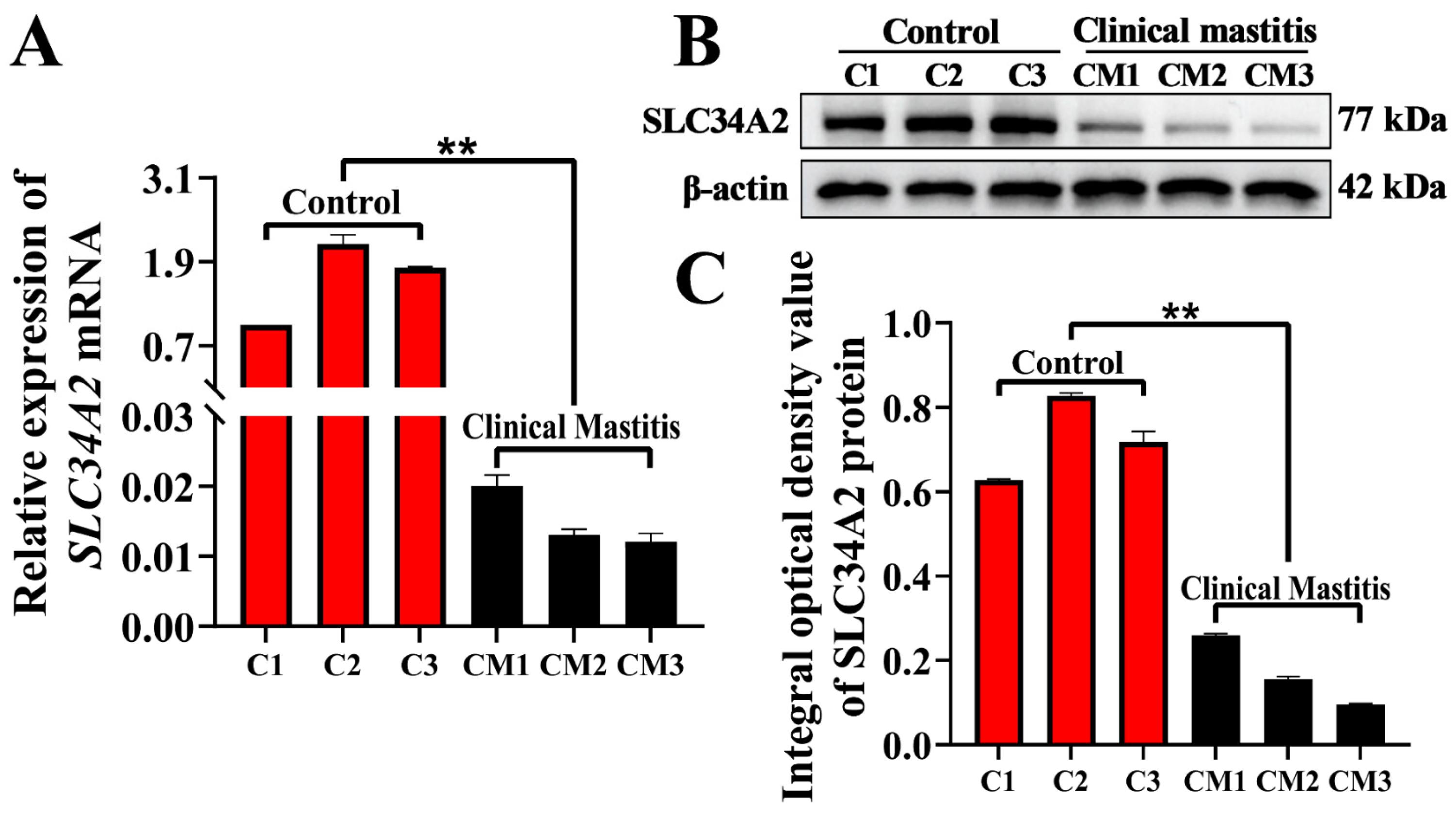
2.4. RNA Isolation, cDNA Synthesis and qRT-PCR
2.5. Western Blot
2.6. Bioinformatics Analysis
2.7. Statistical Analysis
3. Results
3.1. Subcellular Location Analysis of SLC34A2 Protein in the MGs
3.2. Expression Patterns of SLC34A2 mRNA and Protein in MGs
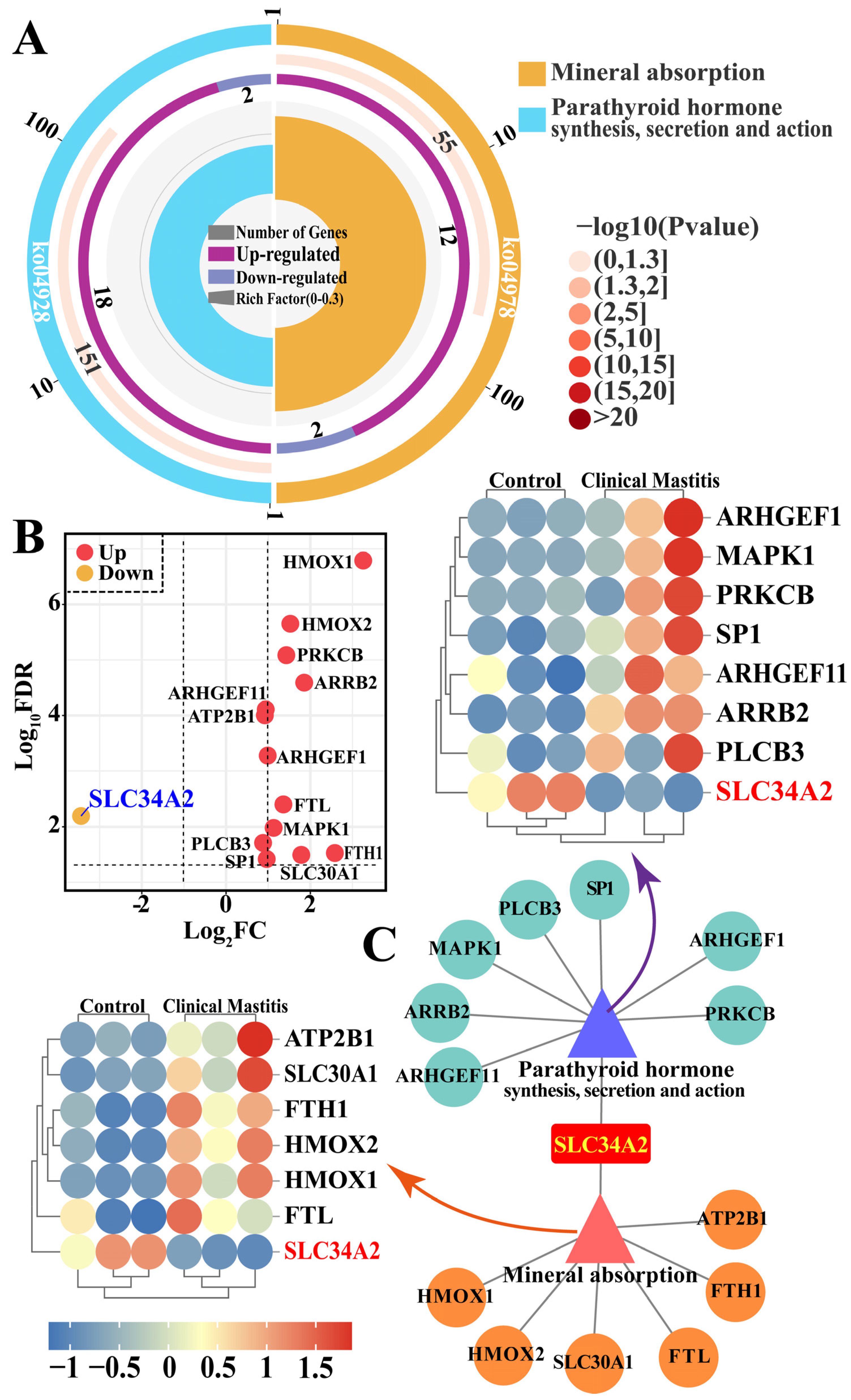
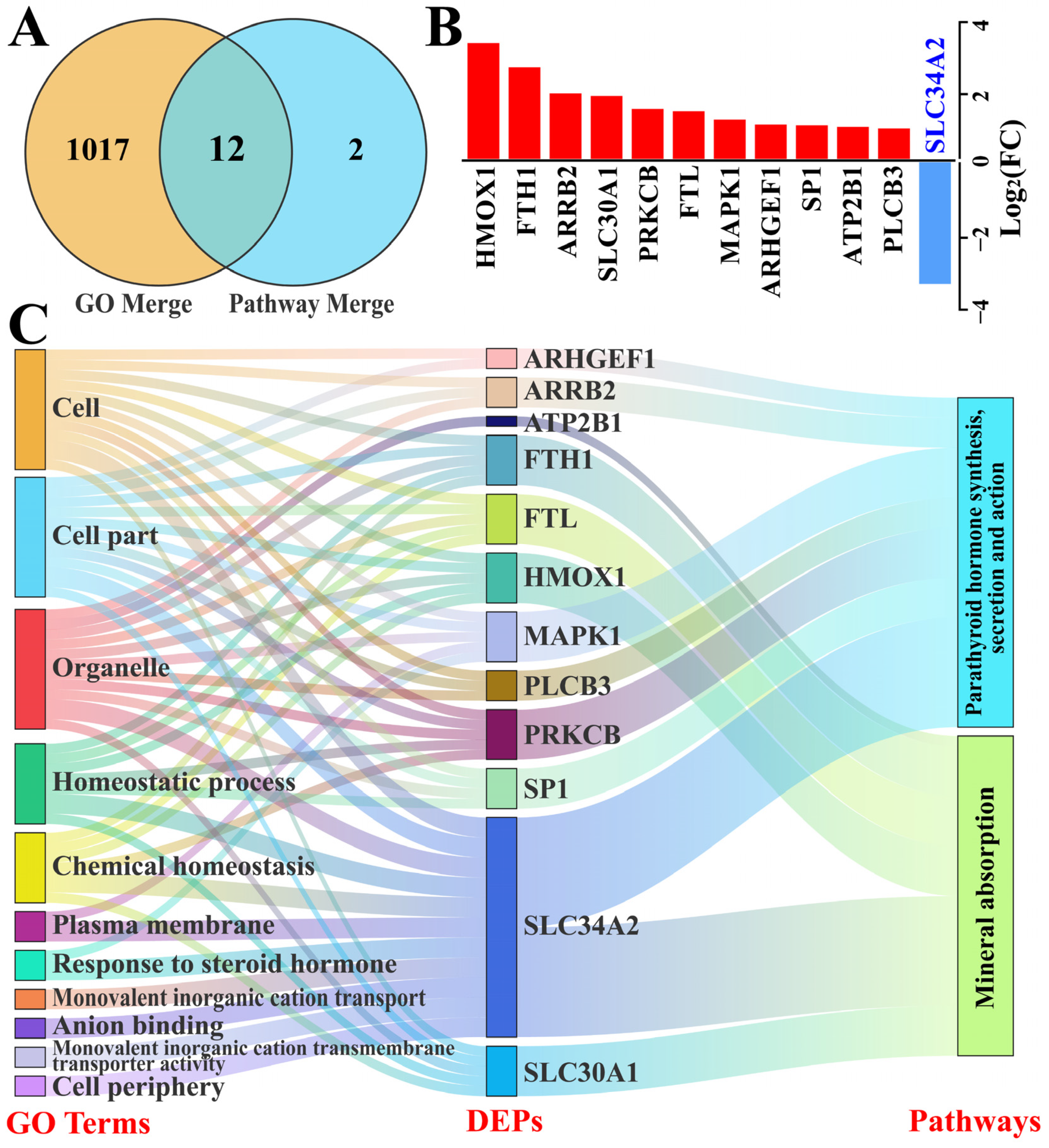
3.3. Identification of GO Terms and Candidate DEPs Related to SLC34A2
3.4. Identification of the Pathways and Candidate DEPs Related to SLC34A2
3.5. Identification of Candidate DEPs Interacting with SLC34A2 Based on GO Terms and Pathways
3.6. PPI Network Analyses of the Candidate DEPs, GO Terms, and Pathways in Ion Metabolism and Homeostasis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Q.; Bai, X.; Lin, T.; Wang, X.; Zhang, B.; Dai, L.; Shi, J.; Zhang, Y.; Zhao, X. HMOX1 Promotes Ferroptosis in Mammary Epithelial Cells via FTH1 and Is Involved in the Development of Clinical Mastitis in Dairy Cows. Antioxidants 2022, 11, 2221. [Google Scholar] [CrossRef]
- Zhang, B.; Lin, T.; Bai, X.; An, X.; Dai, L.; Shi, J.; Zhang, Y.; Zhao, X.; Zhang, Q. Sulfur Amino Acid Metabolism and the Role of Endogenous Cystathionine-γ-lyase/H2S in Holstein Cows with Clinical Mastitis. Animals 2022, 12, 1451. [Google Scholar] [CrossRef]
- Zhang, Q.; Bai, X.; Shi, J.; Wang, X.; Zhang, B.; Dai, L.; Lin, T.; Gao, Y.; Zhang, Y.; Zhao, X. DIA proteomics identified the potential targets associated with angiogenesis in the mammary glands of dairy cows with hemorrhagic mastitis. Front. Vet. Sci. 2022, 9, 980963. [Google Scholar] [CrossRef]
- Dorozhkin, S.V.J.B. Calcium orthophosphates: Occurrence, properties, biomineralization, pathological calcification and biomimetic applications. Biomatter 2011, 1, 121–164. [Google Scholar] [CrossRef]
- Vötterl, J.C.; Klinsoda, J.; Zebeli, Q.; Hennig-Pauka, I.; Kandler, W.; Metzler-Zebeli, B.U. Dietary phytase and lactic acid-treated cereal grains differently affected calcium and phosphorus homeostasis from intestinal uptake to systemic metabolism in a pig model. Nutrients 2020, 12, 1542. [Google Scholar] [CrossRef]
- Jorgensen, R.; Nyengaard, N.; Ham, S.; Enemark, J.; Andersen, P. Rumen motility during induced hyper-and hypocalcaemia. Acta Vet. Scand. 1998, 39, 331–338. [Google Scholar] [CrossRef]
- Ogawa, E.; Kobayashi, K.; Yoshiura, N.; Mukai, J.J.A.J.V.R. Bovine postparturient hemoglobinemia: Hypophosphatemia. Am. J. Vet. Res. 1987, 48, 1300–1303. [Google Scholar]
- Verhoef, W.; Zuidhof, S.; Ross, J.; Beaugrand, K.; Olson, M. Evaluation of a novel dipotassium phosphate bolus for treatment of metabolic disorders in dairy cattle. Front. Vet. Sci. 2023, 10, 1274183. [Google Scholar] [CrossRef]
- Goselink, R.; Klop, G.; Dijkstra, J.; Bannink, A. Phosphorus metabolism in dairy cattle: Literature study on recent developments and gaps in knowledge. Anim. Nutr. Physiol. 2015. Available online: https://edepot.wur.nl/363222 (accessed on 21 April 2024).
- Garel, J.-M.; Barlet, J.-P. Calcium metabolism in newborn animals: The interrelationship of calcium, magnesium, and inorganic phosphorus in newborn rats, foals, lambs, and calves. Pediatr. Res. 1976, 10, 749–754. [Google Scholar] [CrossRef]
- Pastor-Arroyo, E.M.; Rodriguez, J.M.M.; Pellegrini, G.; Bettoni, C.; Levi, M.; Hernando, N.; Wagner, C.A. Constitutive depletion of Slc34a2/NaPi-IIb in rats causes perinatal mortality. Sci. Rep. 2021, 11, 7943. [Google Scholar] [CrossRef]
- Vlasenkova, R.; Nurgalieva, A.; Akberova, N.; Bogdanov, M.; Kiyamova, R. Characterization of SLC34A2 as a potential prognostic marker of oncological diseases. Biomolecules 2021, 11, 1878. [Google Scholar] [CrossRef]
- Xu, H.; Bai, L.; Collins, J.F.; Ghishan, F.K. Molecular cloning, functional characterization, tissue distribution, and chromosomal localization of a human, small intestinal sodium–phosphate (Na+–Pi) transporter (SLC34A2). Genomics 1999, 62, 281–284. [Google Scholar] [CrossRef]
- Chen, D.-R.; Chien, S.-Y.; Kuo, S.-J.; Teng, Y.-H.; Tsai, H.-T.; Kuo, J.-H.; Chung, J.-G. SLC34A2 as a novel marker for diagnosis and targeted therapy of breast cancer. Anticancer Res. 2010, 30, 4135–4140. [Google Scholar]
- Wang, Y.; Yang, W.; Pu, Q.; Yang, Y.; Ye, S.; Ma, Q.; Ren, J.; Cao, Z.; Zhong, G.; Zhang, X. The effects and mechanisms of SLC34A2 in tumorigenesis and progression of human non-small cell lung cancer. J. Biomed. Sci. 2015, 22, 52. [Google Scholar] [CrossRef]
- Bai, X.; Wang, X.; Lin, T.; Dong, W.; Gao, Y.; Ji, P.; Zhang, Y.; Zhao, X.; Zhang, Q. Toll-like receptor 2 is associated with the immune response, apoptosis, and angiogenesis in the mammary glands of dairy cows with clinical mastitis. Int. J. Mol. Sci. 2022, 23, 10717. [Google Scholar] [CrossRef]
- Dai, L.; Zhang, Q.; Shi, J.; Bai, X.; An, X.; Zhang, B.; Zhang, Y.; Zhao, X.J.A. The distribution, expression patterns and functional analysis of NR1D1 and NR4A2 in the reproductive axis tissues of the male Tianzhu White Yak. Animals 2021, 11, 3117. [Google Scholar] [CrossRef]
- Bathla, S.; Sindhu, A.; Kumar, S.; Dubey, S.K.; Pattnaik, S.; Rawat, P.; Chopra, A.; Mohanty, A.K. Quantitative proteomics revealed the putative biomarker for detection of early-stage intra-mammary gland infection in cow. J. Proteins Proteom. 2020, 11, 173–181. [Google Scholar] [CrossRef]
- Sommerfeld, V.; Omotoso, A.O.; Oster, M.; Reyer, H.; Camarinha-Silva, A.; Hasselmann, M.; Huber, K.; Ponsuksili, S.; Seifert, J.; Stefanski, V. Phytate degradation, transcellular mineral transporters, and mineral utilization by two strains of laying hens as affected by dietary phosphorus and calcium. Animals 2020, 10, 1736. [Google Scholar] [CrossRef]
- Li, J.; Yuan, J.; Guo, Y.; Sun, Q.; Hu, X. The influence of dietary calcium and phosphorus imbalance on intestinal NaPi-IIb and calbindin mRNA expression and tibia parameters of broilers. Asian Austral. J. Anim. 2012, 25, 552. [Google Scholar] [CrossRef]
- Wagner, C.A.; Hernando, N.; Forster, I.C.; Biber, J. The SLC34 family of sodium-dependent phosphate transporters. Pflug. Arch. Eur. J. Physiol. 2014, 466, 139–153. [Google Scholar] [CrossRef]
- Shennan, D.; Peaker, M. Transport of milk constituents by the mammary gland. Physiol. Rev. 2000, 80, 925–951. [Google Scholar] [CrossRef]
- Shennan, D.J.C.B.; Physiology, P.P.A. Mechanisms of mammary gland ion transpor. Comp. Biochem. Physiol. Part A Physiol. 1990, 97, 317–324. [Google Scholar] [CrossRef]
- McCoard, S.A.; Hayashi, A.A.; Sciascia, Q.; Rounce, J.; Sinclair, B.; McNabb, W.C.; Roy, N.C. Mammary transcriptome analysis of lactating dairy cows following administration of bovine growth hormone. Animal 2016, 10, 2008–2017. [Google Scholar] [CrossRef] [PubMed]
- Shillingford, J.M.; Miyoshi, K.; Robinson, G.W.; Bierie, B.; Cao, Y.; Karin, M.; Hennighausen, L. Proteotyping of mammary tissue from transgenic and gene knockout mice with immunohistochemical markers: A tool to define developmental lesions. J. Histochem. Cytochem. 2003, 51, 555–565. [Google Scholar] [CrossRef]
- Schenck, P.A.; Chew, D.J.; Nagode, L.A.; Rosol, T.J. Disorders of calcium: Hypercalcemia and hypocalcemia. Fluid Electrolyte Acid-Base Disord. Small Anim. Pract. 2006, 4, 120–194. [Google Scholar]
- Rosol, T.J.; Capen, C.C. Calcium-regulating hormones and diseases of abnormal mineral (calcium, phosphorus, magnesium) metabolism. In Clinical Biochemistry of Domestic Animals; Elsevier: Amsterdam, The Netherlands, 1997; pp. 619–702. [Google Scholar]
- Birkenhäger, J.C.; Seldenrath, H.J.; Hackeng, W.H.L.; Schelleken, A.P.M.; van der Veer, A.L.J.; Roelfsema, F. Calcium and phosphorus metabolism, parathyroid hormone, calcitonin and bone histology in pseudohypoparathyroidism. Eur. J. Clin. Investig. 1973, 3, 27–34. [Google Scholar] [CrossRef]
- Laymon, C.W.; Zelickson, A. Pseudohypoparathyroidism: The Seabright Bantam Syndrome. AMA Arch. Dermatol. 1959, 79, 194–201. [Google Scholar] [CrossRef] [PubMed]
- van Altena, S.; de Klerk, B.; Hettinga, K.; van Neerven, R.; Boeren, S.; Savelkoul, H.; Tijhaar, E. A proteomics-based identification of putative biomarkers for disease in bovine milk. Vet. Immunol. Immunop. 2016, 174, 11–18. [Google Scholar] [CrossRef]
- Marks, J. The role of SLC34A2 in intestinal phosphate absorption and phosphate homeostasis. Pflug. Arch. Eur. J. Physiol. 2019, 471, 165–173. [Google Scholar] [CrossRef]
- Li, T.; Gao, J.; Zhao, X.; Ma, Y. Digital gene expression analyses of mammary glands from meat ewes naturally infected with clinical mastitis. R. Soc. Open Sci. 2019, 6, 181604. [Google Scholar] [CrossRef]
- Wagner, P.; Brügemann, K.; Yin, T.; Engel, P.; König, S.J.G. Inferring Causalities of Environmental and Genetic Factors for Differential Somatic Cell Count and Mastitis Pathogens in Dairy Cows Using Structural Equation Modelling. Genes 2023, 14, 2102. [Google Scholar] [CrossRef] [PubMed]
- Valable, A.S.; Létourneau-Montminy, M.P.; Klein, S.; Lardic, L.; Lecompte, F.; Metayer-Coustard, S.; Metayer-Coustard, N.; Page, G.; Duclos, M.J.; Narcy, A. Early-life conditioning strategies to reduce dietary phosphorus in broilers: Underlying mechanisms. J. Nutr. Sci. 2020, 9, e28. [Google Scholar] [CrossRef] [PubMed]
- Centeno, V.A.; De Barboza, G.E.D.; Marchionatti, A.M.; Alisio, A.E.; Dallorso, M.E.; Nasif, R.; De Talamoni, N.G.T. Dietary calcium deficiency increases Ca2+ uptake and Ca2+ extrusion mechanisms in chick enterocytes. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2004, 139, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Olukosi, O.A.; Adedokun, S.A.; Ajuwon, K.M.; Adeola, O. Early responses of sodium-dependent phosphate transporter type IIb in broiler chicks to dietary phosphorus intervention. Br. Poult. Abstr. 2011, 7, 39. [Google Scholar]
- Takeuchi, A.; Reddy, G.S.; Kobayashi, T.; Okano, T.; Park, J.; Sharma, S. Nuclear factor of activated T cells (NFAT) as a mo-lecular target for 1alpha, 25-dihydroxyvitamin D3-mediated effects. J. Immunol. 1998, 160, 209–218. [Google Scholar] [CrossRef]
- Abdullah, H.I.; Pedraza, P.L.; McGiff, J.C.; Ferreri, N.R. CaR activation increases TNF production by mTAL cells via a Gi-dependent mechanism. Am. J. Physiol. Renal. Physiol. 2008, 294, F345–F354. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Zhang, B.; Dong, W.; Zhao, Y.; Zhao, X.; Zhang, Y.; Zhang, Q. SLC34A2 Targets in Calcium/Phosphorus Homeostasis of Mammary Gland and Involvement in Development of Clinical Mastitis in Dairy Cows. Animals 2024, 14, 1275. https://doi.org/10.3390/ani14091275
Wang X, Zhang B, Dong W, Zhao Y, Zhao X, Zhang Y, Zhang Q. SLC34A2 Targets in Calcium/Phosphorus Homeostasis of Mammary Gland and Involvement in Development of Clinical Mastitis in Dairy Cows. Animals. 2024; 14(9):1275. https://doi.org/10.3390/ani14091275
Chicago/Turabian StyleWang, Xueying, Bohao Zhang, Weitao Dong, Yu Zhao, Xingxu Zhao, Yong Zhang, and Quanwei Zhang. 2024. "SLC34A2 Targets in Calcium/Phosphorus Homeostasis of Mammary Gland and Involvement in Development of Clinical Mastitis in Dairy Cows" Animals 14, no. 9: 1275. https://doi.org/10.3390/ani14091275





