Geopolymer Composites for Potential Applications in Cultural Heritage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.2.1. Geopolymer (GMK)
2.2.2. Geopolymer Composites (GMK-E and GMK-E-MP)
2.2.3. Curing Treatments
2.3. Geopolimers Characterization Methods
2.3.1. Thermogravimetric Analysis (TGA)
2.3.2. Fourier Transform Infrared Spectroscopy (FT-IR)
2.3.3. X-ray Powder Diffraction (XRD)
2.3.4. Compressive Strength Test
2.3.5. SEM Analysis
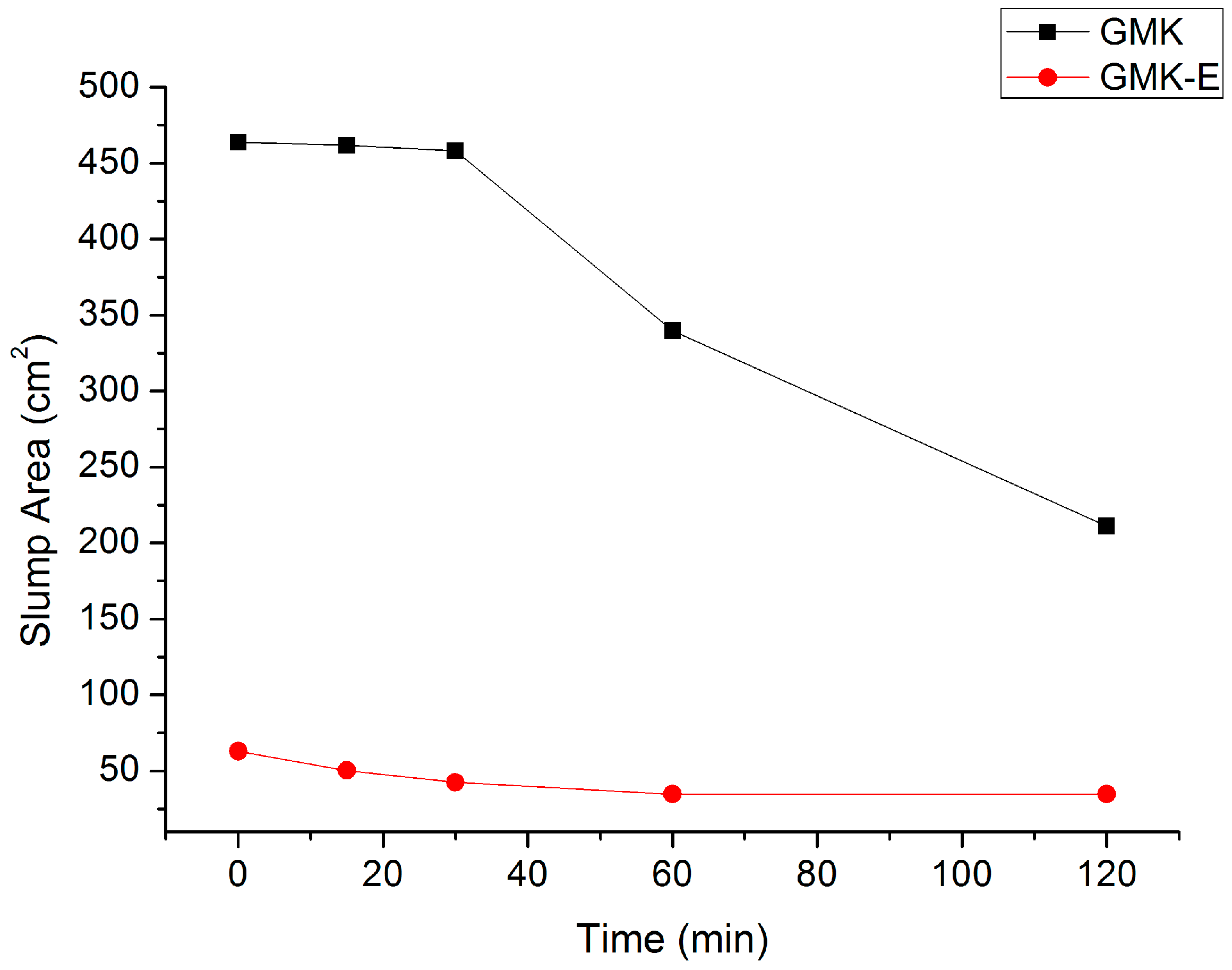
2.3.6. Rheological Measurement
2.3.7. Determination of the Water-Soluble Salt Conten
3. Results and Discussion
3.1. Material Characterization
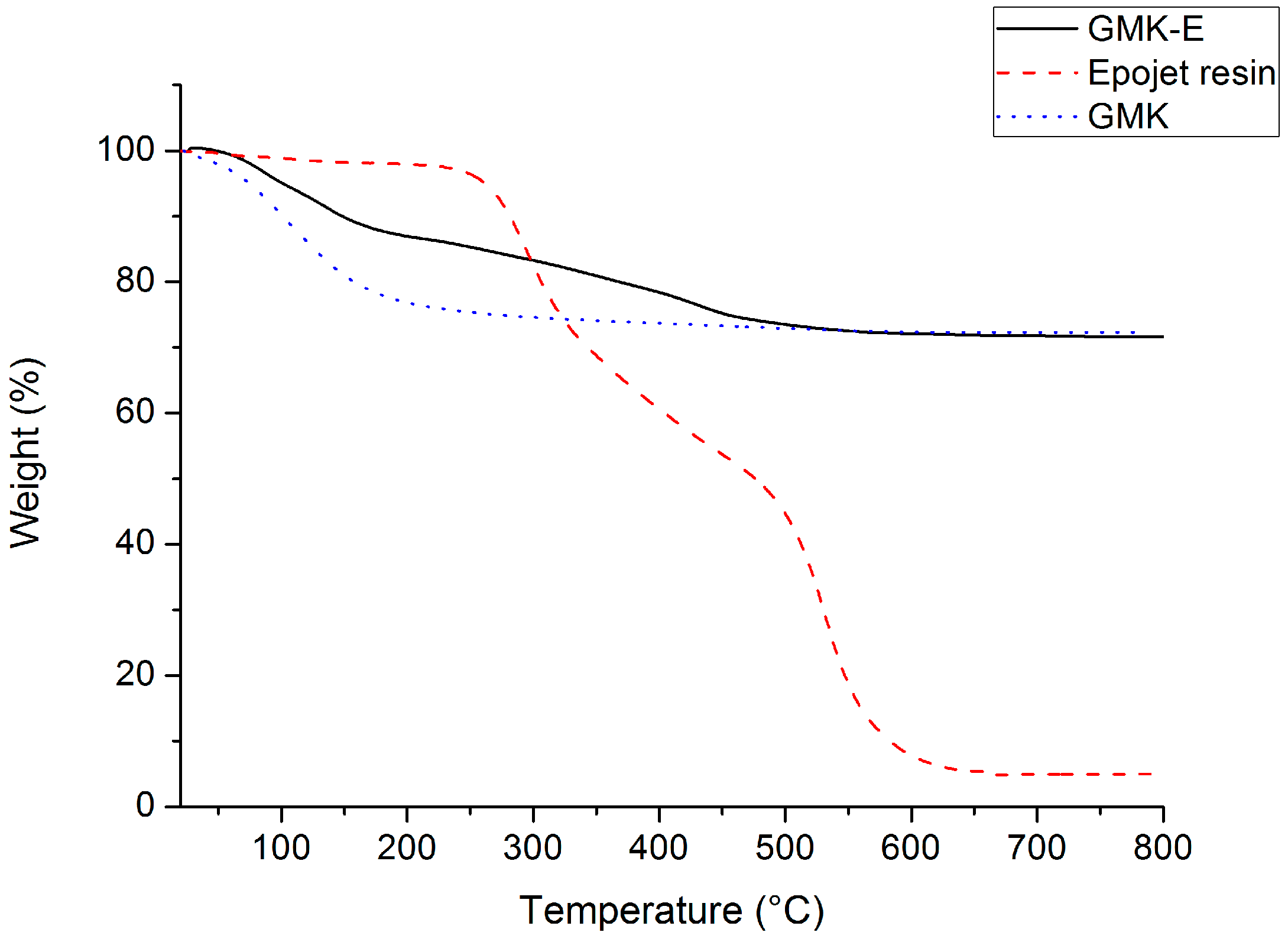
3.1.1. Thermal Analysis (TGA)
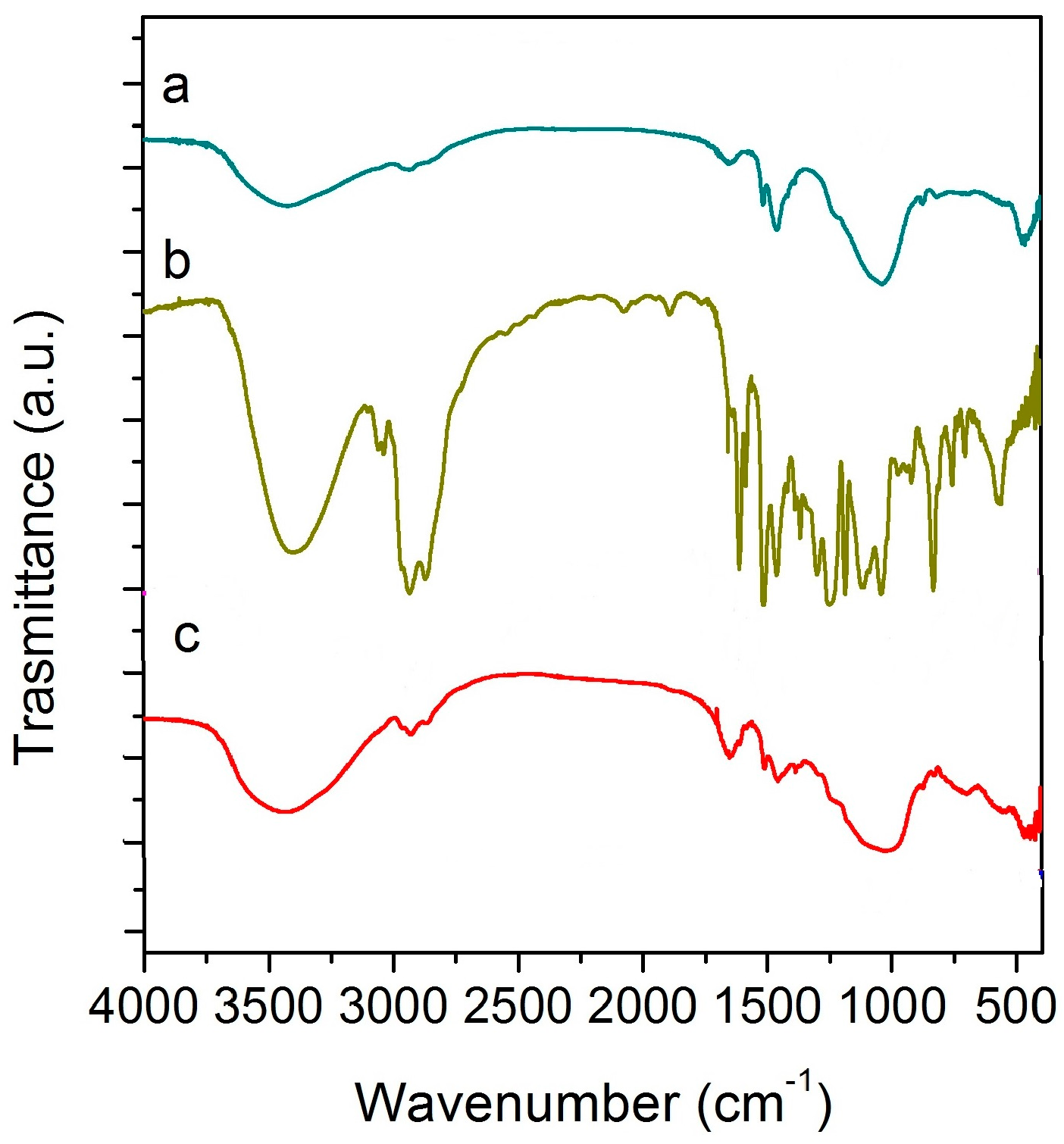
3.1.2. FT-IR Analysis
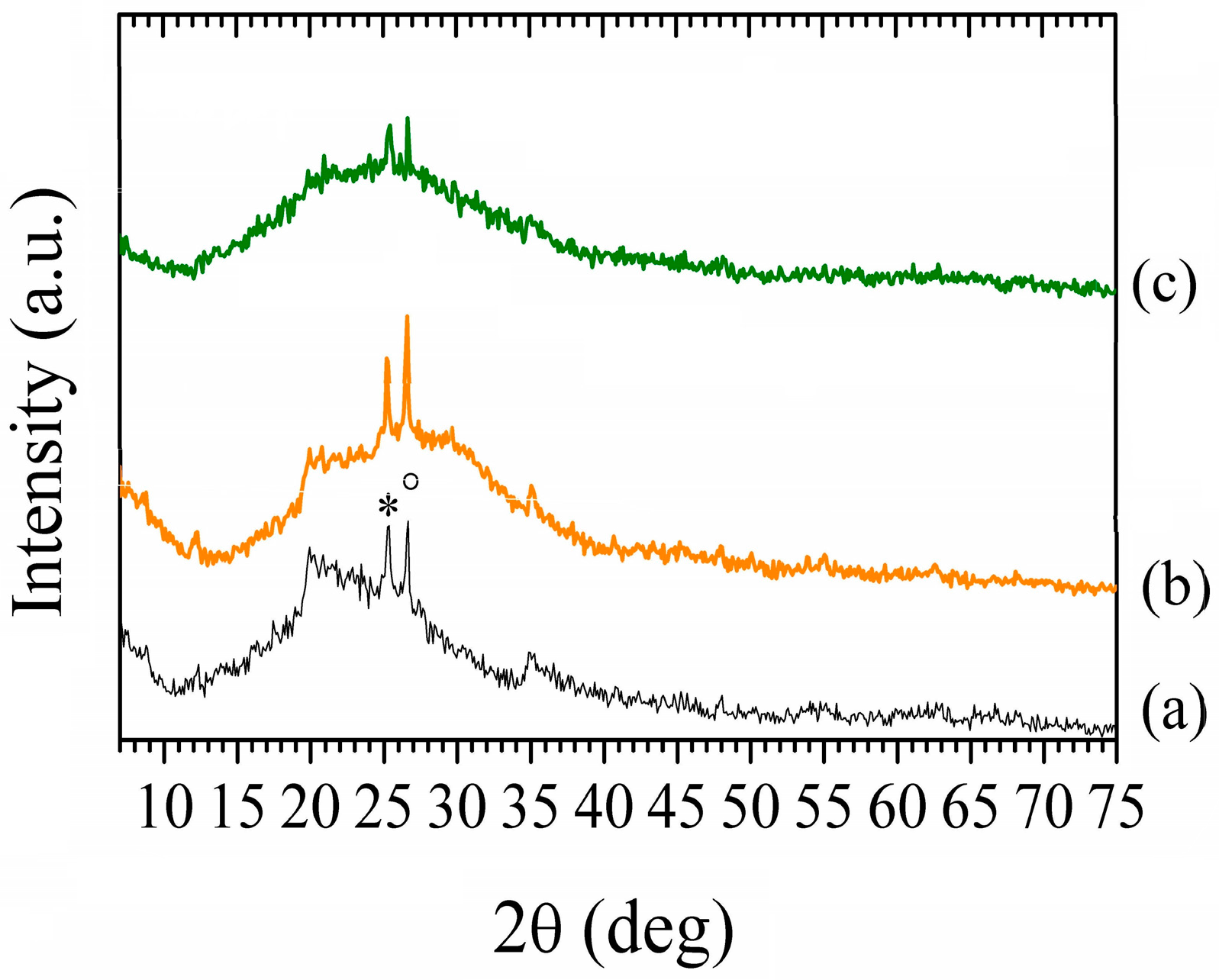
3.1.3. X-ray Diffraction Characterization
3.1.4. Compressive Strength Test
3.1.5. Textural and Microstructural Characterizations
3.1.6. Determination of the Water-Soluble Salt Content
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Riccio, A.; Chianese, E.; Agrillo, G.; Esposito, C.; Ferrara, L.; Tirimberio, G. Source apportion of atmospheric particulate matter: A joint Eulerian/Lagrangian approach. Environ. Sci. Pollut. Res. 2014, 21, 13160–13168. [Google Scholar] [CrossRef] [PubMed]
- Riccio, A.; Chianese, E.; Tirimberio, G.; Prati, M.V. Emission factors of inorganic ions from road traffic: A case study from the city of Naples (Italy). Transp. Res. Part D Transp. Environ. 2017, 54, 239–249. [Google Scholar] [CrossRef]
- Chianese, E.; Riccio, A.; Duro, I.; Trifuoggi, M.; Iovino, P.; Capasso, S.; Barone, G. Measurements for indoor air quality assessment at the Capodimonte Museum in Naples (Italy). Int. J. Environ. Res. 2012, 6, 509–518. [Google Scholar]
- Ozga, I.; Ghedini, N.; Giosuè, C.; Sabbioni, C.; Tittarelli, F.; Bonazza, B. Assessment of air pollutant sources in the deposit on monuments by multivariate analysis. Sci. Total Environ. 2014, 490, 776–784. [Google Scholar] [CrossRef] [PubMed]
- International Council on Monuments and Sites (ICOMOS); International Scientific Committee for Analysis and Restoration of Structures of Architectural Heritage. Recommendations for the Analysis, Conservation and Structural Restoration of Architectural Heritage; ICOMOS: Paris, France, 2003. [Google Scholar]
- Corradi, M.; Tedeschi, C.; Binda, L.; Borri, A. Experimental evaluation of shear and compression strength of masonry wall before and after reinforcement: Deep repointing. Constr. Build. Mater. 2008, 22, 463–472. [Google Scholar] [CrossRef]
- Valluzzi, M.R.; Modena, C.; de Felice, G. Current practice and open issues in strengthening historical buildings with composites. Mater. Struct. 2014, 47, 1971–1985. [Google Scholar] [CrossRef]
- Bergamonti, L.; Alfieri, I.; Lorenzi, A.; Predieri, G.; Barone, G.; Gemelli, G.; Mazzoleni, P.; Raneri, S.; Bersani, D.; Lottic, P.P. Nanocrystalline TiO2 coatings by sol–gel: Photocatalytic activity on Pietra di Noto biocalcarenite. J. Sol-Gel Sci. Technol. 2015, 75, 141–151. [Google Scholar] [CrossRef]
- Cocca, M.; D’Arienzo, L.; D’Orazio, L.; Gentile, G.; Martuscelli, E. Polyacrylates for conservation: Chemico-physical properties and durability of different commercial products. Polym. Test. 2004, 23, 333–342. [Google Scholar] [CrossRef]
- Clausi, M.; Tarantino, S.C.; Magnani, L.L.; Riccardi, M.P.; Tedeschi, C.; Zema, M. Metakaolin as a precursor of materials for applications in Cultural Heritage: Geopolymer-based mortars with ornamental stone aggregates. Appl. Clay Sci. 2016, 132–133, 589–599. [Google Scholar] [CrossRef]
- Geraldes, C.F.M.; Lima, A.M.; Delgado-Rodrigues, J.; Mimoso, J.M.; Pereira, S.R.M. Geopolymers as potential repair material in tiles conservation. Appl. Phys. A 2016, 122, 197. [Google Scholar] [CrossRef]
- Rowles, M.; O’Connor, B. Chemical optimisation of the compressive strength of aluminosilicate geopolymers synthesised by sodium silicate activation of metakaolinite. J. Mater. Chem. 2003, 13, 1161–1165. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications, 3rd ed.; Institut Geopolymere: Saint Quentin, France, 2011. [Google Scholar]
- Habert, G.; Ouellet-Plamondon, C. Recent update on the environmental impact of geopolymers. RILEM Tech. Lett. 2016, 1, 17–23. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Van Deventer, J.S. The role of inorganic polymer technology in the development of ‘green concrete’. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Ferone, C.; Roviello, G.; Colangelo, F.; Cioffi, R.; Tarallo, O. Novel hybrid organic-geopolymer materials. Appl. Clay Sci. 2013, 73, 42–50. [Google Scholar] [CrossRef]
- Ferone, C.; Colangelo, F.; Roviello, G.; Asprone, D.; Menna, C.; Balsamo, A.; Manfredi, G. Application-oriented chemical optimization of a metakaolin based geopolymer. Materials 2013, 6, 1920–1939. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricciotti, L.; Roviello, G.; Tarallo, O.; Borbone, F.; Ferone, C.; Colangelo, F.; Catauro, M.; Cioffi, R. Synthesis and characterizations of melamine-based epoxy resins. Int. J. Mol. Sci. 2013, 14, 18200–18214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roviello, G.; Ricciotti, L.; Ferone, C.; Colangelo, F.; Cioffi, R.; Tarallo, O. Synthesis and Characterization of Novel Epoxy Geopolymer Hybrid Composites. Materials 2013, 6, 3943–3962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiarella, F.; Barra, M.; Ricciotti, L.; Aloisio, A.; Cassinese, A. Morphology, electrical performance and potentiometry of PDIF-CN2 thin-film transistors on HMDS-treated and bare silicon dioxide. Electronics 2014, 3, 76–86. [Google Scholar] [CrossRef]
- Colangelo, F.; Roviello, G.; Ricciotti, L.; Ferone, C.; Cioffi, R. Preparation and characterization of new geopolymer-epoxy resin hybrid mortars. Materials 2013, 6, 2989–3006. [Google Scholar] [CrossRef] [PubMed]
- Strini, A.; Roviello, G.; Ricciotti, L.; Ferone, C.; Messina, F.; Schiavi, L.; Cioffi, R. TiO2-Based Photocatalytic Geopolymers for Nitric Oxide Degradation. Materials 2016, 9, 513. [Google Scholar] [CrossRef] [PubMed]
- Roviello, G.; Ricciotti, L.; Ferone, C.; Colangelo, F.; Tarallo, O. Fire resistant melamine based organic-geopolymer hybrid composites. Cem. Concr. Compos. 2015, 59, 89–99. [Google Scholar] [CrossRef]
- Roviello, G.; Menna, C.; Tarallo, O.; Ricciotti, L.; Ferone, C.; Colangelo, F.; Asprone, D.; di Maggio, R.; Cappelletto, E.; Prota, A.; et al. Preparation, structure and properties of hybrid materials based on geopolymers and polysiloxanes. Mater. Des. 2015, 87, 82–94. [Google Scholar] [CrossRef]
- Colangelo, F.; Cioffi, R.; Roviello, G.; Capasso, I.; Caputo, D.; Aprea, P.; Liguori, B.; Ferone, C. Thermal cycling stability of fly ash based geopolymer mortars. Compos. Part B 2017, 129, 11–17. [Google Scholar] [CrossRef]
- Messina, F.; Ferone, C.; Molino, A.; Roviello, G.; Colangelo, F.; Molino, B.; Cioffi, C. Synergistic recycling of calcined clayey sediments and water potabilization sludge as geopolymer precursors: Upscaling from binders to precast paving cement-free bricks. Constr. Build. Mater. 2017, 133, 14–26. [Google Scholar] [CrossRef]
- Roviello, G.; Menna, C.; Tarallo, O.; Ricciotti, L.; Messina, F.; Ferone, C.; Asprone, D.; Cioffi, R. Lightweight geopolymer-based hybrid materials. Compos. Part B Eng. 2017, 128, 225–237. [Google Scholar] [CrossRef]
- Colangelo, F.; Roviello, G.; Ricciotti, L.; Ferrándiz-Mas, V.; Messina, F.; Ferone, C.; Tarallo, O.; Cioffi, R.; Cheeseman, C.R. Mechanical and thermal properties of lightweight geopolymer composites containing recycled expanded polystyrene. Cem. Concr. Compos. 2018, 86, 266–272. [Google Scholar] [CrossRef]
- Roviello, G.; Ricciotti, L.; Tarallo, O.; Ferone, C.; Colangelo, F.; Roviello, V.; Cioffi, R. Innovative fly ash geopolymer-epoxy composites: Preparation, microstructure and mechanical properties. Materials 2016, 9, 461. [Google Scholar] [CrossRef] [PubMed]
- Kong, D.L.Y.; Sanjayan, J.G.; Sagoe-Crentsil, K. Comparative performance of geopolymers made with metakaolin and fly ash after exposure to elevated temperatures. Cem. Concr. Res. 2007, 37, 1583–1589. [Google Scholar] [CrossRef]
- White, C.E.; Provis, J.L.; Proffen, T.; van Deventer, J.S.J. The effects of temperature on the local structure of metakaolin-based geopolymer binder: A neutron pair distribution function investigation. J. Am. Ceram. Soc. 2010, 93, 3486–3492. [Google Scholar] [CrossRef]
- Duxson, P.; Lukey, G.C.; van Deventer, J.S.J. Physical evolution of Na-geopolymer derived from metakaolin up to 1000 °C. J. Mater. Sci. 2007, 42, 3044–3054. [Google Scholar] [CrossRef]
- Van Jaarsveld, J.G.S.; van Deventer, J.S.J.; Lukey, G.C. The characterisation of source materials in fly ash-based geopolymers. Mater. Lett. 2003, 57, 1272–1280. [Google Scholar] [CrossRef]
- Barbosa, V.F.F.; MacKenzie, K.J.D.; Thaumaturgo, C. Synthesis and characterisation of materials based on inorganic polymers of alumina and silica: Sodium polysialate polymers. Int. J. Inorg. Mater. 2000, 2, 309–317. [Google Scholar] [CrossRef]
- Aronne, A.; Esposito, S.; Ferone, C.; Pansini, M.; Pernice, P. FTIR study of the thermal transformation of barium-exchanged zeolite A to celsian. J. Mater. Chem. 2002, 12, 3039–3045. [Google Scholar] [CrossRef]
- Ortego, J.D.; Barroeta, Y. Leaching effects on silicate polymerization, A FTIR and 29Si NMR study of lead and zinc in Portland cement. Environ. Sci. Technol. 1991, 25, 1171–1174. [Google Scholar] [CrossRef]
- Clayden, N.J.; Esposito, S.; Aronne, A.; Pernice, P. Solid state 27Al NMR and FTIR study of lanthanum aluminosilicate glasses. J. Non-Cryst. Solids 1999, 258, 11–19. [Google Scholar] [CrossRef]
- Fellahi, S.; Chikhi, N.; Bakar, M. Modification of epoxy resin with kaolin as a toughening agent. J. Appl. Polym. Sci. 2001, 82, 861–878. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Yan, F. Synthesis and mechanical properties of metakaolinite-based geopolymer. Colloids Surf. A 2005, 268, 1–6. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Bassler, G.C.; Morrill, T.C. Spectrometric Identification of Organic Compounds, 4th ed.; John Wiley and Sons: New York, NY, USA, 1981. [Google Scholar]
- Barbosa, V.F.F.; MacKenzie, K.J.D. Thermal behaviour of inorganic geopolymers and composites derived from sodium polysialate. Mater. Res. Bull. 2003, 38, 319–331. [Google Scholar] [CrossRef]
- Parker, R.W.; Frost, R.L. The application of drift spectroscopy to the multicomponent analysis of organic chemicals adsorbed on montmorillonite. Clays Clay Miner. 1996, 44, 32–40. [Google Scholar] [CrossRef]
- Frost, R.L.; Fredericks, P.M.; Shurvell, H.F. Raman microscopy of some kaolinite clay minerals. Can. J. Appl. Spectrosc. 1996, 41, 10–14. [Google Scholar]




| Sample | Metakaolin | Marble Powder | Sodium Silicate |
|---|---|---|---|
| SiO2 | 52.90 | 1.12 | 27.40 |
| Al2O3 | 41.90 | 0.37 | - |
| CaO | 0.17 | 52.26 | - |
| Fe2O3 | 1.60 | 0.11 | - |
| MgO | 0.19 | 0.87 | - |
| K2O | 0.77 | 0.10 | - |
| Na2O | - | 0.14 | 8.15 |
| Water | - | - | 64.45 |
| LoI * | 2.47 | 45.03 | - |
| Sample | MK | SS | NaOH | Resin | MP |
|---|---|---|---|---|---|
| GMK | 41.6 | 50.0 | 8.4 | - | - |
| GMK-E | 37.4 | 45.0 | 7.6 | 10 | - |
| GMK-E-MP | 30.0 | 36.0 | 6.0 | 8.0 | 20.0 |
| Sample | Wts (°C) 1 | Wte (°C) 2 | R (Weight %) 3 |
|---|---|---|---|
| GMK | 30 | 500 | 72 |
| GMK-E | 30 | 600 | 70 |
| Epojet® resin | 250 | 650 | 0 |
| Sample 1 | F− (mg/(g Sample)) | Cl− (mg/(g Sample)) | NO3− (mg/(g Sample)) | PO43− (mg/(g Sample)) | SO42− (mg/(g Sample)) | Na+ (mg/(g Sample)) | K+ (mg/(g Sample)) | pH |
|---|---|---|---|---|---|---|---|---|
| GMK-E-AI | 0.020 | 0.106 | 0.027 | 0.056 | 0.266 | 6.701 | 0.100 | 11.1 |
| GMK-E-BI | 0.017 | 0.077 | 0.013 | 0.023 | 0.173 | 5.518 | 0.069 | 10.6 |
| GMK-E-AII | 0.004 | 0.028 | 0.011 | 0.016 | 0.047 | 1.661 | 0.045 | 10.5 |
| GMK-E-BII | 0.004 | 0.017 | 0.007 | 0.013 | 0.040 | 1.634 | 0.025 | 10.4 |
| GMK-E-AIII | 0.000 | 0.016 | 0.011 | 0.011 | 0.030 | 1.260 | 0.044 | 9.8 |
| GMK-E-BIII | 0.000 | 0.016 | 0.007 | 0.008 | 0.031 | 1.108 | 0.032 | 10.1 |
| GMK-E-AIV | 0.000 | 0.005 | 0.011 | 0.010 | 0.021 | 0.736 | 0.031 | 10.0 |
| GMK-E-BIV | 0.000 | 0.005 | 0.007 | 0.007 | 0.022 | 0.726 | 0.019 | 10.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ricciotti, L.; Molino, A.J.; Roviello, V.; Chianese, E.; Cennamo, P.; Roviello, G. Geopolymer Composites for Potential Applications in Cultural Heritage. Environments 2017, 4, 91. https://doi.org/10.3390/environments4040091
Ricciotti L, Molino AJ, Roviello V, Chianese E, Cennamo P, Roviello G. Geopolymer Composites for Potential Applications in Cultural Heritage. Environments. 2017; 4(4):91. https://doi.org/10.3390/environments4040091
Chicago/Turabian StyleRicciotti, Laura, Antonio Jacopo Molino, Valentina Roviello, Elena Chianese, Paola Cennamo, and Giuseppina Roviello. 2017. "Geopolymer Composites for Potential Applications in Cultural Heritage" Environments 4, no. 4: 91. https://doi.org/10.3390/environments4040091





