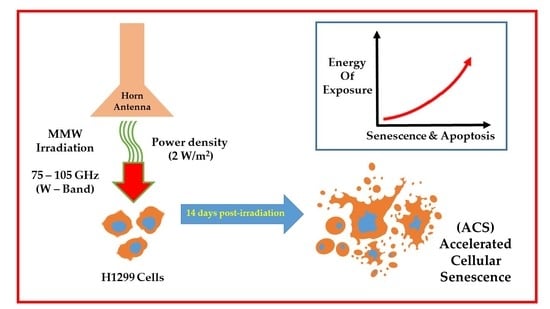
Morphological Changes in H1299 Human Lung Cancer Cells Following W-Band Millimeter-Wave Irradiation
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Irradiation Setup
2.3. Penetration of MMW through Petri Dish and Cell Growth Medium
2.4. Exposure Conditions
2.5. Microscopy and Image Processing
2.6. Statistical Analysis
3. Results
3.1. Millimeter Waves Can Penetrate through Petri Dishes
3.2. Millimeter Waves Transmit through RPMI 1640 Cell Growth Medium without Generating Thermal Heat
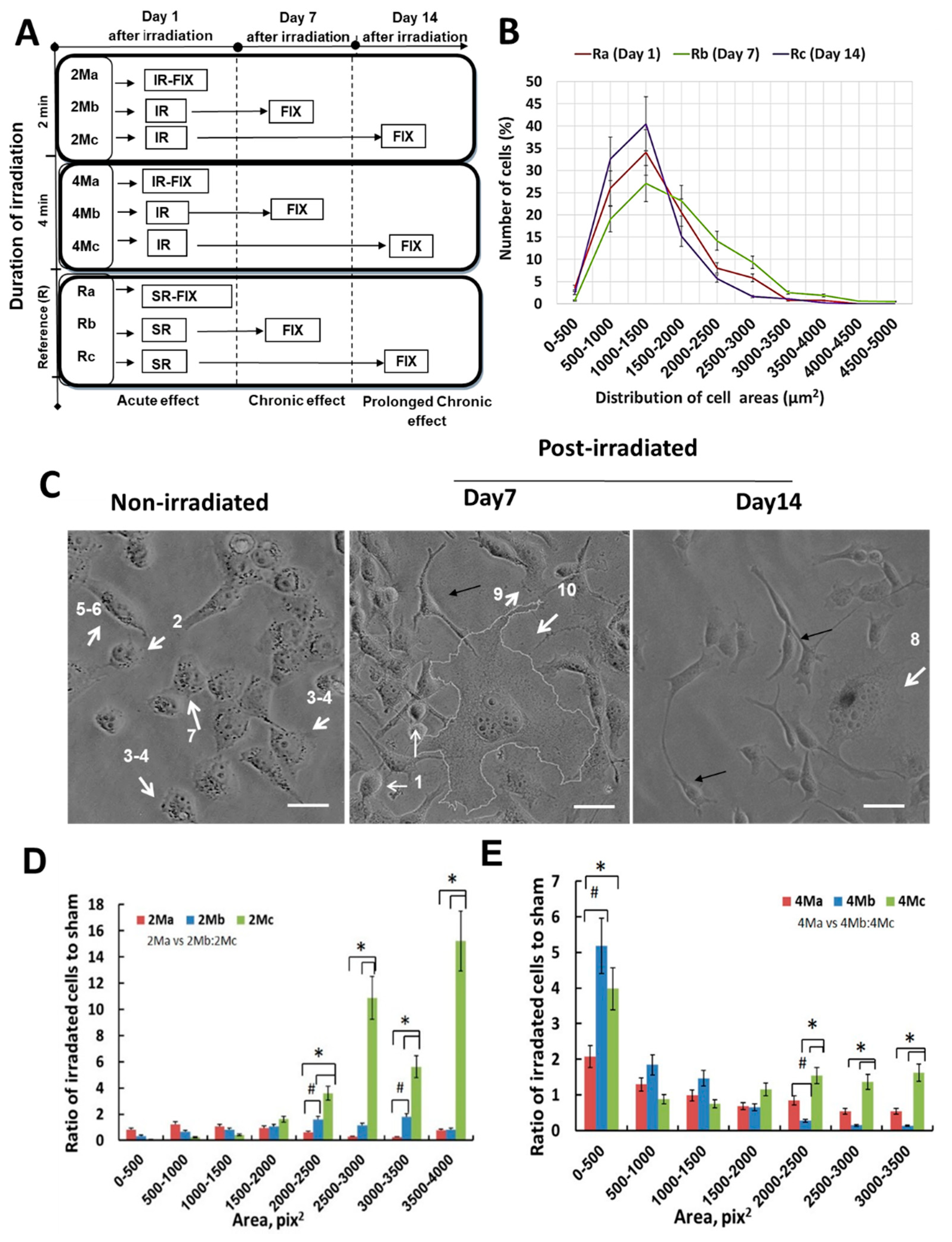
3.3. MMW Irradiation Changes the Morphology and Size of H1299 Cancer Cells
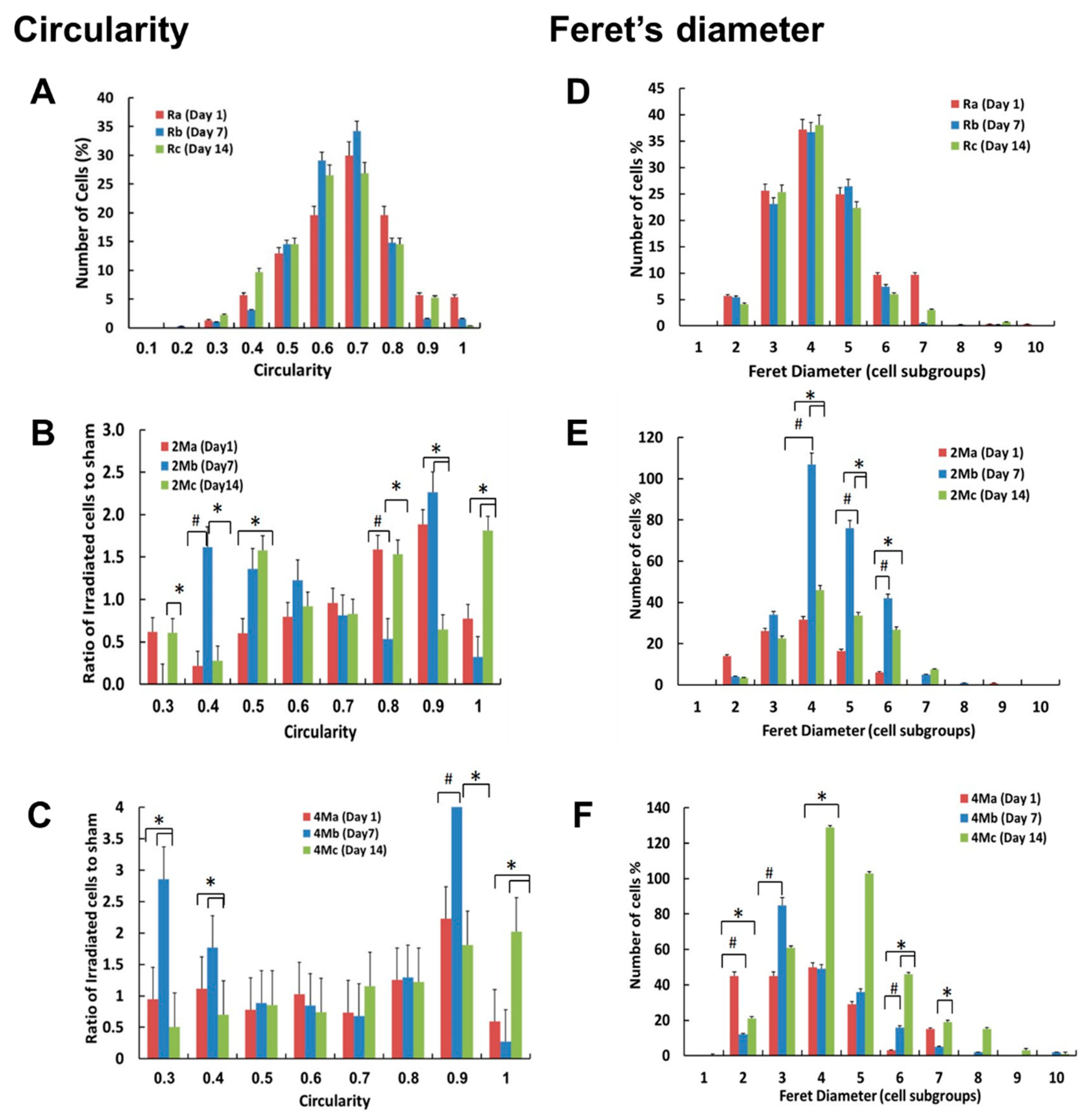
3.4. MMW Irradiation Increases Cell Circularity and Feret’s Diameter of H1299 Cancer Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cruz, C.S.D.; Tanoue, L.T.; Matthay, R.A. Lung cancer: Epidemiology, etiology, and prevention. Clin. Chest Med. 2011, 32, 605–644. [Google Scholar] [CrossRef] [PubMed]
- Hann, C.L.; Rudin, C.M. Management of small-cell lung cancer: Incremental changes but hope for the future. Oncology 2008, 22, 1486–1492. [Google Scholar] [PubMed]
- De Angelis, C.; Salvo, N.; Barnes, E.; Van Draanen, J.; Stacey, E.; Mitera, G.; Breen, D.; Giotis, A.; Czarnota, G.; Pang, J. Prophylaxis and management of acute radiation-induced skin reactions: A systematic review of the literature. Curr. Oncol. 2010, 17, 94–112. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.L. Ionizing Radiation: The Good, the Bad, and the Ugly. J. Investig. Dermatol. 2012, 132, 985–993. [Google Scholar] [CrossRef] [PubMed]
- NSCLC Meta-Analyses Collaborative Group. Chemotherapy in Addition to Supportive Care Improves Survival in Advanced Non–Small-Cell Lung Cancer: A Systematic Review and Meta-Analysis of Individual Patient Data from 16 Randomized Controlled Trials. J. Clin. Oncol. 2008, 26, 4617–4625. [Google Scholar] [CrossRef] [PubMed]
- (ICNIRP) International Commission on Non-Ionizing Radiation Protection. Guidelines for limiting exposure to Electromagnetic Fields (100 kHz to 300 GHz). Health Phys. 2020, 118, 483–524. [Google Scholar] [CrossRef] [PubMed]
- Yaekashiwa, N.; Otsuki, S.; Hayashi, S.; Kawase, K. Investigation of the non-thermal effects of exposing cells to 70–300 GHz irradiation using a widely tunable source. J. Radiat. Res. 2017, 59, 116–121. [Google Scholar] [CrossRef]
- Grundler, W.; Keilmann, F. Sharp Resonances in Yeast Growth Prove Nonthermal Sensitivity to Microwaves. Phys. Rev. Lett. 1983, 51, 1214–1216. [Google Scholar] [CrossRef]
- Hadjiloucas, S.; Chahal, M.S.; Bowen, J. Preliminary results on the non-thermal effects of 200 350 GHz radiation on the growth rate ofS. cerevisiaecells in microcolonies. Phys. Med. Biol. 2002, 47, 3831–3839. [Google Scholar] [CrossRef]
- Beneduci, A. Evaluation of the Potential In Vitro Antiproliferative Effects of Millimeter Waves at Some Therapeutic Frequencies on RPMI 7932 Human Skin Malignant Melanoma Cells. Cell Biophys. 2009, 55, 25–32. [Google Scholar] [CrossRef]
- Siegel, P.; Pikov, V. Impact of low intensity millimetre waves on cell functions. Electron. Lett. 2010, 46, S70. [Google Scholar] [CrossRef]
- Shapiro, M.G.; Priest, M.F.; Siegel, P.H.; Bezanilla, F. Thermal Mechanisms of Millimeter Wave Stimulation of Excitable Cells. Biophys. J. 2013, 104, 2622–2628. [Google Scholar] [CrossRef] [PubMed]
- Curecheriu, L.; Foca-Nici, E.; Vlahovici, A.L.; Avadane, O.; Sandu, D.D.; Creangai, D.; Miclaus, S. Radiofrequency wave effects on DNA and RNA levels in some animal tissues. Rom. J. Phys. 2007, 52, 389–395. [Google Scholar]
- Titushkin, I.A.; Rao, V.S.; Pickard, W.F.; Moros, E.G.; Shafirstein, G.; Cho, M.R. Altered Calcium Dynamics Mediates P19-Derived Neuron-Like Cell Responses to Millimeter-Wave Radiation. Radiat. Res. 2009, 172, 725–736. [Google Scholar] [CrossRef] [PubMed]
- Komoshvili, K.; Levitan, J.; Aronov, S.; Kapilevich, B.; Yahalom, A. Millimeter waves non-thermal effect on human lung cancer cells. In Proceedings of the 2011 IEEE International Conference on Microwaves, Communications, Tel Aviv, Israel, 7–9 November 2011; pp. 1–4. [Google Scholar]
- Homenko, A.; Kapilevich, B.; Kornstein, R.; Firer, M.A. Effects of 100 GHz radiation on alkaline phosphatase activity and antigen-antibody interaction. Bioelectromagnetics 2009, 30, 167–175. [Google Scholar] [CrossRef] [PubMed]
- Beneduci, A.; Chidichimo, G.; Tripepi, S.; Perrotta, E. Transmission electron microscopy study of the effects produced by wide-band low-power millimeter waves on MCF-7 human breast cancer cells in culture. Anticancer. Res. 2005, 25, 1009–1013. [Google Scholar]
- Grenier, K.; Dubuc, D.; Chretiennot, T.; Chen, T.; Artis, F.; Poupot, M.; Fournie, J.-J. Recent Advances in Microwave-Based Dielectric Spectroscopy at the Cellular Level for Cancer Investigations. IEEE Trans. Microw. Theory Tech. 2013, 61, 2023–2030. [Google Scholar] [CrossRef]
- Prohofsky, E.; Eyster, J. Prediction of giant breathing and rocking modes in double helical RNA. Phys. Lett. A 1974, 50, 329–330. [Google Scholar] [CrossRef]
- Gründler, W.; Keilmann, F.; Frohlich, H. Resonant growth rate response of yeast cells irradiated by weak microwaves. Phys. Lett. A 1977, 62, 463–466. [Google Scholar] [CrossRef]
- Frohlich, H. Coherent Processes in Biological Systems. J. Am. Chem. Soc. 1980, 157, 47. [Google Scholar]
- Belyaev, I.Y.; Alipov, Y.D.; Polunin, V.A.; Shcheglov, V.S. Evidence for Dependence of Resonant Frequency of Millimeter Wave Interaction with Escherichia coli K12 Cells on Haploid Genome Length. Electro Magnetobiol. 1993, 12, 39–49. [Google Scholar] [CrossRef]
- Burrell, R.A.; Swanton, C. Tumour heterogeneity and the evolution of polyclonal drug resistance. Mol. Oncol. 2014, 8, 1095–1111. [Google Scholar] [CrossRef] [PubMed]
- Saraste, A.; Pulkki, K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc. Res. 2000, 45, 528–537. [Google Scholar] [CrossRef]
- Cho, K.A.; Ryu, S.J.; Oh, Y.S.; Park, J.H.; Lee, J.W.; Kim, H.-P.; Kim, K.T.; Jang, I.S.; Park, S.C. Morphological Adjustment of Senescent Cells by Modulating Caveolin-1 Status. J. Biol. Chem. 2004, 279, 42270–42278. [Google Scholar] [CrossRef]
- Bharadwaj, D.; Mandal, M. Senescence in polyploid giant cancer cells: A road that leads to chemoresistance. Cytokine Growth Factor Rev. 2020, 52, 68–75. [Google Scholar] [CrossRef]
- Helmy, I.M.; Azim, A.M.A. Efficacy of ImageJ in the assessment of apoptosis. Diagn. Pathol. 2012, 7, 15. [Google Scholar] [CrossRef]
- Choi, J.Y.; Hong, W.G.; Cho, J.H.; Kim, E.M.; Kim, J.; Jung, C.-H.; Hwang, S.-G.; Um, H.-D.; Park, J.K. Podophyllotoxin acetate triggers anticancer effects against non-small cell lung cancer cells by promoting cell death via cell cycle arrest, ER stress and autophagy. Int. J. Oncol. 2015, 47, 1257–1265. [Google Scholar] [CrossRef]
- Liu, N.; Wang, Y.A.; Sun, Y.; Ecsedy, J.; Sun, J.; Li, X.; Wang, P. Inhibition of Aurora A enhances radiosensitivity in selected lung cancer cell lines. Respir. Res. 2019, 20, 230. [Google Scholar] [CrossRef]
- Hernandez-Segura, A.; Nehme, J.; DeMaria, M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef]
- Conlon, I.J.; Raff, M.C. Size Control in Animal Development. Cell 1999, 96, 235–244. [Google Scholar] [CrossRef]
- Zhadobov, M.; Chahat, N.; Sauleau, R.; Le Quément, C.; Le Dréan, Y. Millimeter-wave interactions with the human body: State of knowledge and recent advances. Int. J. Microw. Wirel. Technol. 2011, 3, 237–247. [Google Scholar] [CrossRef]
- Apollonio, F.; Liberti, M.; Paffi, A.; Merla, C.; Marracino, P.; Denzi, A.; Marino, C.; D’Inzeo, G. Feasibility for Microwaves Energy to Affect Biological Systems Via Nonthermal Mechanisms: A Systematic Approach. IEEE Trans. Microw. Theory Tech. 2013, 61, 2031–2045. [Google Scholar] [CrossRef]
- Lee, H.O.; Davidson, J.; Duronio, R.J. Endoreplication: Polyploidy with purpose. Genes Dev. 2009, 23, 2461–2477. [Google Scholar] [CrossRef] [PubMed]
- Weihua, Z.; Lin, Q.; Ramoth, A.J.; Fan, M.; Fidler, I.J. Formation of solid tumors by a single multinucleated cancer cell. Cancer 2011, 117, 4092–4099. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Wu, P.C.; Dong, D.Z.; Ivanova, I.; Chu, E.; Zeliadt, S.; Vesselle, H.; Wu, D.Y. Polyploidy road to therapy-induced cellular senescence and escape. Int. J. Cancer 2012, 132, 1505–1515. [Google Scholar] [CrossRef] [PubMed]
- Le Quément, C.; Nicolaz, C.N.; Habauzit, D.; Zhadobov, M.; Sauleau, R.; Le Dréan, Y. Impact of 60-GHz millimeter waves and corresponding heat effect on endoplasmic reticulum stress sensor gene expression. Bioelectromagnetics 2014, 35, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Koyama, S.; Narita, E.; Shimizu, Y.; Suzuki, Y.; Shiina, T.; Taki, M.; Shinohara, N.; Miyakoshi, J. Effects of Long-Term Exposure to 60 GHz Millimeter-Wavelength Radiation on the Genotoxicity and Heat Shock Protein (Hsp) Expression of Cells Derived from Human Eye. Int. J. Environ. Res. Public Health 2016, 13, 802. [Google Scholar] [CrossRef]
- Kim, K.S.; Cho, C.H.; Park, E.K.; Jung, M.-H.; Yoon, K.-S.; Park, H.-K. AFM-Detected Apoptotic Changes in Morphology and Biophysical Property Caused by Paclitaxel in Ishikawa and HeLa Cells. PLoS ONE 2012, 7, e30066. [Google Scholar] [CrossRef]
- Osborne, L.D.; Li, G.Z.; How, T.; O’Brien, E.T.; Blobe, G.C.; Superfine, R.; Mythreye, K. TGF-β regulates LARG and GEF-H1 during EMT to affect stiffening response to force and cell invasion. Mol. Biol. Cell 2014, 25, 3528–3540. [Google Scholar] [CrossRef]
- Lin, H.-H.; Lin, H.-K.; Lin, I.-H.; Chiou, Y.-W.; Chen, H.-W.; Liu, C.-Y.; Harn, H.I.-C.; Chiu, W.-T.; Wang, Y.-K.; Shen, M.-R.; et al. Mechanical phenotype of cancer cells: Cell softening and loss of stiffness sensing. Oncotarget 2015, 6, 20946–20958. [Google Scholar] [CrossRef]
- Raudenska, M.; Kratochvilova, M.; Vicar, T.; Gumulec, J.; Balvan, J.; Polanska, H.; Pribyl, J.; Masarik, M. Cisplatin enhances cell stiffness and decreases invasiveness rate in prostate cancer cells by actin accumulation. Sci. Rep. 2019, 9, 1660. [Google Scholar] [CrossRef] [PubMed]
- Roberson, R.S.; Kussick, S.J.; Vallieres, E.; Chen, S.-Y.J.; Wu, D.Y. Escape from Therapy-Induced Accelerated Cellular Senescence in p53-Null Lung Cancer Cells and in Human Lung Cancers. Cancer Res. 2005, 65, 2795–2803. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.-L.; Lan, K.-C.; Jang, L.-S. Electrical characteristics analysis of various cancer cells using a microfluidic device based on single-cell impedance measurement. Sens. Actuators B Chem. 2012, 173, 927–934. [Google Scholar] [CrossRef]
- Garg, A.; Jones, T.; Moss, S.M.; Mishra, S.; Kaul, K.; Ahirwar, D.K.; Ferree, J.; Kumar, P.; Subramaniam, D.; Ganju, R.K.; et al. Electromagnetic fields alter the motility of metastatic breast cancer cells. Commun. Biol. 2019, 2, 303–316. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Liu, Y.; Liu, S.; Luo, T.; Zhong, G.; Liu, A.; Zeng, Q.; Xin, X. Millimeter wave exposure induces apoptosis in human melanoma A375 cells in vitro. Nan Fang Yi Ke Da Xue Xue Bao 2019, 39, 76–81. [Google Scholar] [CrossRef]
- Yuan, H.-B.; Wang, X.-Y.; Sun, J.-Y.; Xie, F.-F.; Zheng, X.-X.; Tao, G.-Y.; Pan, L.; Hogarth, D.K. Flexible bronchoscopy-guided microwave ablation in peripheral porcine lung: A new minimally-invasive ablation. Transl. Lung Cancer Res. 2019, 8, 787–796. [Google Scholar] [CrossRef]
- Orlacchio, R.; Le Page, Y.; Le Dréan, Y.; Le Guével, R.; Sauleau, R.; Alekseev, S.; Zhadobov, M. Millimeter-wave pulsed heating in vitro: Cell mortality and heat shock response. Sci. Rep. 2019, 9, 15249. [Google Scholar] [CrossRef]
- Logani, M.K.; Szabó, I.; Makar, V.; Bhanushali, A.; Alekseev, S.; Ziskin, M.C. Effect of millimeter wave irradiation on tumor metastasis. Bioelectromagnetics 2006, 27, 258–264. [Google Scholar] [CrossRef]
- Makar, V.; Logani, M.; Szabó, I.; Ziskin, M. Effect of millimeter waves on cyclophosphamide induced suppression of T cell functions. Bioelectromagnetics 2003, 24, 356–365. [Google Scholar] [CrossRef]
- Wao, H.; Mhaskar, R.; Kumar, A.; Miladinovic, B.; Djulbegovic, B. Survival of patients with non-small cell lung cancer without treatment: A systematic review and meta-analysis. Syst. Rev. 2013, 2, 10. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komoshvili, K.; Becker, T.; Levitan, J.; Yahalom, A.; Barbora, A.; Liberman-Aronov, S. Morphological Changes in H1299 Human Lung Cancer Cells Following W-Band Millimeter-Wave Irradiation. Appl. Sci. 2020, 10, 3187. https://doi.org/10.3390/app10093187
Komoshvili K, Becker T, Levitan J, Yahalom A, Barbora A, Liberman-Aronov S. Morphological Changes in H1299 Human Lung Cancer Cells Following W-Band Millimeter-Wave Irradiation. Applied Sciences. 2020; 10(9):3187. https://doi.org/10.3390/app10093187
Chicago/Turabian StyleKomoshvili, Konstantin, Tzippi Becker, Jacob Levitan, Asher Yahalom, Ayan Barbora, and Stella Liberman-Aronov. 2020. "Morphological Changes in H1299 Human Lung Cancer Cells Following W-Band Millimeter-Wave Irradiation" Applied Sciences 10, no. 9: 3187. https://doi.org/10.3390/app10093187
APA StyleKomoshvili, K., Becker, T., Levitan, J., Yahalom, A., Barbora, A., & Liberman-Aronov, S. (2020). Morphological Changes in H1299 Human Lung Cancer Cells Following W-Band Millimeter-Wave Irradiation. Applied Sciences, 10(9), 3187. https://doi.org/10.3390/app10093187