Ball Milling Effect on the CO2 Uptake of Mafic and Ultramafic Rocks: A Review
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Selection and Preparation
2.2. Mechanical Activation
2.3. Characterization of Unmilled and Ball-Milled Samples
3. Results and Discussion
3.1. Petrography
3.2. Powder X-ray Diffraction (PXRD)
3.3. Surface Texture
3.4. Scanning Electron Microscopy (SEM)
3.5. Transmission Electron Microscopy (TEM and HRTEM)
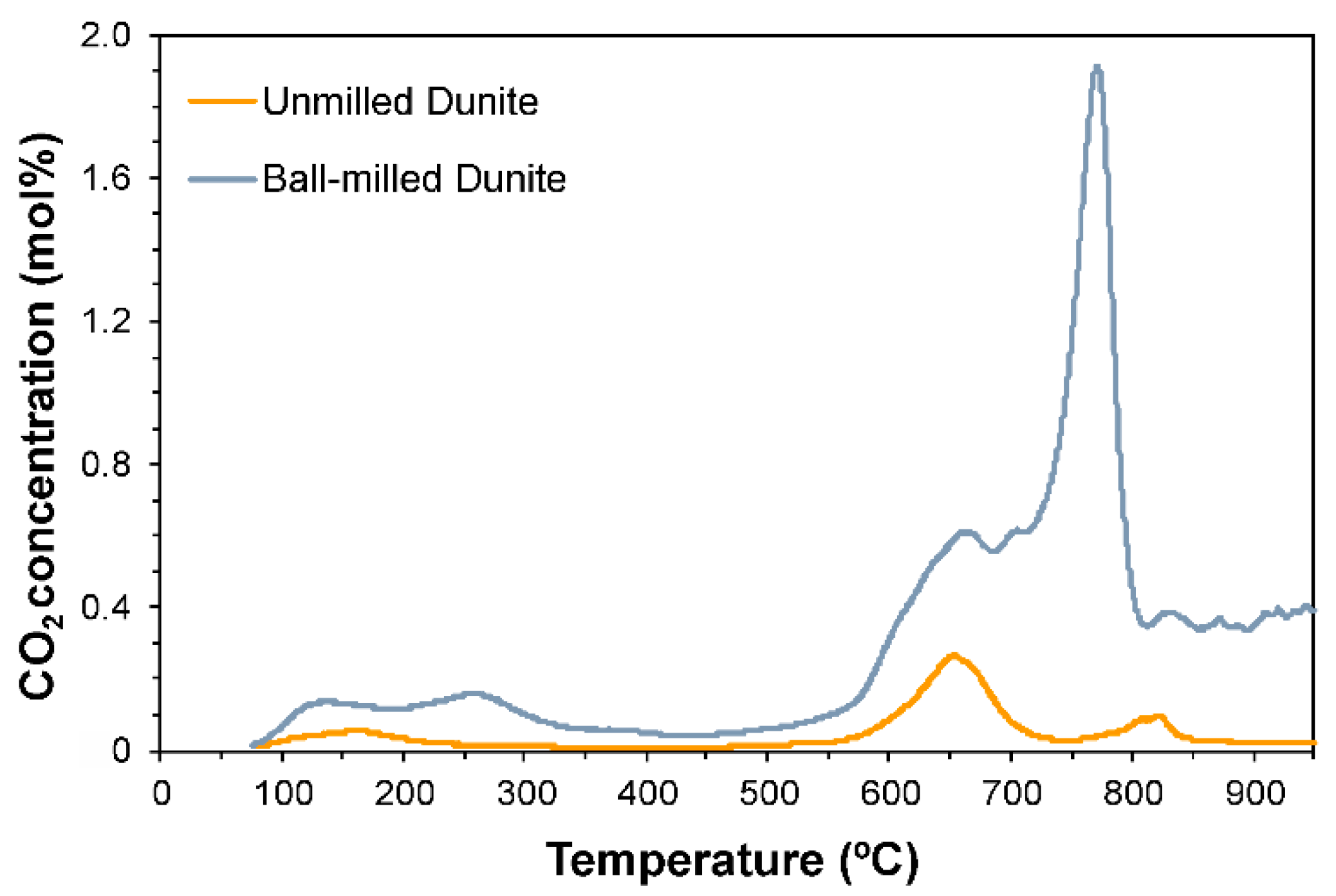
3.6. CO2 Selective Chemisorption Followed by TPD
4. Practical Significance of the Results
5. Conclusions
- Ball milling can be used for the development of ultrafine mafic/ultramafic powders with substantially enhanced CO2 uptake compared to the initial rock materials. This is due to the fact that the ball milling process modifies the crystal structure, surface morphology and size of the primary crystals of the minerals, thus increasing the number and strength of the surface basic sites that are responsible for the carbonation process;
- Prolonged ball milling resulted in a reduction of the CO2 uptake due to the increased agglomeration of nanoparticles;
- The use of 50 wt% ethanol as milling liquid resulted in the development of nanoscale powders with the highest CO2-storage capacity;
- The optimum ball milling conditions, in terms of CO2 uptake, were not the same for all samples. The dunite showed the highest CO2 uptake after ball milling, followed by the olivine basalt, the pyroxenite and the dolerite;
- Τhe exact petrographic characteristics (i.e., type of primary mafic minerals, degree of alteration, participation of flexible minerals) of each rock type are the main factors controlling the structural and morphological characteristics, as well as the carbon sequestration efficiency, of the final ultrafine powders;
- The incorporation of the ball milling process in the production of aggregates and other raw materials, as well as the use of suitable quarry fines, could reduce to some extent the total energy required for the size reduction of the initial rock materials;
- The end-products of mineral carbonation could be used by the construction industry as additives. Alternatively, the ultrafine rock powders could be used, before carbonation, as nano-additives in composite building materials (in replacement to cement or lime), in order to enhance their carbonation process.
Author Contributions
Funding
Conflicts of Interest
References
- Intergovernmental Panel on Climate Change (IPCC). Climate Change 2014: Mitigation of Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- Solomon, S.; Plattner, G.K.; Knutti, R.; Friedlingstein, P. Irreversible climate change due to carbon dioxide emissions. Proc. Nat. Acad. Sci. USA 2009, 106, 1704–1709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerdemann, S.J.; O’Connor, W.K.; Dahlin, D.C.; Panner, L.R.; Rush, H. Ex situ aqueous mineral carbonation. Environ. Sci. Technol. 2007, 41, 2587–2593. [Google Scholar] [CrossRef] [PubMed]
- Matter, J.M.; Stute, M.; Snæbjörnsdottir, S.Ó.; Oelkers, E.H.; Gislason, S.R.; Aradottir, E.S.; Sigfusson, B.; Gunnarsson, I.; Sigurdardottir, H.; Gunnlaugsson, E.; et al. Rapid carbon mineralization for permanent disposal of anthropogenic carbon dioxide emissions. Science 2016, 352, 1312–1314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Power, I.M.; Harrison, A.L.; Dipple, G.M. Accelerating mineral carbonation using carbonic anhydrase. Environ. Sci. Technol. 2016, 50, 2610–2618. [Google Scholar] [CrossRef] [PubMed]
- Snæbjörnsdóttir, S.Ó.; Gislason, S.R.; Galeczka, I.M.; Oelkers, E.H. Reaction path modelling of in-situ mineralisation of CO2 at the CarbFix site at Hellisheidi, SW-Iceland. Geochim. Cosmochim. Acta 2018, 220, 348–366. [Google Scholar] [CrossRef]
- Seifritz, W. CO2 disposal by means of silicates. Nature 1990, 345, 486. [Google Scholar] [CrossRef]
- Lackner, K.S.; Wendt, C.H.; Butt, D.P.; Joyce, E.L.; Sharp, D.H. Carbon dioxide disposal in carbonate minerals. Energy 1995, 20, 1153–1170. [Google Scholar] [CrossRef]
- Oelkers, E.H.; Gislason, S.R.; Matter, J. Mineral carbonation of CO2. Elements 2008, 4, 333–337. [Google Scholar] [CrossRef]
- Sanna, A.; Uibu, M.; Caramanna, G.; Kuusik, R.; Maroto-Valer, M. A review of mineral carbonation technologies to sequester CO2. Chem. Soc. Rev. 2014, 43, 8049–8080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, R.M.; Audenaerde, A.V.; Chiang, Y.W.; Iacobescu, R.I.; Knops, P.; Gerven, T.V. Nickel extraction from olivine: Effect of carbonation pre-treatment. Metals 2015, 5, 1620–1644. [Google Scholar] [CrossRef] [Green Version]
- Gislason, S.R.; Oelkers, E.H. Carbon storage in basalt. Science 2014, 344, 373. [Google Scholar] [CrossRef] [PubMed]
- Matter, J.M.; Kelemen, P.B. Permanent storage of carbon dioxide in geological reservoirs by mineral carbonation. Nat. Geosci. 2009, 2, 837–841. [Google Scholar] [CrossRef]
- Rigopoulos, I.; Delimitis, A.; Ioannou, I.; Efstathiou, A.M.; Kyratsi, T. Effect of ball milling on the carbon sequestration efficiency of serpentinized peridotites. Miner. Eng. 2018, 120, 66–74. [Google Scholar] [CrossRef]
- Sanna, A.; Hall, M.R.; Maroto-Valer, M. Post-processing pathways in carbon capture and storage by mineral carbonation (CCSM) towards the introduction of carbon neutral materials. Energy Environ. Sci. 2012, 5, 7781–7796. [Google Scholar] [CrossRef]
- Haug, A.H.; Kleiv, R.A.; Munz, I.A. Investigating dissolution of mechanically activated olivine for carbonation purposes. Appl. Geochem. 2010, 25, 1547–1563. [Google Scholar] [CrossRef]
- Declercq, J.; Bosc, O.; Oelkers, E.H. Do organic ligands affect forsterite dissolution rates? Appl. Geochem. 2013, 39, 69–77. [Google Scholar] [CrossRef]
- Li, J.; Hitch, M. Characterization of the microstructure of mechanically-activated olivine using X-ray diffraction pattern analysis. Miner. Eng. 2016, 86, 24–33. [Google Scholar] [CrossRef]
- Rigopoulos, I.; Vasiliades, M.A.; Ioannou, I.; Efstathiou, A.M.; Godelitsas, A.; Kyratsi, T. Enhancing the rate of ex situ mineral carbonation in dunites. Adv. Powder Technol. 2016, 27, 360–371. [Google Scholar] [CrossRef]
- Kleiv, R.A.; Thornhill, M. Mechanical activation of olivine. Miner. Eng. 2006, 19, 340–347. [Google Scholar] [CrossRef]
- Kleiv, R.A.; Thornhill, M. The effect of mechanical activation in the production of olivine surface area. Miner. Eng. 2016, 89, 19–23. [Google Scholar] [CrossRef]
- Baláž, P.; Turianicová, E.; Fabián, M.; Kleiv, R.A.; Briančin, J.; Obut, A. Structural changes in olivine (Mg,Fe)2SiO4 mechanically activated in high-energy mills. Int. J. Miner. Process. 2008, 88, 1–6. [Google Scholar] [CrossRef]
- Turianicová, E.; Baláž, P.; Tuček, L.; Zorkovská, A.; Zeleňák, V.; Németh, Z.; Šatka, A.; Kováč, J. A comparison of the reactivity of activated and non-activated olivine with CO2. Int. J. Miner. Process. 2013, 123, 73–77. [Google Scholar] [CrossRef]
- Li, J.; Hitch, M. Mechanical activation of magnesium silicates for mineral carbonation: A review. Miner. Eng. 2018, 128, 69–83. [Google Scholar] [CrossRef]
- Rigopoulos, I.; Petallidou, K.C.; Vasiliades, M.A.; Delimitis, A.; Ioannou, I.; Efstathiou, A.M.; Kyratsi, T. Carbon dioxide storage in olivine basalts: Effect of ball milling process. Powder Technol. 2015, 273, 220–229. [Google Scholar] [CrossRef]
- Rigopoulos, I.; Harrison, A.L.; Delimitis, A.; Ioannou, I.; Efstathiou, A.M.; Kyratsi, T.; Oelkers, E.H. Carbon sequestration via enhanced weathering of peridotites and basalts in seawater. Appl. Geochem. 2018, 91, 197–207. [Google Scholar] [CrossRef]
- Power, I.M.; Harrison, A.L.; Dipple, G.M.; Wilson, S.A.; Kelemen, P.B.; Hitch, M.; Southam, G. Carbon mineralization: from natural analogues to engineered systems. Rev. Mineral. Geochem. 2013, 77, 305–360. [Google Scholar] [CrossRef]
- Wilson, S.A.; Harrison, A.L.; Dipple, G.M.; Power, I.M.; Barker, S.L.L.; Ulrich Mayer, K.; Fallon, S.J.; Raudsepp, M.; Southam, G. Offsetting of CO2 emissions by air capture in mine tailings at the Mount Keith Nickel Mine, Western Australia: Rates, controls and prospects for carbon neutral mining. Int. J. Greenh. Gas Control 2014, 25, 121–140. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Hitch, M. Ultra-fine grinding and mechanical activation of mine waste rock using a planetary mill for mineral carbonation. Int. J. Miner. Process. 2017, 158, 18–26. [Google Scholar] [CrossRef]
- Li, J.; Hitch, M. Structural and chemical changes in mine waste mechanically-activated in various milling environments. Powder Technol. 2017, 308, 13–19. [Google Scholar] [CrossRef]
- Li, J.; Hitch, M.; Power, I.M.; Pan, Y. Integrated mineral carbonation of ultramafic mine deposits—A review. Minerals 2018, 8, 147. [Google Scholar] [CrossRef]
- Rigopoulos, I.; Vasiliades, M.A.; Petallidou, K.C.; Ioannou, I.; Efstathiou, A.M.; Kyratsi, T. A method to enhance the CO2 storage capacity of pyroxenitic rocks. Greenh. Gas. Sci. Technol. 2015, 5, 1–14. [Google Scholar] [CrossRef]
- Rigopoulos, I.; Petallidou, K.C.; Vasiliades, M.A.; Delimitis, A.; Ioannou, I.; Efstathiou, A.M.; Kyratsi, T. On the potential use of quarry waste material for CO2 sequestration. J. CO2 Util. 2016, 16, 361–370. [Google Scholar] [CrossRef]
- Mukasa, S.B.; Ludden, J.N. Uranium–lead ages of plagiogranites from the Troodos ophiolite, Cyprus, and their tectonic significance. Geology 1987, 1, 825–828. [Google Scholar] [CrossRef]
- Robinson, P.T.; Malpas, J. The Troodos Ophiolite of Cyprus: New perspectives on its origin and emplacement. In Ophiolites: Oceanic Crustal Analogues; Malpas, J., Moores, E.M., Panayiotou, A., Xenophontos, C., Eds.; Cyprus Geological Survey Department: Nicosia, Cyprus, 1990; pp. 13–36. [Google Scholar]
- Robertson, A.H.F. Overview of the genesis and emplacement of Mesozoic ophiolites in the Eastern Mediterranean Tethyan region. Lithos 2002, 65, 1–67. [Google Scholar] [CrossRef]
- Hochella, M.F.J. Nanogeoscience: From origins to cutting-edge applications. Elements 2008, 4, 373–379. [Google Scholar] [CrossRef]
- Costa, C.N.; Anastasiadou, T.; Efstathiou, A.M. The selective catalytic reduction of nitric oxide with methane over La2O3–CaO systems: Synergistic effects and surface reactivity studies of NO, CH4, O2, and CO2 by transient techniques. J. Catal. 2000, 194, 250–265. [Google Scholar] [CrossRef]
- Efstathiou, A.M.; Bennett, C.O. Enthalpy and entropy of H2 adsorption on Rh/Al2O3 measured by Temperature-Programmed Desorption. J. Catal. 1990, 124, 116–126. [Google Scholar] [CrossRef]
- Streckeisen, A.L. To each plutonic rock its proper name. Earth Sci. Rev. 1976, 12, 1–33. [Google Scholar] [CrossRef]
- Munz, I.A.; Brandvoll, Ø.; Haug, T.A.; Iden, K.; Smeets, R.; Kihle, J.; Johansen, H. Mechanisms and rates of plagioclase carbonation reactions. Geochim. Cosmochim. Acta 2012, 77, 27–51. [Google Scholar] [CrossRef]
- Rigopoulos, I.; Tsikouras, B.; Pomonis, P.; Hatzipanagiotou, K. Petrographic investigation of microcrack initiation in mafic ophiolitic rocks under uniaxial compression. Rock Mech. Rock Eng. 2013, 46, 1061–1072. [Google Scholar] [CrossRef]
- Rigopoulos, I.; Tsikouras, B.; Pomonis, P.; Hatzipanagiotou, K. Microcracks in ultrabasic rocks under uniaxial compressive stress. Eng. Geol. 2011, 117, 104–113. [Google Scholar] [CrossRef]
- Sissmann, O.; Brunet, F.; Martinez, I.; Guyot, F.; Verlaguet, A.; Pinquier, Y.; Daval, D. Enhanced olivine carbonation within a basalt as compared to single-phase experiments: reevaluating the potential of CO2 mineral sequestration. Environ. Sci. Technol. 2014, 48, 5512–5519. [Google Scholar] [CrossRef] [PubMed]
- Ounoughene, G.; Buskens, E.; Santos, R.M.; Cizer, Ö.; Gerven, T.V. Solvochemical carbonation of lime using ethanol: Mechanism and enhancement for direct atmospheric CO2 capture. J. CO2 Util. 2018, 26, 143–151. [Google Scholar] [CrossRef]
- O’Connor, W.K.; Dahlin, D.C.; Rush, G.E.; Gerdemann, S.J.; Penner, L.R.; Nilsen, D.N. Aqueous Mineral Carbonation: Mineral Availability, Pretreatment, Reaction Parameters, and Process Studies; United States Department of Energy: Washington, DC, USA, 2005.
- Rigopoulos, I.; Tsikouras, B.; Pomonis, P.; Hatzipanagiotou, K. The influence of alteration on the engineering properties of dolerites: The examples from the Pindos and Vourinos ophiolites (northern Greece). Int. J. Rock Mech. Min. Sci. 2010, 47, 69–80. [Google Scholar] [CrossRef]
- Rigopoulos, I.; Tsikouras, B.; Pomonis, P.; Hatzipanagiotou, K. Assessment of the engineering behavior of ultramafic and mafic rocks using chemical alteration indices. Eng. Geol. 2015, 196, 222–237. [Google Scholar] [CrossRef]
- Engidasew, T.A.; Barbieri, G. Geo-engineering evaluation of Termaber basalt rock mass for crushed stone aggregate and building stone from Central Ethiopia. J. Afr. Earth Sci. 2014, 99, 581–594. [Google Scholar] [CrossRef]
- Rigopoulos, I.; Török, Á.; Kyratsi, T.; Delimitis, A.; Ioannou, I. Sustainable exploitation of mafic rock quarry waste for carbon sequestration following ball milling. Resour. Policy 2018, in press. [Google Scholar] [CrossRef]
- Intergovernmental Panel on Climate Change. Guidelines for National Greenhouse Gas Inventories; IPCC National Greenhouse Gas Inventories Programme: Hayama, Japan, 2006; Volume 3, Available online: https://www.ipcc-nggip.iges.or.jp/public/2006gl/pdf/3_Volume3/V3_2_Ch2_Mineral_Industry.pdf (accessed on 6 November 2018).
- Hills, T.P.; Sceats, M.; Rennie, D.; Fennell, P. LEILAC: Low cost CO2 capture for the cement and lime industries. Energy Proc. 2017, 114, 6166–6170. [Google Scholar] [CrossRef]
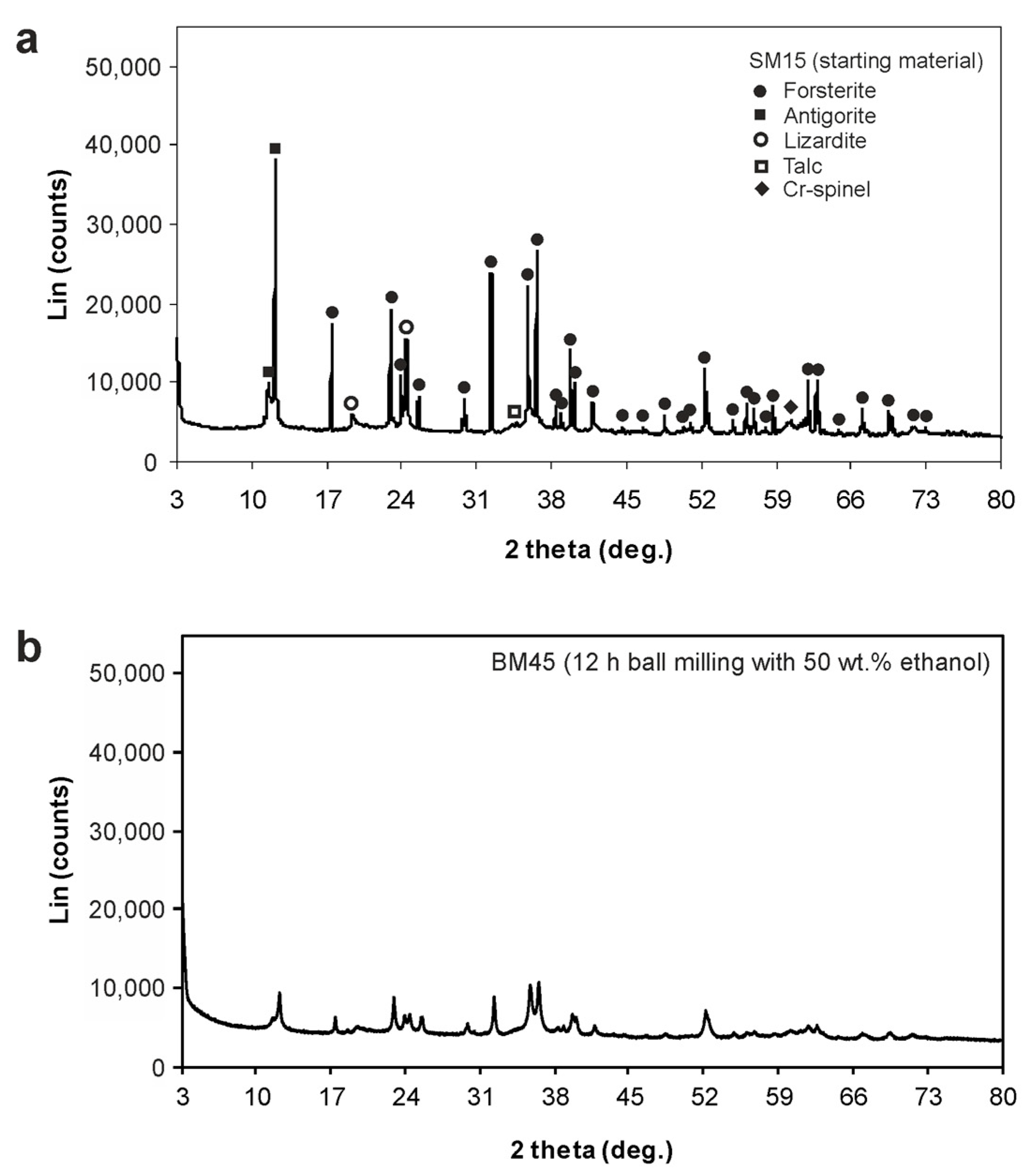
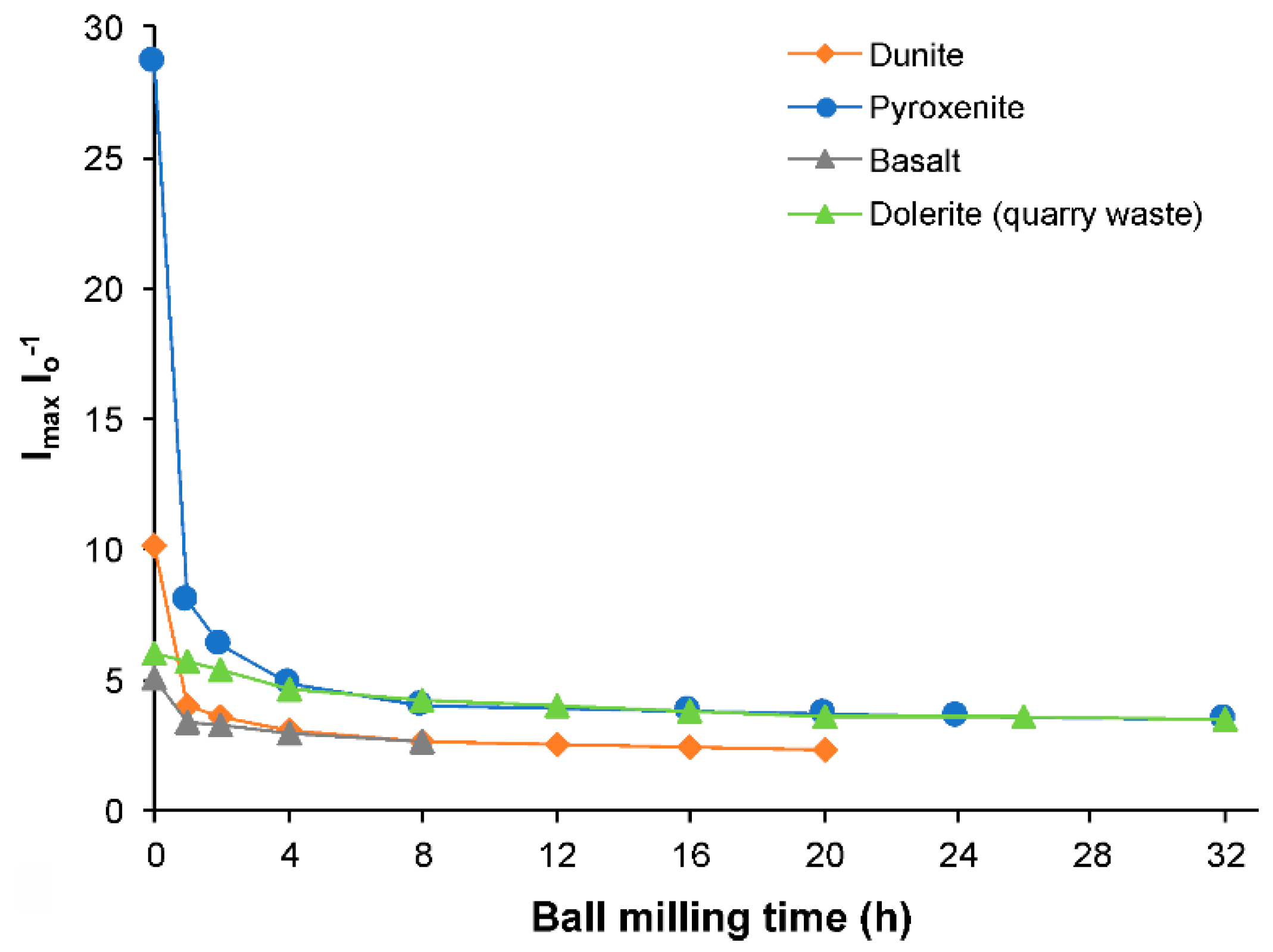

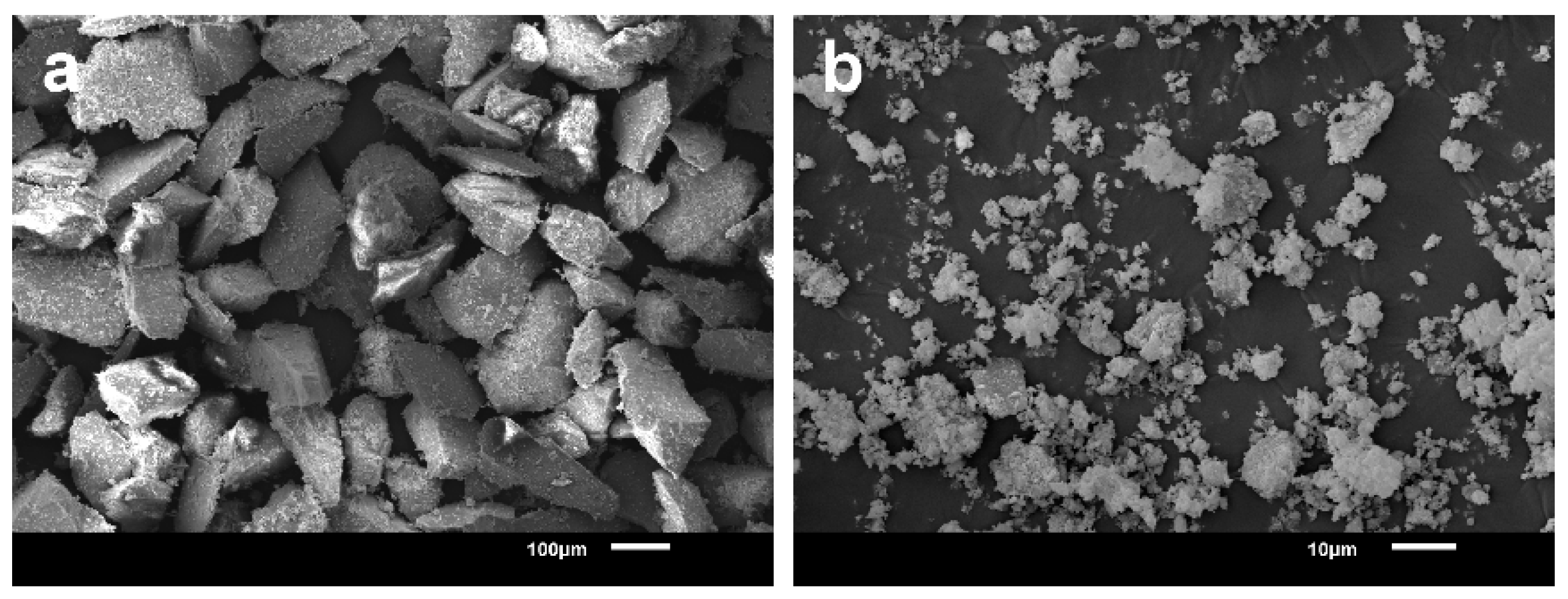


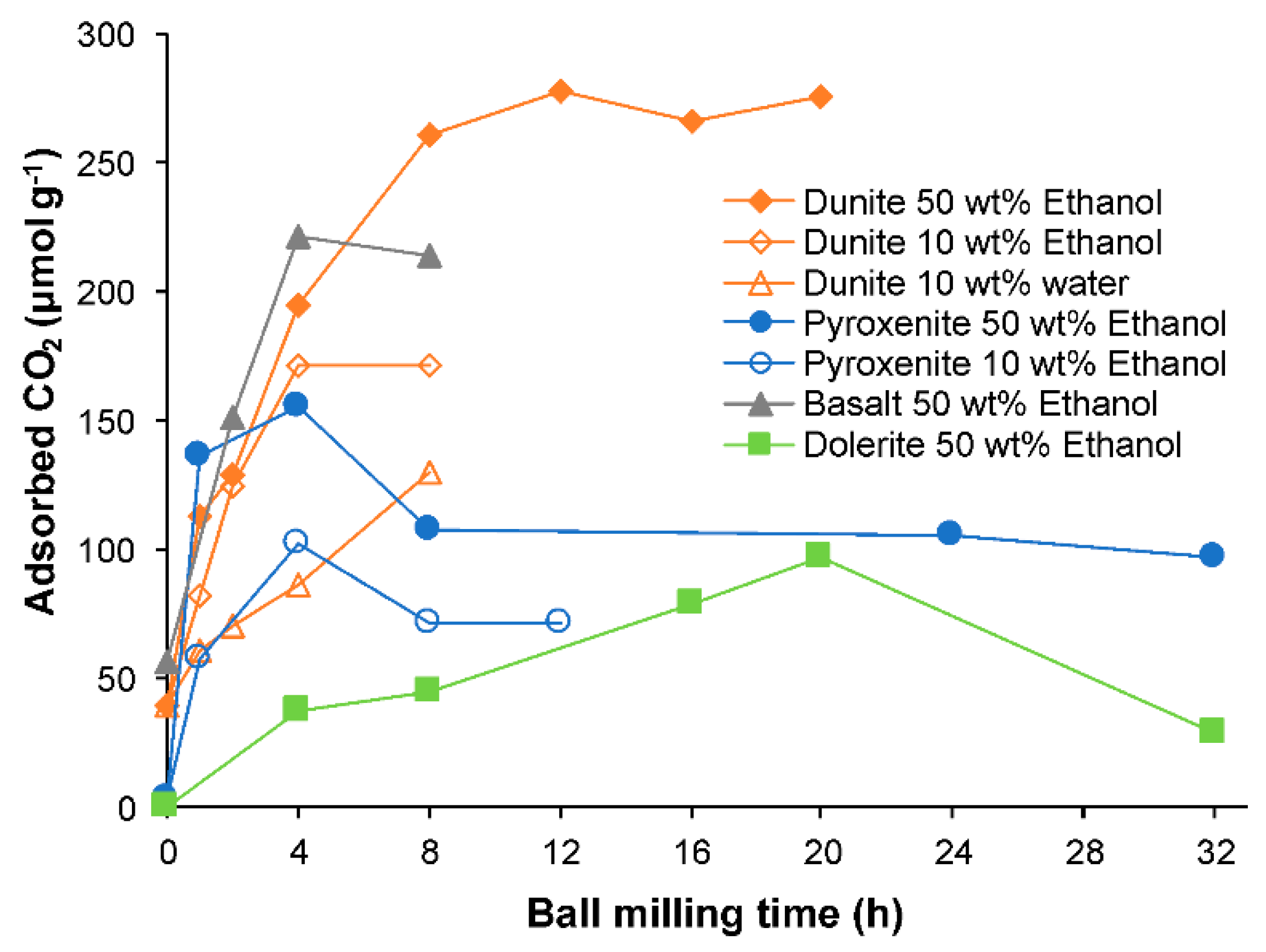
| Ball Milling Conditions | Textural Properties | ||||
|---|---|---|---|---|---|
| Rock Type/Sample Code | Milling Time (h) | Type of Milling | BET (m2 g−1) | Specific Pore Volume (cm3 g−1) | Average Pore Diameter (nm) |
| Dunite | |||||
| SM15 * | - | - | 6.8 | 0.011 | 5.4 |
| BM30 | 1 | Wet (10 wt% H2O) | 24.5 | 0.048 | 6.5 |
| BM31 | 2 | Wet (10 wt% H2O) | 36.9 | 0.055 | 4.9 |
| BM41 | 4 | Wet (10 wt% H2O) | 45.9 | 0.059 | 4.2 |
| BM42 | 8 | Wet (10 wt% H2O) | 52.2 | 0.052 | 3.9 |
| BM26 | 1 | Wet (10 wt% Ethanol) | 29.5 | 0.097 | 11.2 |
| BM27 | 2 | Wet (10 wt% Ethanol) | 38.0 | 0.104 | 9.3 |
| BM34 | 4 | Wet (10 wt% Ethanol) | 47.7 | 0.109 | 7.9 |
| BM35 | 8 | Wet (10 wt% Ethanol) | 32.5 | 0.089 | 9.0 |
| BM44 | 1 | Wet (50 wt% Ethanol) | 25.2 | 0.081 | 11.4 |
| BM36 | 2 | Wet (50 wt% Ethanol) | 30.3 | 0.104 | 12.2 |
| BM38 | 4 | Wet (50 wt% Ethanol) | 35.7 | 0.121 | 11.8 |
| BM39 | 8 | Wet (50 wt% Ethanol) | 41.5 | 0.170 | 13.7 |
| BM45 | 12 | Wet (50 wt% Ethanol) | 51.9 | 0.173 | 11.4 |
| BM40 | 16 | Wet (50 wt% Ethanol) | 49.9 | 0.157 | 10.9 |
| BM46 | 20 | Wet (50 wt% Ethanol) | 64.6 | 0.179 | 9.3 |
| Pyroxenite | |||||
| SMP1 * | - | - | 1.8 | 0.005 | 9.7 |
| BM3 | 1 | Wet (10 wt% Ethanol) | 12.8 | 0.034 | 9.7 |
| BM8 | 1 | Wet (50 wt% Ethanol) | 11.8 | 0.017 | 5.3 |
| BM9 | 2 | Wet (50 wt% Ethanol) | 17.7 | 0.047 | 9.0 |
| BM10 | 4 | Wet (50 wt% Ethanol) | 23.3 | 0.067 | 9.5 |
| BM13 | 2 | Wet (10 wt% Ethanol) | 15.1 | 0.030 | 6.7 |
| BM14 | 4 | Wet (10 wt% Ethanol) | 27.2 | 0.044 | 6.7 |
| BM16 | 8 | Wet (50 wt% Ethanol) | 32.7 | 0.088 | 8.7 |
| BM18 | 8 | Wet (10 wt% Ethanol) | 48.9 | 0.071 | 4.8 |
| BM20 | 16 | Wet (50 wt% Ethanol) | 42.3 | 0.123 | 9.9 |
| BM24 | 20 | Wet (50 wt% Ethanol) | 59.9 | 0.209 | 11.2 |
| BM25 | 12 | Wet (10 wt% Ethanol) | 59.0 | 0.092 | 5.1 |
| BM32 | 24 | Wet (50 wt% Ethanol) | 67.7 | 0.180 | 8.4 |
| BM33 | 32 | Wet (50 wt% Ethanol) | 53.7 | 0.175 | 10.5 |
| Ball Milling Conditions | Textural Properties | ||||
|---|---|---|---|---|---|
| Rock Type/Sample Code | Milling Time (h) | Type of Milling | BET (m2 g−1) | Specific Pore Volume (cm3 g−1) | Average Pore Diameter (nm) |
| Basalt | |||||
| SM1 * | - | - | 9.0 | 0.014 | 6.4 |
| BM1 | 1 | Wet (10 wt% Ethanol) | 40.2 | 0.081 | 7.3 |
| BM2 | 1 | Wet (10 wt% H2O) | 34.1 | 0.041 | 4.1 |
| BM5 | 1 | Wet (50 wt% Ethanol) | 31.2 | 0.076 | 8.9 |
| BM6 | 2 | Wet (50 wt% Ethanol) | 42.9 | 0.078 | 6.4 |
| BM7 | 4 | Wet (50 wt% Ethanol) | 58.9 | 0.106 | 6.2 |
| BM11 | 2 | Wet (10 wt% Ethanol) | 64.3 | 0.085 | 4.6 |
| BM12 | 4 | Wet (10 wt% Ethanol) | 55.0 | 0.081 | 6.2 |
| BM15 | 8 | Wet (50 wt% Ethanol) | 44.3 | 0.104 | 8.7 |
| Dolerite (quarry waste) | |||||
| SM16 * | - | - | 8.4 | 0.015 | 7.0 |
| BM47 | 1 | Wet (50 wt% Ethanol) | 16.3 | 0.028 | 7.2 |
| BM48 | 2 | Wet (50 wt% Ethanol) | 20.2 | 0.046 | 8.3 |
| BM49 | 4 | Wet (50 wt% Ethanol) | 27.0 | 0.115 | 14.9 |
| BM50 | 8 | Wet (50 wt% Ethanol) | 28.8 | 0.145 | 18.1 |
| BM51 | 12 | Wet (50 wt% Ethanol) | 28.1 | 0.121 | 14.1 |
| BM52 | 16 | Wet (50 wt% Ethanol) | 29.6 | 0.115 | 13.7 |
| BM53 | 20 | Wet (50 wt% Ethanol) | 32.9 | 0.151 | 16.0 |
| BM54 | 26 | Wet (50 wt% Ethanol) | 32.4 | 0.094 | 10.9 |
| BM56 | 32 | Wet (50 wt% Ethanol) | 34.8 | 0.111 | 11.3 |
| Sample Code | Tmax1 (°C) | Tmax2 (°C) | Tmax3 (°C) | Tmax4 (°C) | Tmax5 (°C) | Tmax6 (°C) | Tmax7 (°C) | Tmax8 (°C) | CO2 Uptake (μmol g−1) | CO2 Uptake (μmol m−2) | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dunite | |||||||||||
| SM15 1 | 160 | 654 | 807 | 824 | - | - | - | - | 40.1 | 5.9 | Rigopoulos et al. [19] |
| BM45 2 | 134 | 259 | 664 | 705 | 772 | 831 | - | - | 278.1 | 5.4 | Rigopoulos et al. [19] |
| Basalt | |||||||||||
| SM1 1 | 85 | 720 | 815 | 875 | - | - | - | - | 56.3 | 6.3 | Rigopoulos et al. [25] |
| BM7 2 | 143 | 276 | 390 | 680 | 699 | 738 | 812 | - | 222.1 | 3.8 | Rigopoulos et al. [25] |
| Pyroxenite | |||||||||||
| SMP1 1 | 165 | 602 | 628 | 700 | 723 | 805 | 868 | 903 | 3.8 | 2.1 | Rigopoulos et al. [32] |
| BM10 2 | 150 | 688 | 765 | 899 | 931 | - | - | - | 155.6 | 6.7 | Rigopoulos et al. [32] |
| Sample Code | Tmax1 (°C) | Tmax2 (°C) | Tmax3 (°C) | Tmax4 (°C) | Tmax5 (°C) | Tmax6 (°C) | Desorbed CO2 (μmol g−1) | CO2 Uptake (μmol g−1) | CO2 Uptake (μmol m−2) | Reference | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| SM16 1 | Chem. | 133 | 783 | - | - | - | - | 337.6 | 0.8 | 0.1 | Rigopoulos et al. [33] |
| No chem. | 770 | - | - | - | - | - | 336.8 | ||||
| BM53 2 | Chem. | 138 | 275 | 677 | 730 | 790 | 873 | 541.9 | 96.9 | 2.9 | Rigopoulos et al. [33] |
| No chem. | 605 | 727 | 783 | 880 | - | - | 445.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rigopoulos, I.; Ioannou, I.; Delimitis, A.; Efstathiou, A.M.; Kyratsi, T. Ball Milling Effect on the CO2 Uptake of Mafic and Ultramafic Rocks: A Review. Geosciences 2018, 8, 406. https://doi.org/10.3390/geosciences8110406
Rigopoulos I, Ioannou I, Delimitis A, Efstathiou AM, Kyratsi T. Ball Milling Effect on the CO2 Uptake of Mafic and Ultramafic Rocks: A Review. Geosciences. 2018; 8(11):406. https://doi.org/10.3390/geosciences8110406
Chicago/Turabian StyleRigopoulos, Ioannis, Ioannis Ioannou, Andreas Delimitis, Angelos M. Efstathiou, and Theodora Kyratsi. 2018. "Ball Milling Effect on the CO2 Uptake of Mafic and Ultramafic Rocks: A Review" Geosciences 8, no. 11: 406. https://doi.org/10.3390/geosciences8110406






