Assessing the Dynamic Performance of Microbots in Complex Fluid Flows
Abstract
:1. Introduction
2. Microbots
2.1. Applicability
2.2. Classification
- Electrostatic microbotsAs it is well known, an electric field, which is created by an electric charge, applies a force to any other charged particles. Coulomb’s law states that the electrical force between two charged objects is directly proportional to the product of the quantity of charge on the objects and inversely proportional to the square of the separation distance between the two objects. This is the principle on which some Micro Electromechanical Systems (MEMS) are based for their control and propulsion. Specifically, electrostatic actuators have been highly explored to convert electrical energy into mechanical deformation for the propulsion of microbots [36]. For instance, Donald et al. [37] designed and fabricated MEMS microbots with an untethered scratch drive actuator used for their propulsion in order to allow the microbot to move freely without wires and rails. Other work carried out by Graule et al. [38] used electrostatic adhesion to perform robotic insets able to perch and take off.
- Magnetic microbotsA magnetic field is the magnetic effect of electric currents and magnetic materials. Magnetic fields have been used to apply forces and torques on magnetic microrobots, providing a valuable actuation method. The controlled magnetic fields can be generated in a variety of ways, and Nelson et al. [9] published a review article in which they detailed the power and locomotion of different microbots with special attention to magnetic microbots. Several works have been developed in this area. Pawashe et al. [39] reported the control of multiple robots by an array of electrostatic anchoring pads on the surface using also some fixed microbots to prevent translation. Later on, Mahoney and Abbott [40] demonstrated magnetic three-degree-of-freedom closed-loop position and two degree-of-freedom open-loop orientation control of a capsule used for endoscopy with a single permanent magnet. Diller et al. [41] achieved the control of multiple microbots in 3D using magnetic gradient. More recently, Diller et al. [42] proposed a theoretical framework and design for six degree-of-freedom actuation of a magnetic micro robot, and Floyd et al. [43] developed methods for controlling multiple untethered magnetic micro-robots.
- Optically actuated microbotsIt is very common to use optical tweezers to trap particles at the scale of nanometers, and, therefore, this technique has also been being used as an actuator in microbots. However, several issues are limiting its applicability as they are low force generated, and a high aperture lens is needed to focus the laser, allowing only a very small workspace and the unsuitability for its use in vitro and in vivo [44]. Hu et al. [45] developed microrobot actuation and control using optically induced thermocapillary effects. The thermocapillary effect has been used to manipulate gas bubbles in oil using optically induced heating. Lozano et al. [46] demonstrated experimentally the orientational response and phototactic motion of spherical active colloids in an inhomogeneous laser field. Palagi et al. [47] reported that soft microrobots consisting of photoactive liquid-crystal elastomers can be driven by structured monochromatic light to carry out specific motions.
- Catalytic microbotsThe idea of catalytic microbots is to mimic the biological motors able to convert chemical into mechanical energy [18]. Sanchez et al. [48] reported the use of catalytic microbots able to transport cells in a controllable manner and their delivery on desired targets. Moreover, Srivastava et al. [49] successfully used catalytic micro motors for the degradation of nitroaromatic pollutants in water, and other authors such as Dey et al. [50] described the pH taxis of a microbot self-propelled in the presence of an external pH gradient.
- Bio-hybrid microbotsThese kinds of microbots are typically designed to be propelled by bacterial cells by integrating these biological cells into synthetic components [51,52]. On the other hand, these bio-hybrid microbots can also be designed by adding microhelices to artificially motorize biological cells, as, for example, sperm cells [53].
3. Complex Fluids in the Human Body for Blood Flow
4. Fluid Dynamic Performance
4.1. Factors
4.1.1. Size and Shape of Microbots
4.1.2. Complex Fluid Nature, Viscosity and Elasticity
4.1.3. Steady and Unsteady Blood Flows
4.2. Experimental Techniques
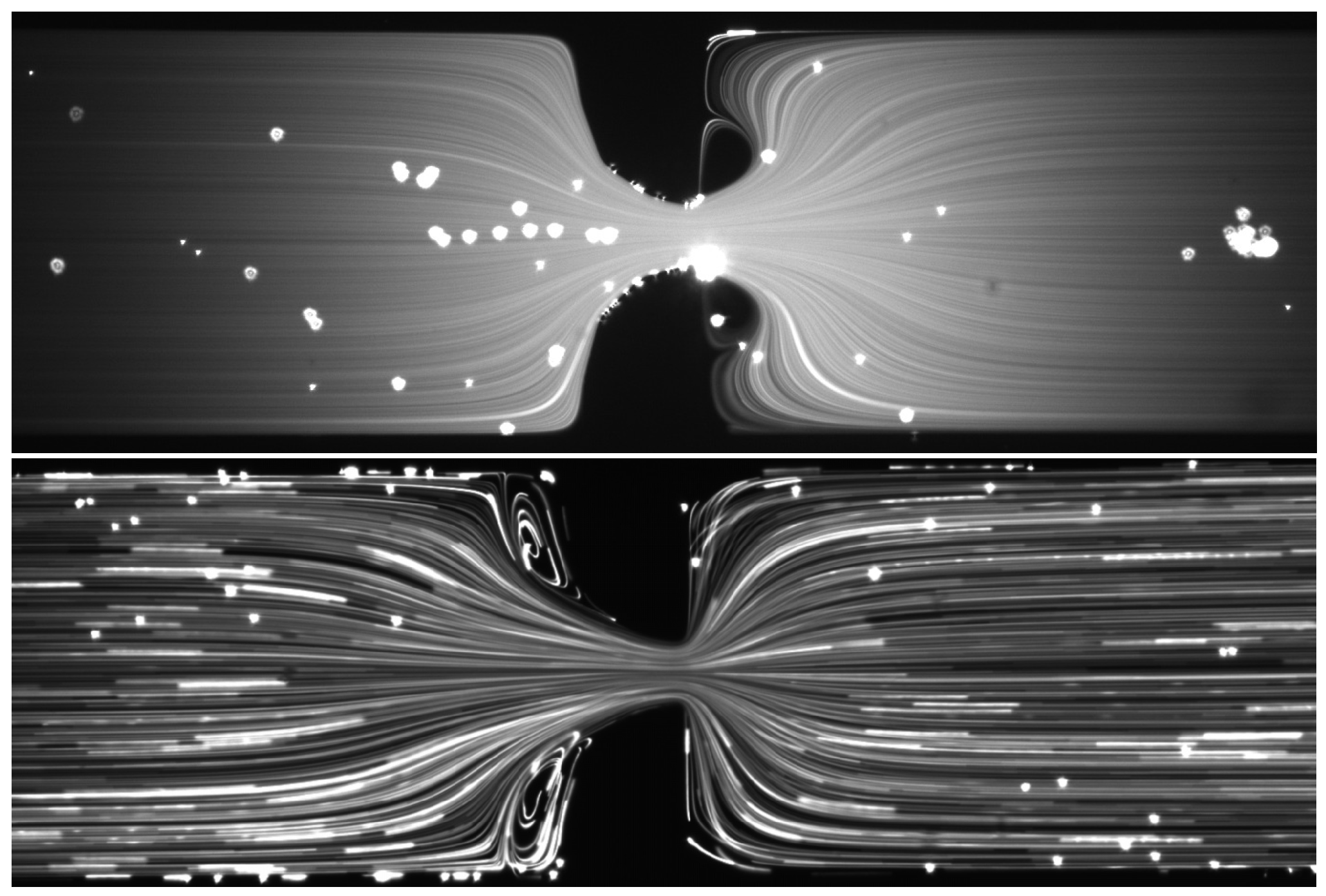
4.2.1. Flow Visualization
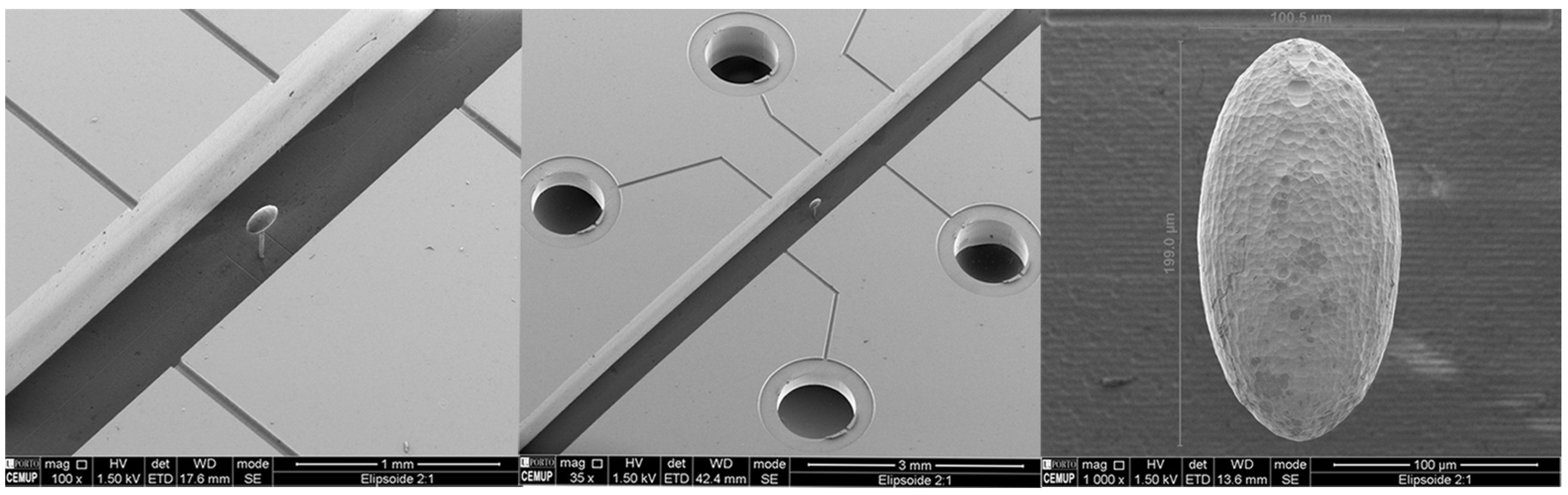
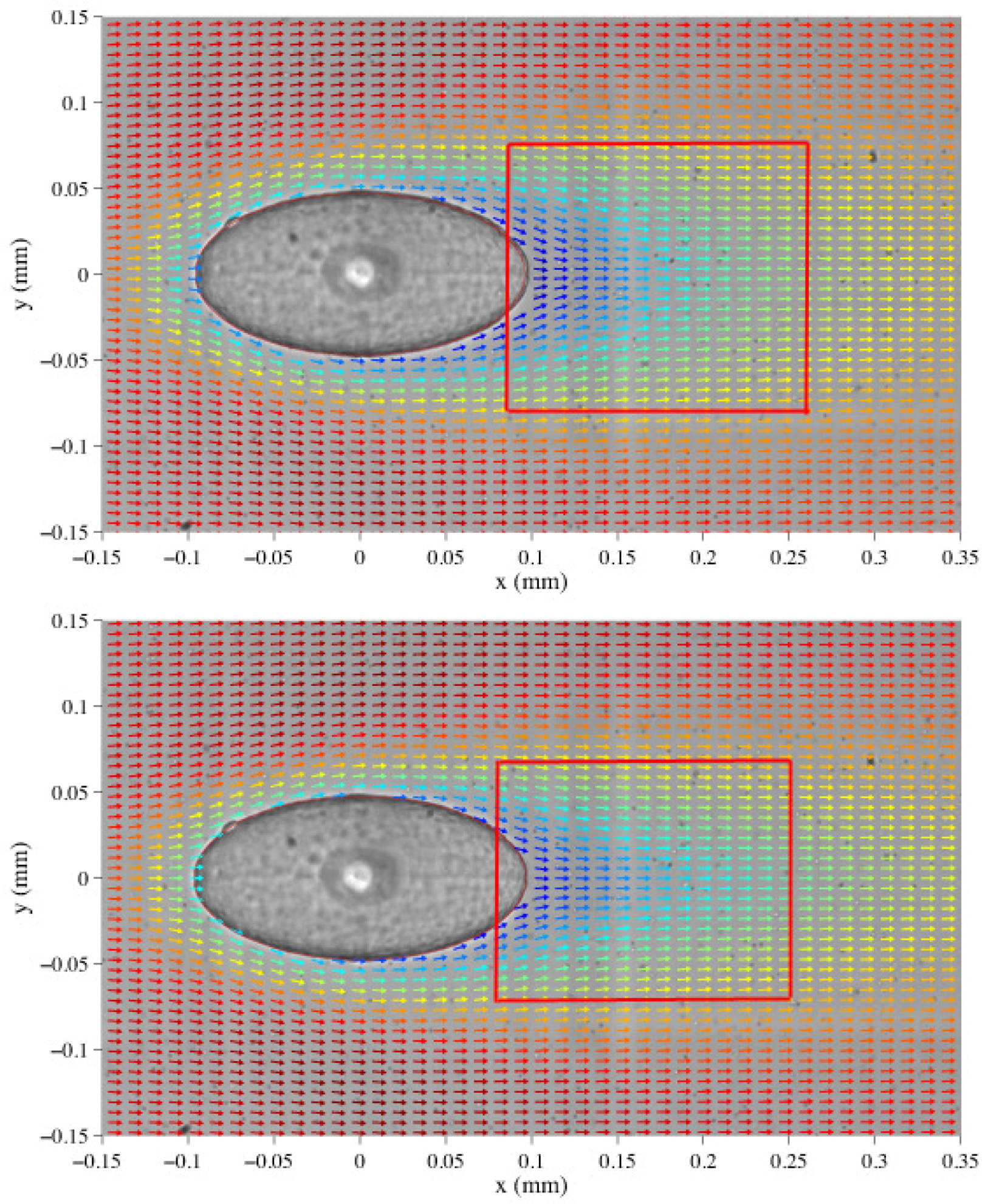
4.2.2. -PIV
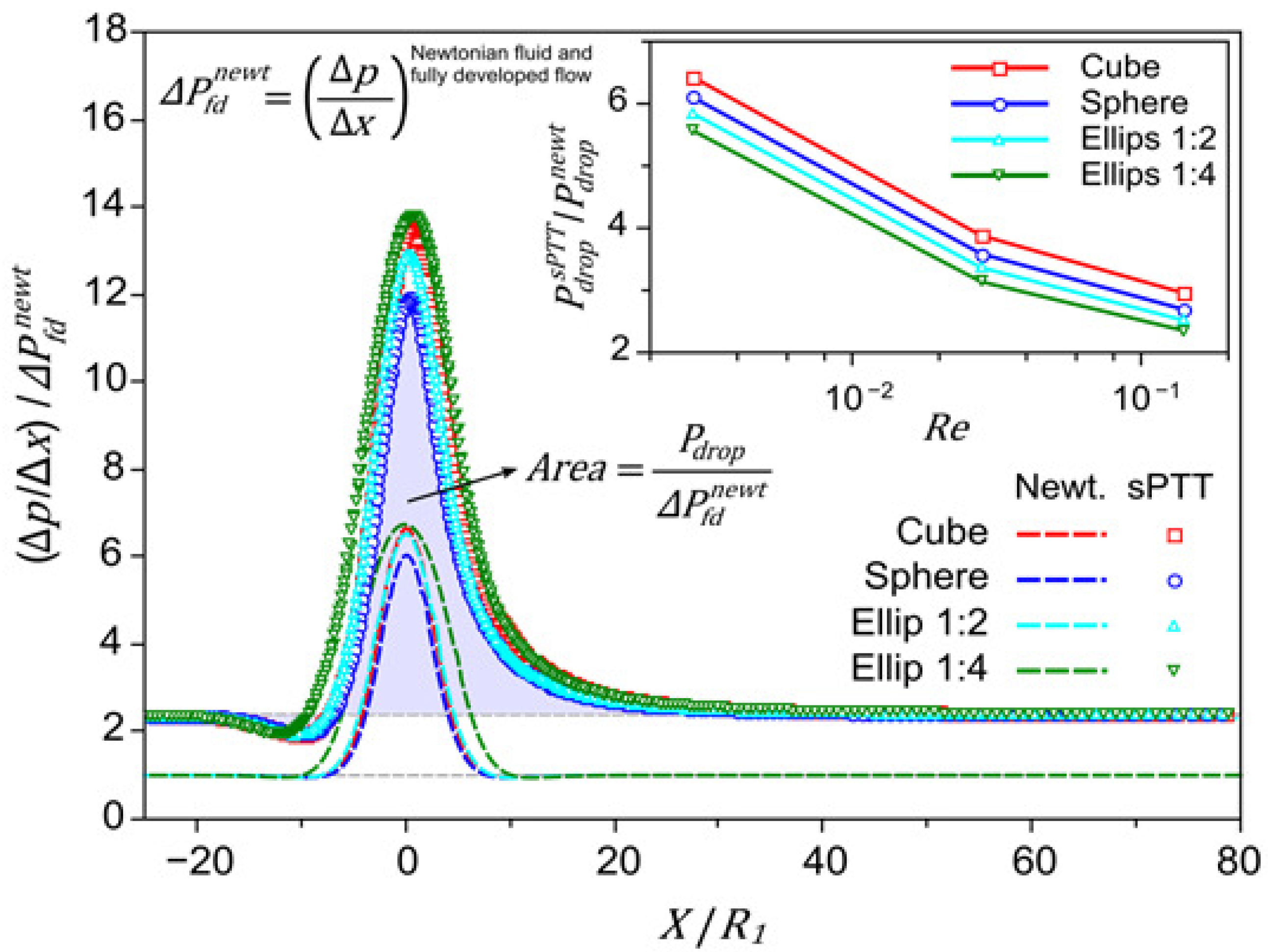
4.2.3. Pressure Drop Measurements
4.2.4. Optical Birefringence
4.3. Comparison of Experimental Techniques
4.4. Performance Assessment
5. Conclusions
Acknowledgments
Conflicts of Interest
Abbreviations
| Re | Reynolds number |
| Wi | Weissemberg number |
| G’ | storage modulus |
| G” | loss modulus |
| -PIV | micro-Particle Image Velocimetry |
| CFD | Computational Fluid Dynamics |
| FSI | Fluid Structure Interaction |
| shear stress | |
| first normal stress difference |
References
- Thurston, G.B. Rheological parameters for the viscosity, viscoelasticity and thixotropy of blood. Biorheology 1979, 16, 149–162. [Google Scholar] [PubMed]
- Dintenfass, L. Blood Rheology in Cardio-vascular Diseases. Nature 1963, 199, 813–815. [Google Scholar] [CrossRef] [PubMed]
- Langstroth, L. Blood Viscosity. I Conditions affecting the viscosity of blood after withdrawal from the body. J. Exp. Med. 1919, 30, 597–606. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.; Usami, S.; Dellenback, R.J.; Gregersen, M.I. Blood viscosity: Influence of erythrocyte deformation. Science 1967, 157, 827–829. [Google Scholar] [CrossRef] [PubMed]
- Chien, S.; Usami, S.; Dellenback, R.J.; Gregersen, M.I.; Nanninga, L.B.; Guest, M.M. Blood viscosity: Influence of erythrocyte aggregation. Science 1967, 157, 829–831. [Google Scholar] [CrossRef] [PubMed]
- Morrison, F.A. Understanding Rheology; Oxford University Press, Inc.: Oxford, UK, 2001. [Google Scholar]
- Galindo-Rosales, F.J.; Campo-Deaño, L.; Sousa, P.C.; Ribeiro, V.M.; Oliveira, M.S.N.; Alves, M.M.; Pinho, F.T. Viscoelastic instabilities in micro-scale flows. Exp. Ther. Fluid Sci. 2014, 59, 128–139. [Google Scholar] [CrossRef] [Green Version]
- Haberzettl, C.A. Nanomedicine: Destination or journey? Nanotechnology 2002, 13, R9–R13. [Google Scholar] [CrossRef]
- Nelson, B.J.; Kaliakatsos, I.K.; Abbott, J.J. Microrobots for minimally invasive medicine. Annu. Rev. Biomed. Eng. 2010, 12, 55–85. [Google Scholar] [CrossRef] [PubMed]
- Takeda, M. Applications of MEMS to Industrial Inspection. In Proceedings of the 14th IEEE International Conference on Micro Electro Mechanical Systems, Interlaken, Switzerland, 21–25 January 2001.
- Ghanbari, A.; Bahrami, M. A Novel Swimming Microrobot Based on Artificial Cilia for Biomedical Applications. J. Intell. Robot. Syst. 2011, 63, 399–416. [Google Scholar] [CrossRef]
- Martel, S. Magnetic Microbots to Fight Cancer; IEEE Spectrum: New York, NY, USA, 2012. [Google Scholar]
- Kummer, M.P.; Abbott, J.J.; Dinser, S.; Nelson, B.J. Artificial Vitreous Humor for in vitro experiments. In Proceedings of the 29th Annual International Conference of the IEEE EMBS Cite Internationale, Lyon, France, 23–26 August 2007.
- Ergeneman, O.; Chatzipirpiridis, G.; Pokki, J.; Marin-Suarez, M.; Sotiriou, G.A.; Medina-Rodriguez, S.; Sanchez, J.F.; Fernandez-Gutierrez, A.; Pane, S.; Nelson, B.J. In vitro oxygen sensing using intraocular microrobots. IEEE Trans. Biomed. Eng. 2012, 59, 3014–3109. [Google Scholar] [CrossRef] [PubMed]
- Peyer, K.E.; Siringil, E.C.; Zhang, L.; Suter, M.; Nelson, B.J. Bacteria-inspired magnetic polymer composite microrobots. Lect. Notes Comput. Sci. 2013, 8064, 216–227. [Google Scholar]
- Zeeshan, M.; Grisch, R.; Pellicer, E.; Sivaraman, K.M.; Peyer, K.E.; Sort, J.; Ozkale, B.; Sakar, M.S.; Nelson, B.J.; Pane, S. Hybrid helical magnetic microrobots obtained by 3D template-assisted electrodeposition. Small 2014, 10, 1284–1288. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Dey, K.K.; Chattopadhyay, A.; Mandal, T.K.; Bandyopadhyay, D. Multimodal chemo-magnetic control of self-propelling microbots. Nanoscale 2014, 6, 1398. [Google Scholar] [CrossRef] [PubMed]
- Solovev, A.A.; Sanchez, S.; Pumera, M.; Mei, Y.F.; Schmidt, O.G. Magnetic control of tubular catalytic microbots for the transport, assembly, and delivery of micro-objects. Adv. Funct. Mater. 2010, 20, 2430–2435. [Google Scholar] [CrossRef]
- Fountain, T.W.R.; Kailat, P.V.; Abbott, J.J. Wireless control of magnetic helical microrobots using a rotating-permanent-magnet manipulator. In Proceedings of the IEEE International Conference on Robotics and Automation, Anchorage, AK, USA, 3–7 May 2010.
- Abbott, J.J.; Peyer, K.E.; Dong, L.X.; Nelson, B.J. How should microrobots swim? Springer Tracts Adv. Robot. 2011, 66, 157–167. [Google Scholar]
- Belharet, K.; Folio, D.; Ferreira, A. Three-dimensional controlled motion of a microrobot using magnetic gradients. Adv. Robot. 2011, 25, 1069–1083. [Google Scholar] [CrossRef]
- Palagi, S.; Jager, E.W.H.; Mazzolai, B.; Beccai, L. Propulsion of swimming microrobots inspired by metachronal waves in ciliates: from biology to material specifications. Bioinspir. Biomim. 2013, 8, 046004. [Google Scholar] [CrossRef] [PubMed]
- Sharafi, A.; Olamaei, N.; Martel, S. A new communication method for untethered intelligent microrobots. In Proceedings of the IEEE/ASME International Conference on Advanced Intelligent Mechatronics (AIM), Wollongong, Australia, 9–12 July 2013.
- Corradi, P.; Scholz, O.; Knoll, T.; Menciassi, A.; Dario, P. An optical system for communication and sensing in millimeter-sized swarming microrobots. J. Micromech. Microeng. 2009, 19, 015022. [Google Scholar] [CrossRef]
- Boillot, N.; Dhoutaut, D.; Bourgeois, J. Using nano-wireless communications in micro-robots applications. In Proceedings of the NANOCOM 2014, 1st ACM International Conference on Nanoscale Computing and Communication, Atlanta, GA, USA, 6–9 May 2014.
- Taylor, G.I. Analysis of the swimming of microscopic organisms. Proc. R. Soc. Lond. A 1951, 209, 447–461. [Google Scholar] [CrossRef]
- Berg, H.C.; Anderson, A.R. Bacteria swim by rotating their flagellar filaments. Nature 1973, 245, 380–382. [Google Scholar] [CrossRef] [PubMed]
- Peyer, K.E.; Zhang, L.; Nelson, B.J. Bio-inspired magnetic swimming microrobots for biomedical applications. Nanoscale 2013, 5, 1259–1272. [Google Scholar] [CrossRef] [PubMed]
- Mellal, L.; Belharet, K.; Folio, D.; Ferreira, A. Optimal structure of particles-based super paramagnetic micro robots: Application to MRI guided targeted drug therapy. J. Nanopart. Res. 2015, 17, 2–18. [Google Scholar] [CrossRef]
- Qiu, T.; Lee, T.C.; Mark, A.G.; Morozov, K.I.; Munster, R.; Mierka, O.; Turek, S.; Leshansky, A.M.; Fischer, P. Swimming by reciprocal motion at low Reynolds number. Nat. Commun. 2014, 4, 5119. [Google Scholar] [CrossRef] [PubMed]
- Walker, D.; Kubler, M.; Morozov, K.I.; Fischer, P.; Leshansky, A.M. Optimal length of low Reynolds Number nano propellers. Nano Lett. 2015, 15, 4412–4416. [Google Scholar] [CrossRef] [PubMed]
- Venugopalan, P.L.; Sai, R.; Chandorkar, Y.; Basu, B.; Shivashankar, S.; Ghosh, A. Conformal Cytocompatible Ferrite Coatings Facilitate the Realization of a Nanovoyager in Human Blood. Nano Lett. 2014, 14, 1968–1975. [Google Scholar] [CrossRef] [PubMed]
- Schamel, D.; Mark, A.G.; Gibbs, J.G.; Miksch, C.; Morozov, K.I.; Leshansky, A.M.; Fischer, P. Nanopropellers and their actuation in complex viscoelastic media. ACS Nano 2014, 8, 8794–8801. [Google Scholar] [CrossRef] [PubMed]
- Temel, F.Z.; Yesilyurt, S. Simulation-based analysis of micro-robots swimming at the center and near the wall of circular mini-channels. Microfluid. Nanofluid. 2013, 14, 287–298. [Google Scholar] [CrossRef] [Green Version]
- Temel, F.Z.; Yesilyurt, S. Confined swimming of bio-inspired microrobots in rectangular channels. Bioinspir. Biomim. 2015, 10, 016015. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Chen, C.; Khaligh, A. An ultra compact dual-stage converter for driving electrostatic actuators in mobile microrobots. IEEE Trans. Power Electron. 2014, 29, 2991–3000. [Google Scholar] [CrossRef]
- Donald, B.R.; Levey, C.G.; McGray, C.D.; Paprotny, I.; Rus, D. An untethered, electrostatic, globally controllable MEMS micro-robot. J. Microelectromech. Syst. 2006, 15, 1–15. [Google Scholar] [CrossRef]
- Graule, M.A.; Chirarattananon, P.; Fuller, S.B.; Jafferis, N.T.; Ma, K.Y.; Spenko, M.; Kornbluh, R.; Wood, R.J. Perching and Takeoff of a robotic insect on overhangs using switchable electrostatic adhesion. Science 2016, 352, 978–982. [Google Scholar] [CrossRef] [PubMed]
- Pawashe, C.; Floyd, S.; Sitti, M. Multiple magnetic micro robot control using electrostatic anchoring. Appl. Phys. Lett. 2009, 94, 164108. [Google Scholar] [CrossRef]
- Mahoney, A.W.; Abbott, J.J. Five-degree-of-freedom manipulation of an untethered magnetic device in fluid using a single permanent magnet with application in stomach cap sue endoscopy. Int. J. Robot. Res. 2016, 35, 129–147. [Google Scholar] [CrossRef]
- Diller, E.; Giltinan, J.; Sitti, M. Independent control of multiple magnetic micro robots in three dimensions. Int. J. Robot. Res. 2013, 32, 614–631. [Google Scholar] [CrossRef]
- Diller, E.; Giltinan, J.; Lum, G.Z.; Ye, Z.; Sitti, M. Six-degree-of-freedom magnetic actuation for wireless micro robotics. Int. J. Robot. Res. 2016, 35, 114–128. [Google Scholar] [CrossRef]
- Floyd, S.; Diller, E.; Pawashe, C.; Sitti, M. IControl methodologies for a heterogeneous group of untethered magnetic micro-robots. Int. J. Robot. Res. 2011, 30, 1553–1565. [Google Scholar] [CrossRef]
- Chowdhury, S.; Jing, W.; Cappelleri, D.J. Controlling multiple micro robots: Recent progress and future challenges. J. Micro-Bio Robot. 2015, 10, 1–11. [Google Scholar] [CrossRef]
- Hu, W.; Ishii, K.S.; Ohta, A.T. Micro-assembly using optically controlled bubble microrobots. Appl. Phys. Lett. 2011, 99, 094103. [Google Scholar] [CrossRef]
- Lozano, C.; Hagen, B.T.; Löwen, H.; Bechinher, C. Phototaxis of synthetic micro swimmers in optical landscapes. Nat. Commun. 2016, 7, 12828. [Google Scholar] [CrossRef]
- Palagi, S.; Mark, A.G.; Reigh, S.Y.; Melde, K.; Qiu, T.; Zeng, H.; Parmeggiani, C.; Martella, D.; Sanchez-Castillo, A.; Kapernaum, N.; et al. Structured light enables biomimetic swimming and versatile locomotion of photo responsive soft microrobots. Nat. Mater. 2016, 15, 647–653. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, S.; Solovev, A.A.; Schulze, S.; Schmidt, O.G. Controlled manipulation of multiple cells using catalytic microbots. Chem. Commun. 2011, 47, 698–700. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.K.; Guix, M.; Schmidt, O.G. Wastewater mediated activation of micro motors for efficient water cleaning. Nano Lett. 2016, 16, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Dey, K.K.; Bhandari, S.; Bandyopadhyay, D.; Basu, S.; Chattopadhyay, A. The pH taxis of an intelligent catalytic microbot. Small 2013, 9, 1916–1920. [Google Scholar] [CrossRef] [PubMed]
- Carlsen, R.W.; Edwards, M.R.; Zhuang, J.; Pacoret, C.; Sitti, M. Magnetic steering control of multi-cellular bio-hybrid microswimmers. Lab Chip 2014, 14, 3850. [Google Scholar] [CrossRef] [PubMed]
- Williams, B.J.; Anand, S.V.; Rajagopalan, J.; Saif, M.T.A. A self-propelled biohybrid swimmer at low Reynolds number. Nat. Commun. 2014, 5, 3081. [Google Scholar] [CrossRef] [PubMed]
- Medina-Sánchez, M.; Schwarz, L.; Meyer, A.K.; Hebenstreit, F.; Schmidt, O.G. Cellular cargo delivery: Toward assisted fertilization by sperm-carrying micromotors. Nano Lett. 2016, 16, 555–561. [Google Scholar] [CrossRef] [PubMed]
- Campo-Deaño, L.; Galindo-Rosales, F.J.; Pinho, F.T.; Alves, M.M.; Oliveira, M.S.N. Flow of low viscosity Boger fluids through a microfluidic hyperbolic contraction. J. Non-Newton. Fluid Mech. 2011, 166, 1286–1296. [Google Scholar] [CrossRef] [Green Version]
- Galindo-Rosales, F.J.; Campo-Deaño, L.; Pinho, F.T.; van Bokhorst, E.; Hamersma, P.J.; Oliveira, M.S.N.; Alves, M.M. Microfluidic system for the analysis of viscoelastic effects in flow through porous media. Microfluid. Nanofluid. 2012, 12, 485–498. [Google Scholar] [CrossRef] [Green Version]
- Sousa, P.C.; Pinho, F.T.; Alves, M.A.; Oliveira, M.S.N. A review of hemorheology: measuring techniques and recent advances. Korea-Aust. Rheol. J. 2016, 28, 1–22. [Google Scholar] [CrossRef] [Green Version]
- Campo-Deaño, L.; Oliveira, M.S.N.; Pinho, F.T. A review of computational hemodynamics in middle cerebral aneurysms and rheological models for blood flow. Appl. Mech. Rev. 2015, 67, 030801. [Google Scholar] [CrossRef]
- Chen, D.T.N. Microrheology of Soft Matter. Ph.D. Thesis, University of Pennsylvania, Philadelphia, PA, USA, 2010. [Google Scholar]
- Schultz, K.M.; Furst, E.M. Microrheology of biomaterial hydrogelators. Soft Matter 2012, 8, 6198–6205. [Google Scholar] [CrossRef]
- Campo-Deaño, L.; Dullens, R.P.A.; Aarts, D.G.L.A.; Pinho, F.T.; Oliveira, M.S.N. Viscoelasticity of blood and viscoelastic blood analogues for use in polydymethylsiloxane in vitro models of the circulatory system. Biomicrofluidics 2013, 7, 034102. [Google Scholar] [CrossRef] [PubMed]
- Jeong, H.H.; Mark, A.G.; Lee, T.C.; Alarcón-Correa, M.; Eslami, S.; Qiu, T.; Gibbs, J.G.; Fischer, P. Active Nanorheology with Plasmonics. Nano Lett. 2016, 16, 4887–4894. [Google Scholar] [CrossRef] [PubMed]
- Landau, L.D.; Akhiezer, A.I.; Lifshitz, E.M. General Physics: Mechanics and Molecular Physics; Pergamon Press: Oxford, UK, 1967. [Google Scholar]
- Martínez-Aranda, S.; Galindo-Rosales, F.J.; Campo-Deaño, L. Complex Fluid Dynamics of Swimming Microbots; Flowing Matter 2014: Lisboa, Portugal, 2014. [Google Scholar]
- Coelho, P.M.; Pinho, F.T. Vortex shedding in cylinder flow of shear-thinning fluids III. Pressure measurements. J. Non-Newton. Fluid Mech. 2004, 121, 55–68. [Google Scholar]
- Coelho, P.M.; Pinho, F.T. Vortex shedding in cylinder flow of shear-thinning fluids II. Flow characteristics. J. Non-Newton. Fluid Mech. 2003, 110, 177–193. [Google Scholar] [CrossRef]
- Coelho, P.M.; Pinho, F.T. Vortex shedding in cylinder flow of shear-thinning fluids I. Identification and demarcation of flow regimes. J. Non-Newton. Fluid Mech. 2003, 110, 143–176. [Google Scholar] [CrossRef]
- Sousa, P.C.; Pinho, F.T.; Oliveira, M.S.N.; Alves, M.M. Purely elastic flow instabilities in micro scale cross-slot devices. Soft Matter 2015, 11, 8856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berg, H.C.; Turner, L. Movement of microorganisms in viscous environments. Nature 1979, 278, 349–351. [Google Scholar] [CrossRef] [PubMed]
- Kimsey, R.B.; Spielman, A. Motility of Lyme Disease Spirochetes in Fluids as Viscous as the Extracellular Matrix. J. Infect. Dis. 1990, 162, 1205–1208. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.N.; Arratia, P.E. Undulatory Swimming in Viscoelastic Fluids. Phys. Rev. Lett. 2011, 106, 208101. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Lauga, E.; Brandt, L. Self-propulsion in viscoelastic fluids: Pushers vr. pullers. Phys. Fluids 2012, 24, 051902. [Google Scholar] [CrossRef]
- Dasgupta, M.; Liu, B.; Fu, H.C.; Berhanu, M.; Breuer, K.S.; Powers, T.R.; Kudrolli, A. Speed of a swimming sheet in Newtonian and viscoelastic fluids. Phys. Rev. E 2013, 87, 013015. [Google Scholar] [CrossRef] [PubMed]
- Riley, E.E.; Lauga, E. Small-amplitude swimmers can self-propel faster in viscoelastic fluids. J. Theor. Biol. 2015, 382, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Berger, S.A.; Goldsmith, W.; Lewis, E.R. Introduction to Bioengineering; Oxford University Press: Oxford, UK, 1996. [Google Scholar]
- Ku, D.N. Blood Flow in Arteries. Annu. Rev. Fluid Mech. 1997, 29, 399–434. [Google Scholar] [CrossRef]
- Womersley, J.R. Method for the calculation of velocity, rate of flow and viscous drag in arteries when the pressure gradient is known. J. Physiol. 1955, 127, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Aranda, S.; Galindo-Rosales, F.J.; Campo-Deaño, L. Complex flow dynamics around 3D microbot prototypes. Soft Matter 2016, 12, 2334. [Google Scholar] [CrossRef] [PubMed]
- Santiago, J.G.; Werely, S.T.; Meinhart, C.D.; Beebe, D.J.; Adrian, R.J. A particle image velocimetry system for microfluidics. Exp. Fluids 1998, 25, 316–319. [Google Scholar] [CrossRef]
- Meinhart, C.D.; Werely, S.T.; Gray, M.H.B. Volume illumination for two-dimensional particle image velocimetry. Meas. Sci. Technol. 2000, 11, 809. [Google Scholar] [CrossRef]
- Martínez-Aranda, S.; Galindo-Rosales, F.J.; Campo-Deaño, L. Blood flow dynamics around bioinspired microbots. In Proceedings of the 6th Iberian Meeting on Colloids and Interfaces (RICI6), Guimarẽs, Portugal, 8–10 July 2015.
- Martínez-Aranda, S.; Galindo-Rosales, F.J.; Campo-Deaño, L. Numerical Study on the Influence of the Swimming Microbot’s Morphology in Human Blood Flow. In Proceedings of the Annual Meeting on Rheology of the Society of Rheology, Philadelphia, PA, USA, 5–9 October 2014.
- Mitsoulis, E. Numerical simulation of confined flow of polyethylene melts around a cylinder in a planar channel. J. Non-Newton. Fluid Mech. 1998, 76, 327–350. [Google Scholar] [CrossRef]
- Takahashi, T.; Fuller, G. Stress tensor measurement using birefringence in oblique transmission. Rheol. Acta 1996, 35, 297–302. [Google Scholar] [CrossRef]
- Larson, R.G.; Khan, S.A.; Raju, V.R. Relaxation of Stress and Birefringence in Polymers of High Molecular Weight. J. Rheol. 1988, 32, 145–161. [Google Scholar] [CrossRef]
- Jensen, K.E.; Szabo, P.; Okkels, F. Topology optimization of viscoelastic rectifiers. Appl. Phys. Lett. 2012, 100, 234102. [Google Scholar] [CrossRef] [Green Version]
- Galindo-Rosales, F.J.; Oliveira, M.S.N.; Alves, M.M. Optimized cross-slot microdevices for homogeneous extension. RSC Adv. 2014, 4, 7799–7804. [Google Scholar] [CrossRef] [Green Version]


| Vessel | Inner Diameter (mm) | Blood Velocity (mm/s) | Reynolds Number |
|---|---|---|---|
| Aorta | 25 | 400 | 3000 |
| Vena cava | 30 | 50–300 | 3000 |
| Medium artery | 4 | 100–400 | 500 |
| Vein | 5 | 3–50 | 150 |
| small arteriole | 0.03 | 1–100 | 0.7 |
| Venule | 0.02 | <3 | 0.01 |
| Capillary | 0.008 | <1 | 0.002 |
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campo-Deaño, L. Assessing the Dynamic Performance of Microbots in Complex Fluid Flows. Appl. Sci. 2016, 6, 410. https://doi.org/10.3390/app6120410
Campo-Deaño L. Assessing the Dynamic Performance of Microbots in Complex Fluid Flows. Applied Sciences. 2016; 6(12):410. https://doi.org/10.3390/app6120410
Chicago/Turabian StyleCampo-Deaño, Laura. 2016. "Assessing the Dynamic Performance of Microbots in Complex Fluid Flows" Applied Sciences 6, no. 12: 410. https://doi.org/10.3390/app6120410






