Electricity Production from Marine Water by Sulfide-Driven Fuel Cell
Abstract
:Featured Application
Abstract
1. Introduction
2. Experimental Part
2.1. Methodology
- No discharge of any toxic or environmentally unfriendly waste into the sea. Therefore, no use of chemical oxidizers for the cathode processes (e.g., hexacyanoferrate, etc.) instead of oxygen is admissible;
- No use of any other supporting electrolytes but marine water (no sodium or potassium hydroxides);
- Use of HS− or S2− as substrates only;
- No metal parts in the fuel cell design;
- Metal electrodes are undesired.
- i—electric current, A;
- m—mass of reacting substance, g;
- t—time, s;
- M—molar mass of reacting substance, g;
- n—number of exchanged electrons;
- F = 96,484 C mol−1, Faraday constant.
- Activation losses, expressed by the measured overpotential at the electrochemical reactions;
- Concentration (or mass transfer) losses;
- Losses due to incomplete conversion of reactants to products;
- Ohmic losses and;
- Losses due to fuel crossover and internal current.
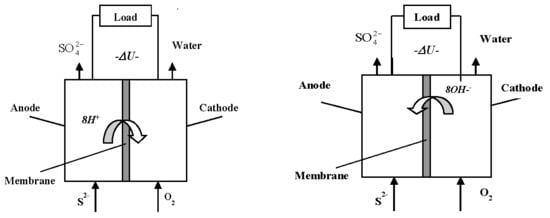
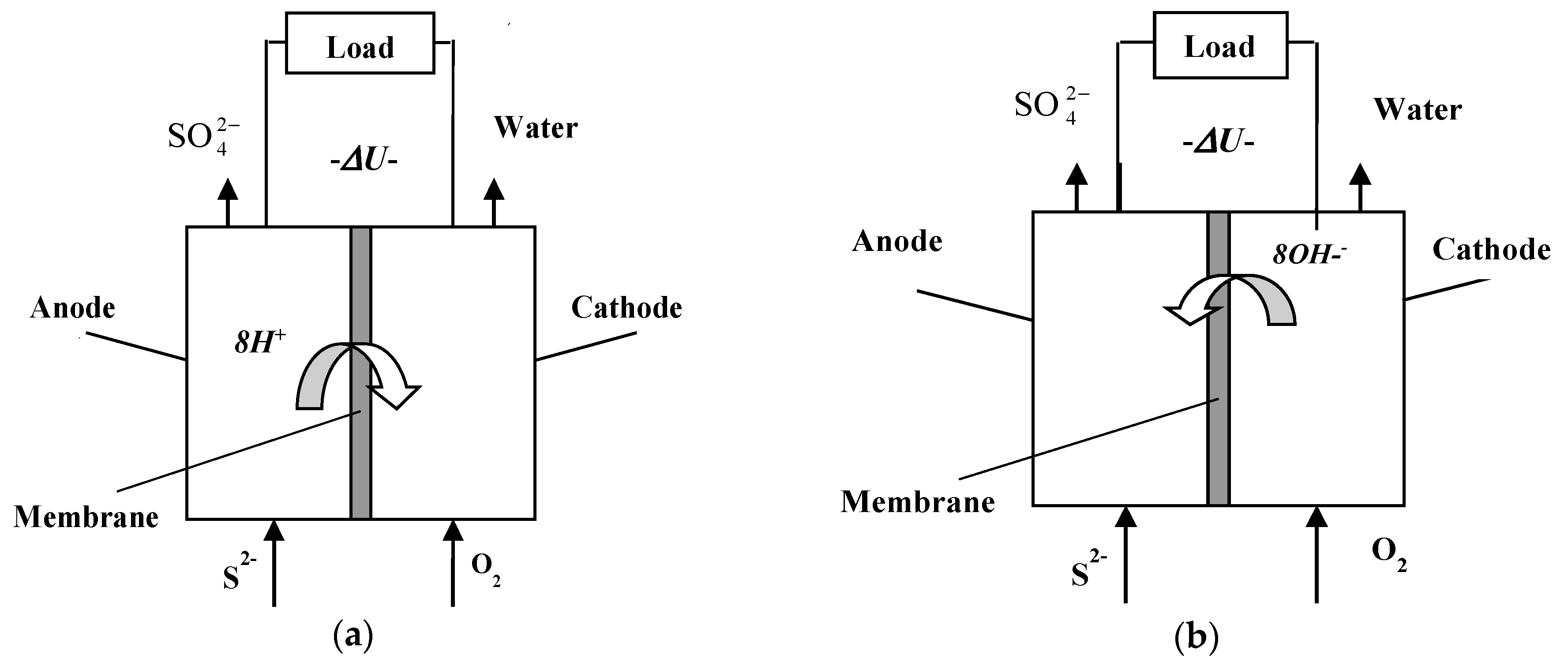
2.2. Fuel Cell Design
2.3. Experimental Conditions
2.4. Chemicals
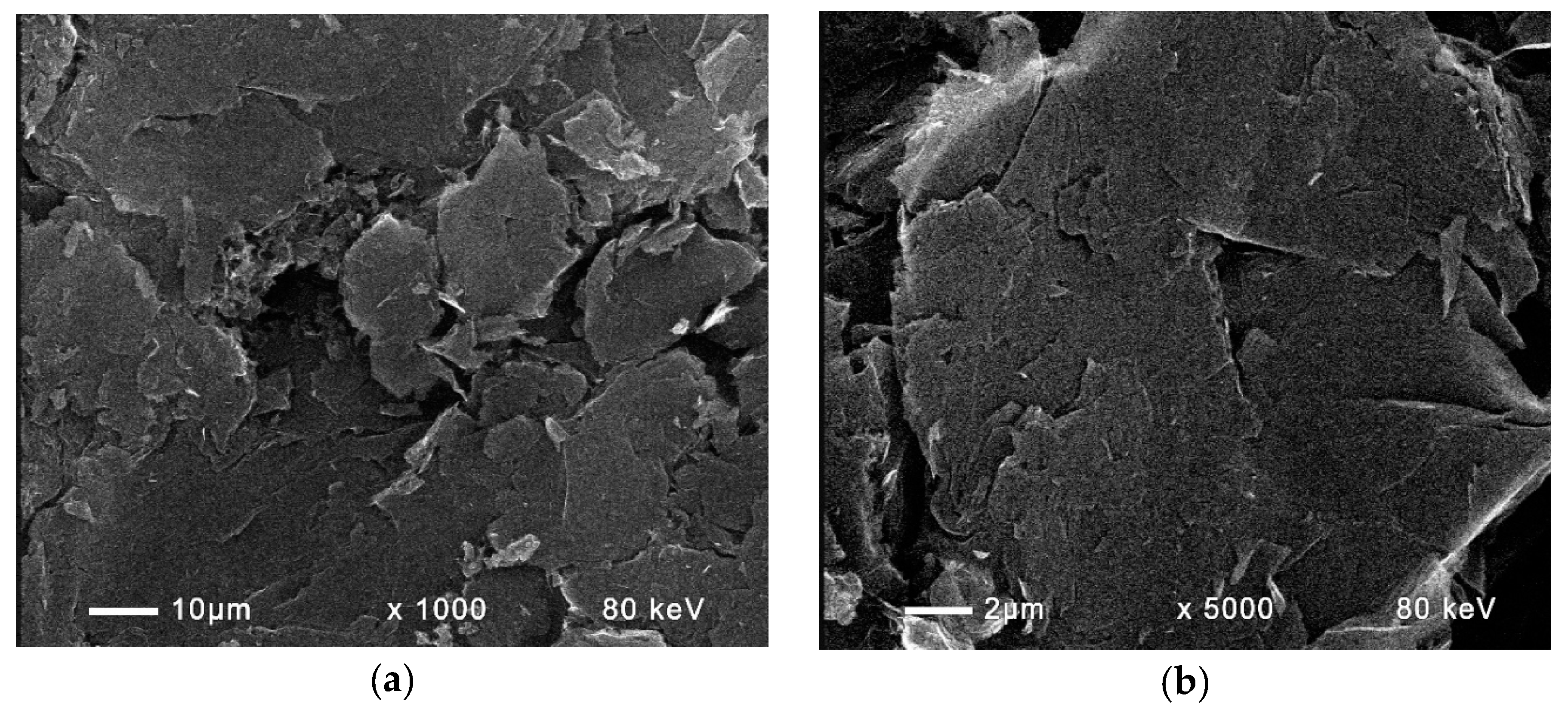
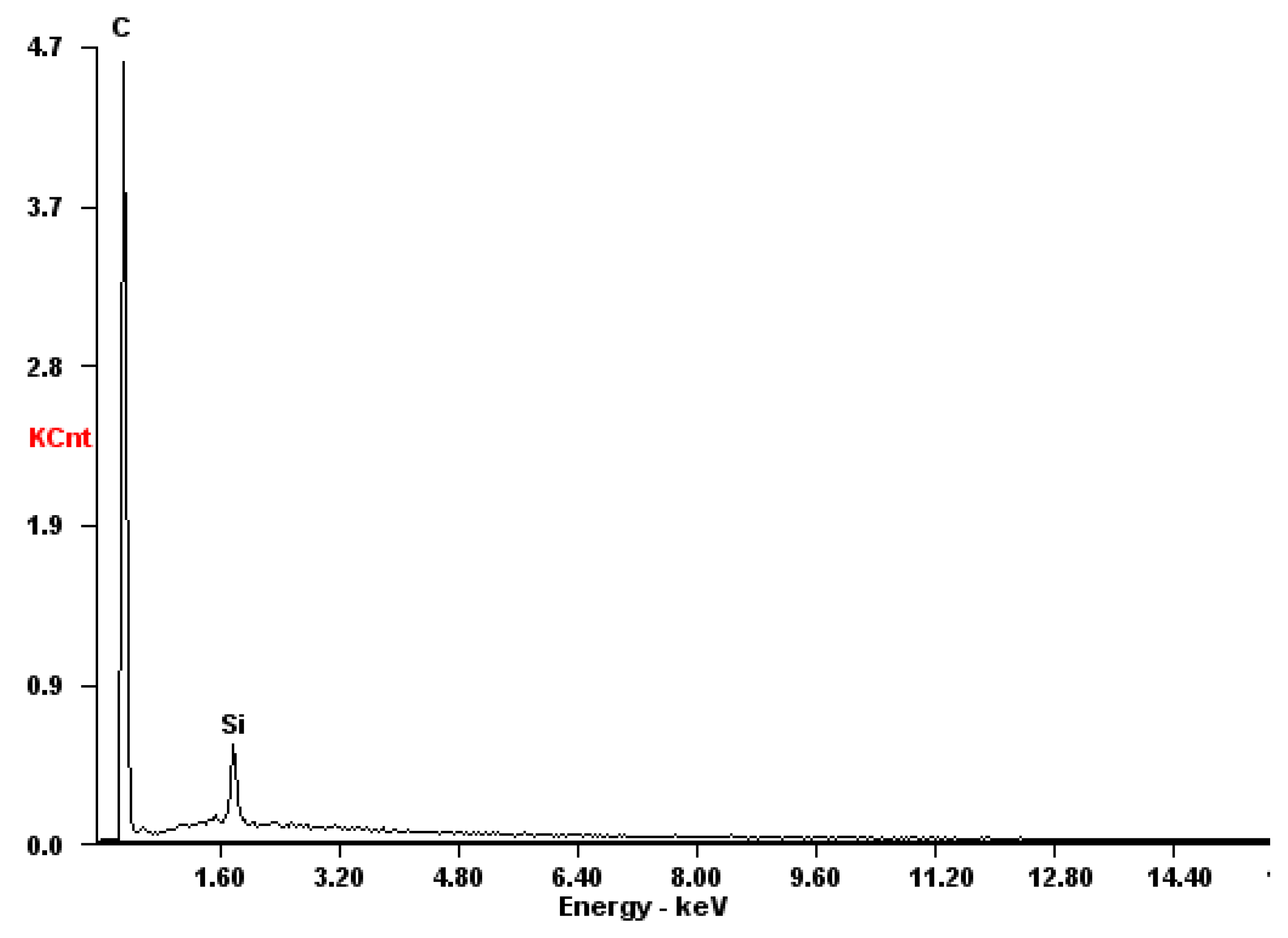
2.5. Analyses
3. Results and Discussion
3.1. Initial Sulfide Concentrations
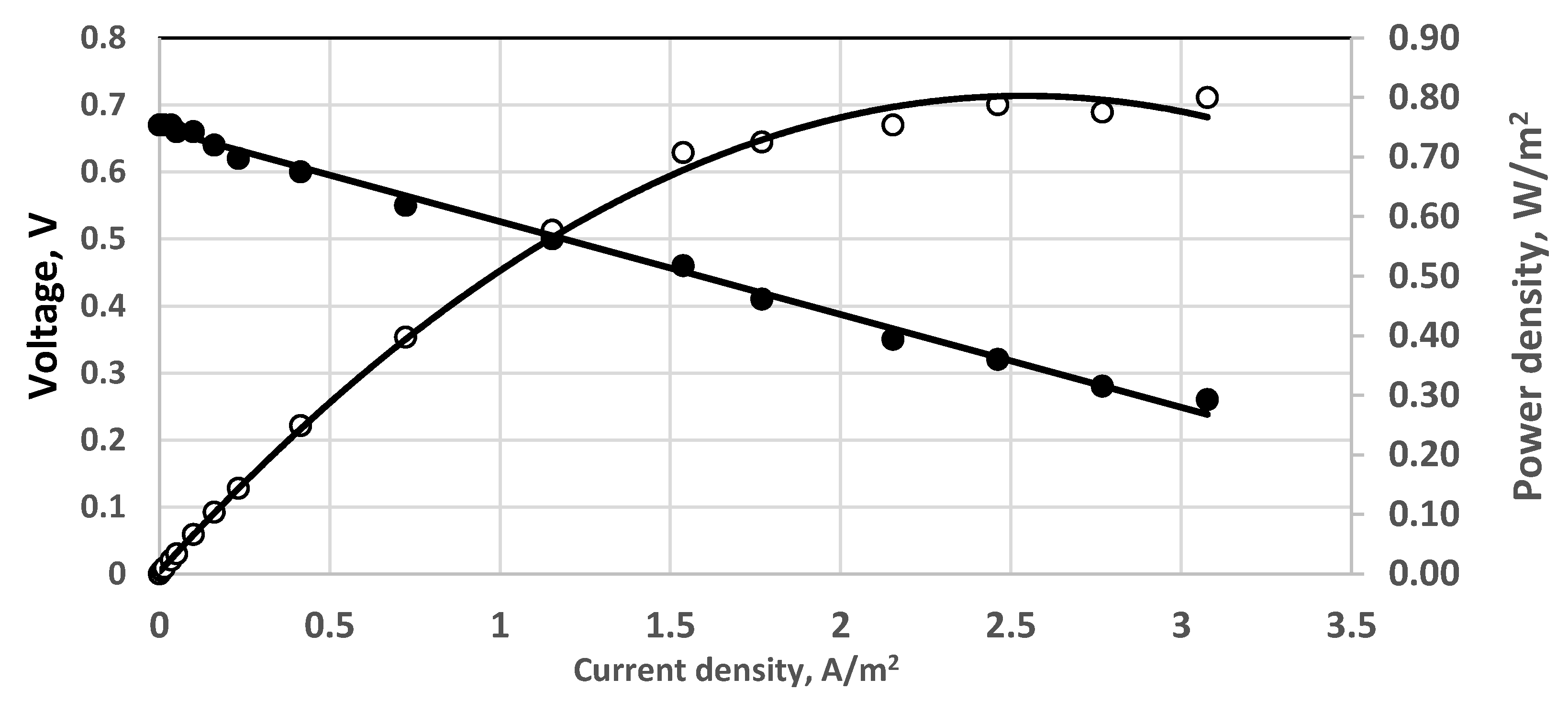
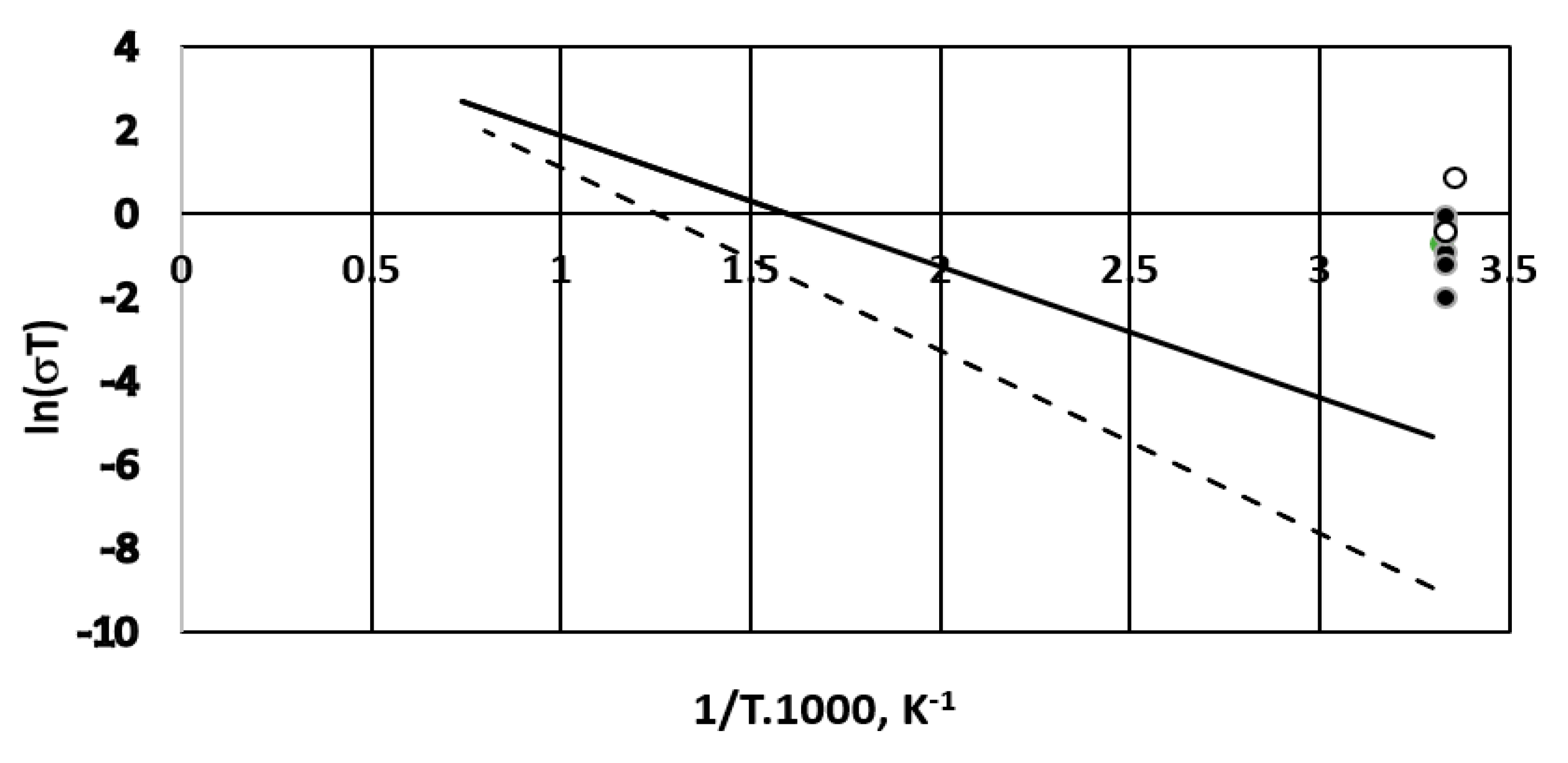
3.2. Overpotential Measurements
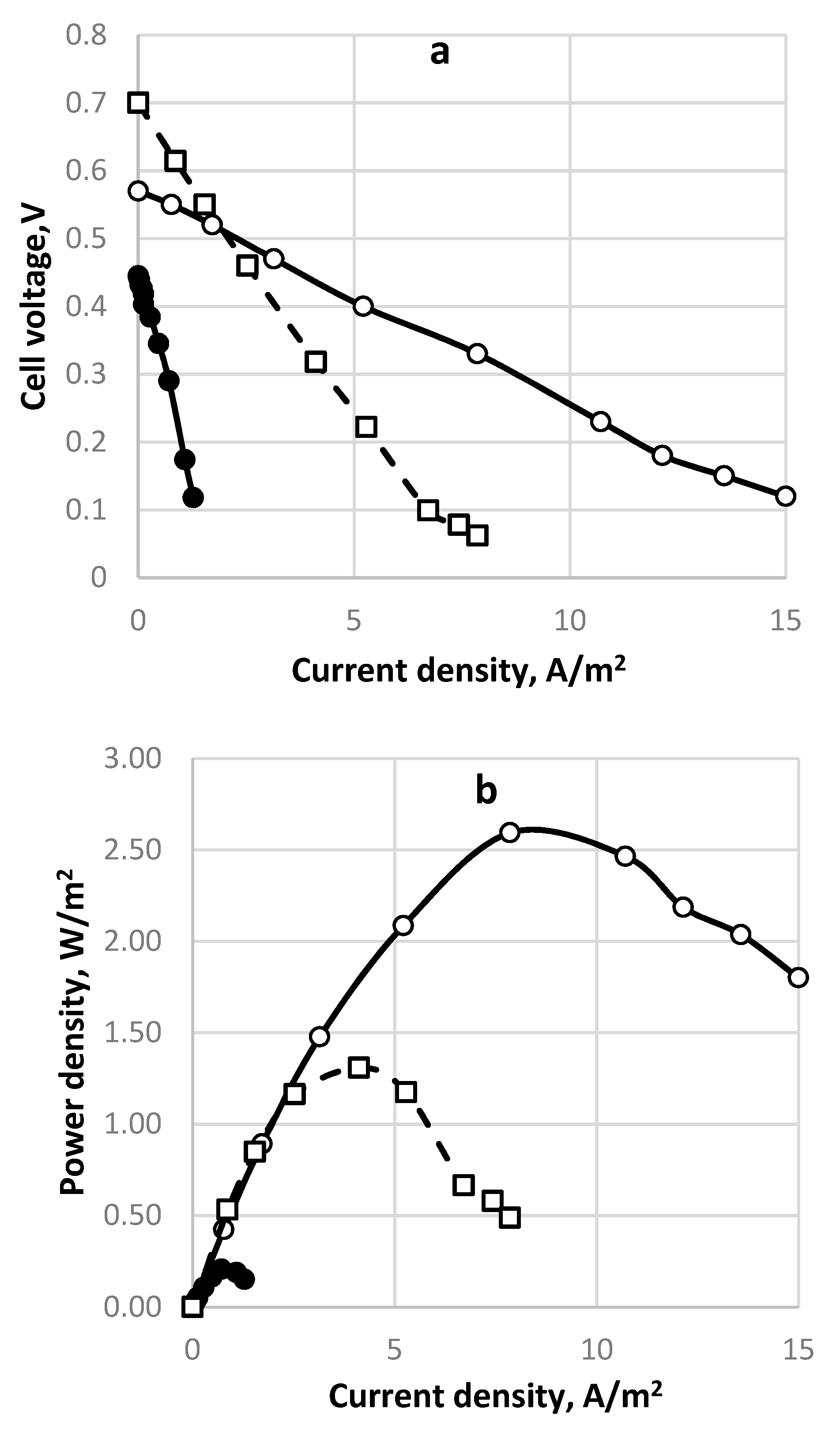
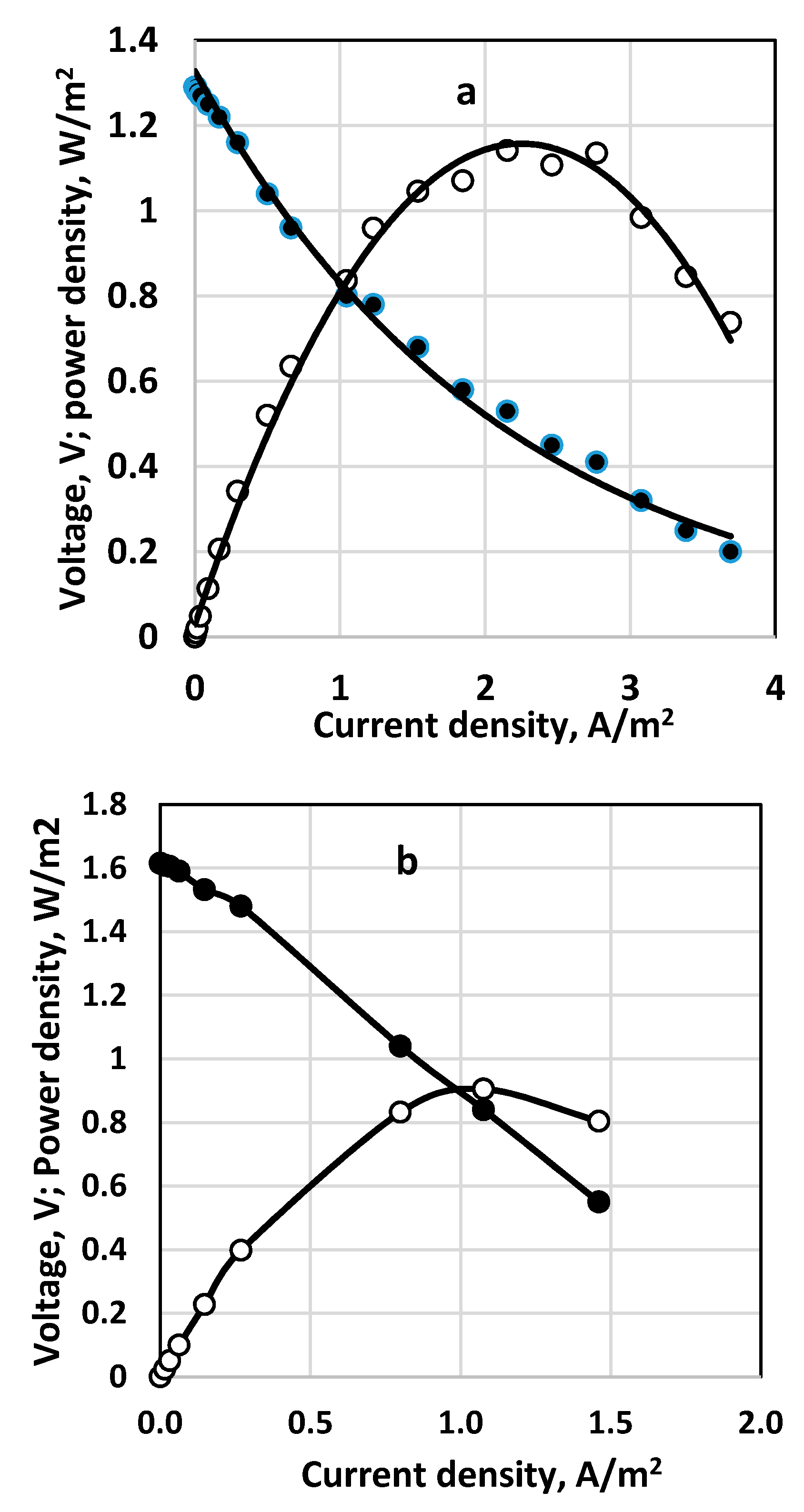
3.3. Effect of Aeration Type
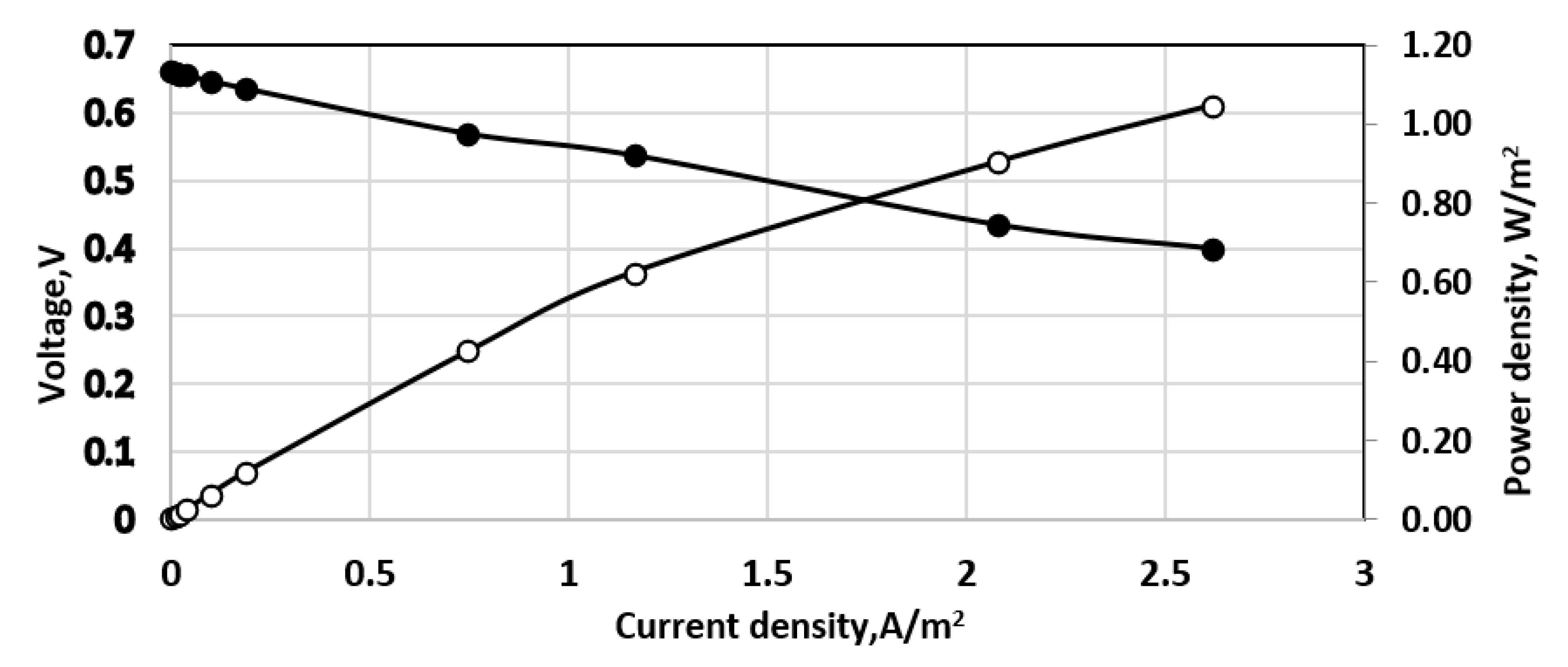
3.4. Study with Different Membranes
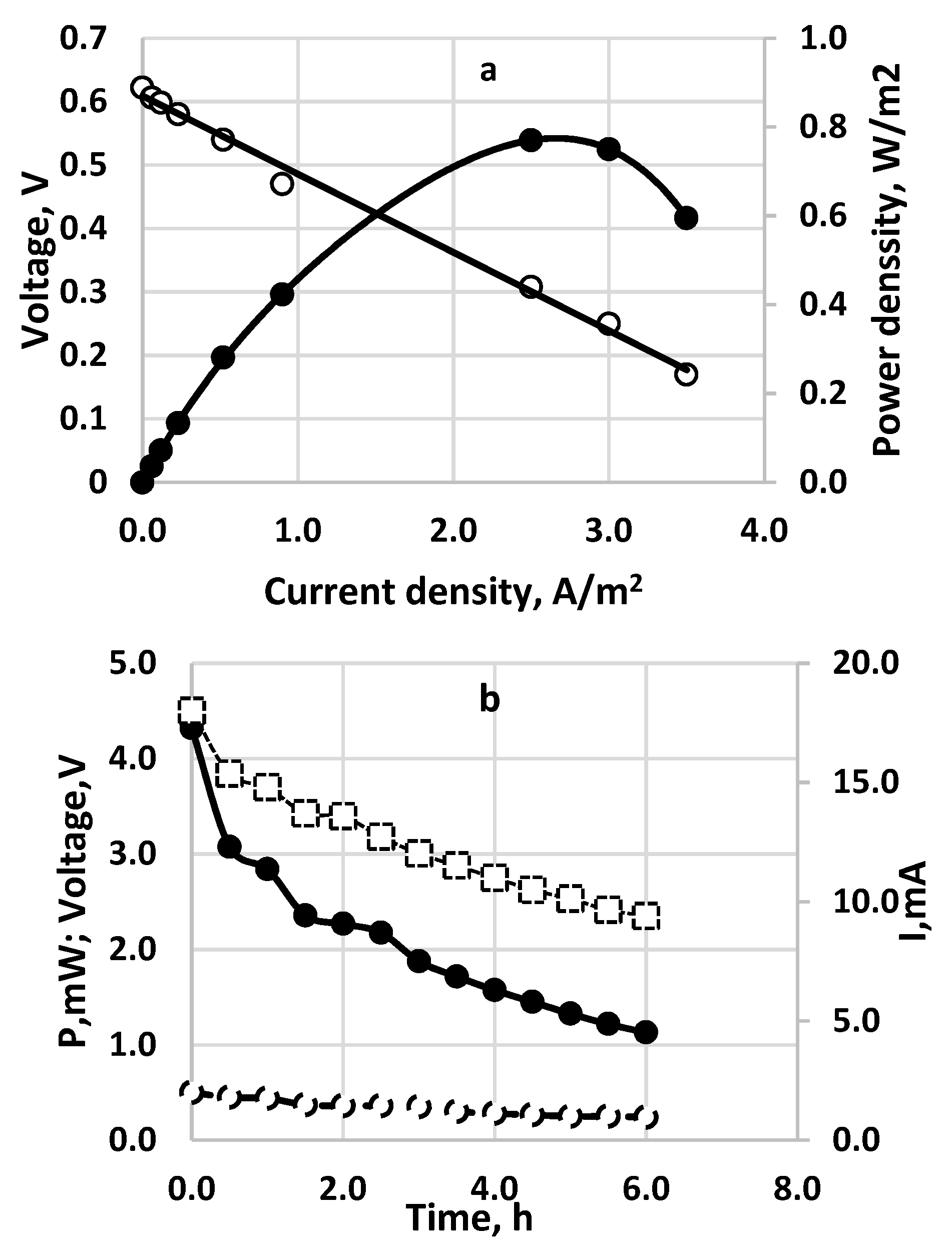
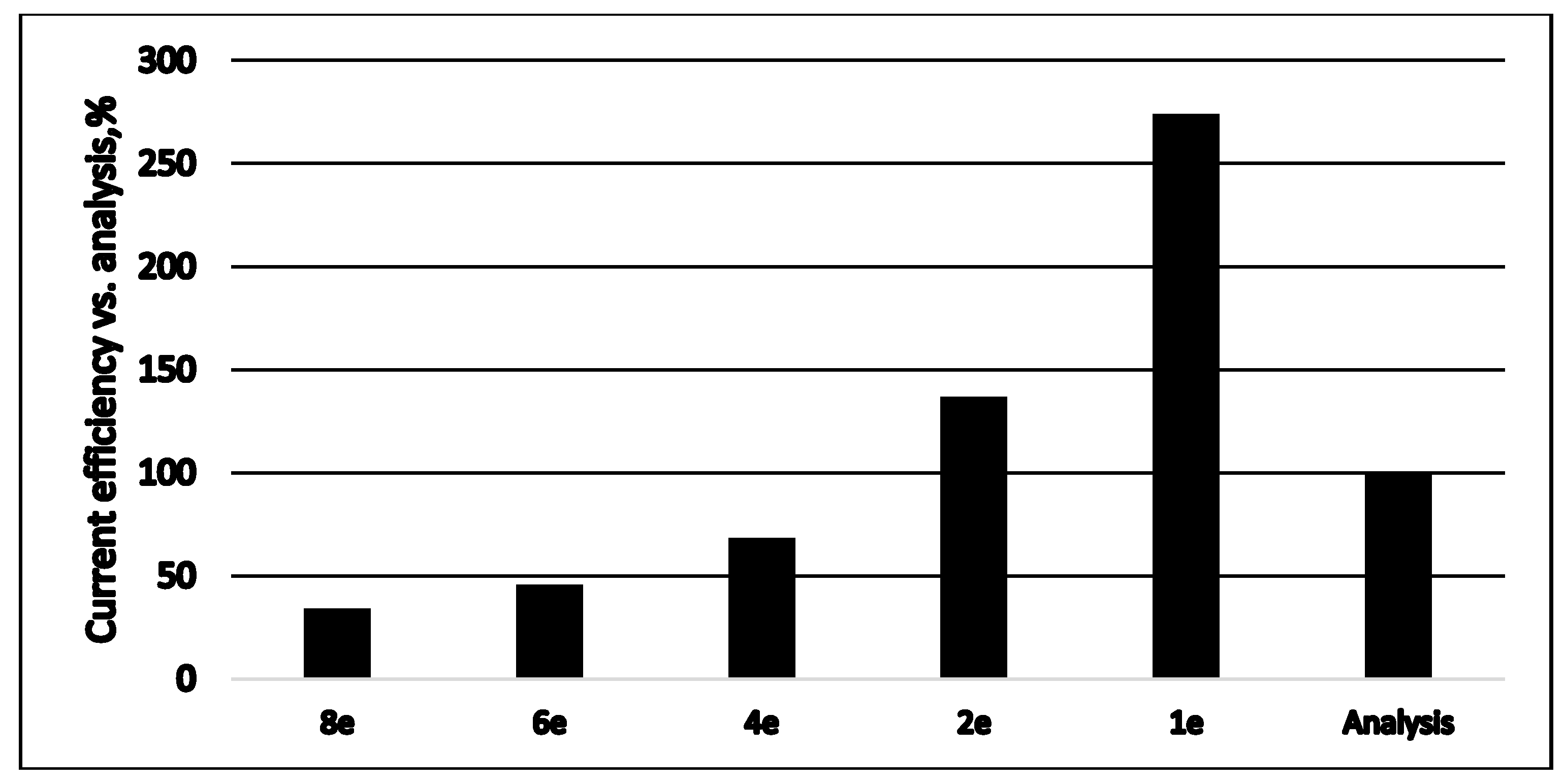
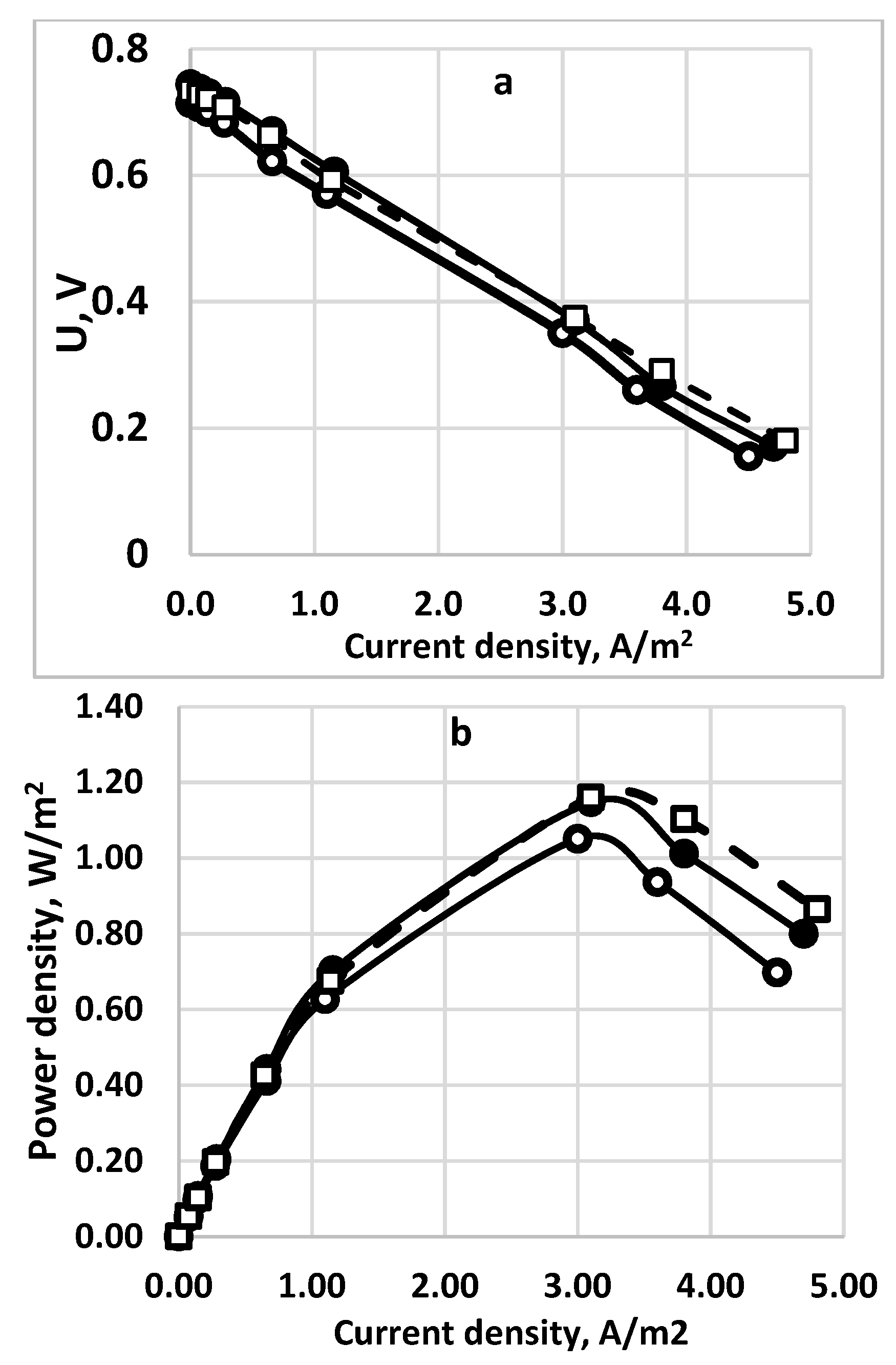
3.5. Stack and Consecutive Fuel Cell Performance
4. Conclusions
- Hydrogen sulfide is a prospective fuel, being a substrate of a liquid-phase fuel cell operating in sulfide form in aqueous media. It is suitable to use in water basins rich in hydrogen sulfide, as it can be relied on to simultaneously produce electric energy and cleanse water.
- Operational parameters of the tested fuel cell show reasonable current and power yields especially for sulfide concentrations in the medium range of 200–300 mg dm−3. The concentration of sulfite and sulfate ions provides for the main products of the electrochemical processes. Above this range parasitic reactions take place, i.e., polysulfide and thio-compounds with low power yield are obtained. Conversion rate is relatively low, (around 30%). Therefore, efforts should be made to increase sulfide to sulfate conversion rate. Further minimization of parasitic reactions in the bulk is required.
- Three different ion-exchange membranes were tested. In all cases the total cell resistance was found to be lower than some reference values. Fumapem and Neosepta membranes showed high sensitivity to hydroxyl ions whereas Celgard 3501 showed better durability.
- However, the achieved current and power densities are still low, and cannot compete with the traditional fuel cells. Efforts should be directed to further reduction of cell resistance by different methods such as membrane selection, new catalyst application to speed-up oxidation and reduction processes to improve the aeration system, etc.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rozhdestvenskiy, A. Hydrochemistry of the Bulgarian Sector of the Black Sea (in Bulgarian); Publishing House of the Bulgarian Academy of Science: Sofia, Bulgaria, 1986; pp. 145–150. [Google Scholar]
- Midilli, A.; Ay, M.; Kale, A.; Nejat Veziroglu, T. A parametric investigation of hydrogen energy potential based on H2S in Black Sea deep waters. Int. J. Hydrogen Energy 2007, 32, 117–124. [Google Scholar] [CrossRef]
- Neklyudov, I.K.; Bortz, B.V.; Polevich, O.W.; Tkachenko, V.I.; Shilyaev, B.A. Alternative hydrogen sulfide energetics in the Black Sea. Part I. State of the art, problems and perspectives (in Russian). Int. Sci. J. Altern. Energy Ecol. (ISJAEE) 2006, 12, 23–30. [Google Scholar]
- Ouali, S.; Chader, S.; Belhamel, M.; Benziada, M. The exploitation of hydrogen sulfide for hydrogen production in geothermal areas. Int. J. Hydrogen Energy 2011, 36, 4103–4109. [Google Scholar] [CrossRef]
- Linga Reddy, E.; Biju, V.M.; Challapalli, S. Production of hydrogen and sulfur from hydrogen sulfide assisted by nonthermal plasma. Appl. Energy 2012, 95, 87–92. [Google Scholar] [CrossRef]
- Vasilevsky, V.V.; Gutsevich, E.I.; Rusanov, V.D. Problems of hydrogen sulfide and its treatment in the Black Sea. Khim. Vys. Energy 1995, 25, 382–386. (In Russian) [Google Scholar]
- Mahmadmuratov, A.; Gruzdkov, Y.A.; Savinov, E.N. Photocatalytical decomposition of cadmium and zinc sulfides immobilized on cation-exchange thin film (in Russian). Kinet. Catal. 1986, 27, 133–136. [Google Scholar]
- Diver, R.B.; Fletcher, E.A. Hydrogen and sulfur from H2S—III. The economics of a quench process. Energy 1985, 10, 831–842. [Google Scholar] [CrossRef]
- Beschkov, V.; Razkazova-Velkova, E. Utilization of sulphide from Black Sea water by electrolysis. Int. J. Curr. Chem. 2010, 1, 7–15. [Google Scholar]
- Ipsakis, D.; Kraia, T.; Konsolakis, M.; Marnellos, G. Remediation of Black Sea ecosystem and pure H2 generation via H2S-H2O co-electrolysis in a proton-conducting membrane cell stack reactor: A feasibility study of the integrated and autonomous approach. Renew. Energy 2018, 125, 806–818. [Google Scholar] [CrossRef]
- Bogautdinov, A.Z.; Eremenko, Y.I.; Rusanov, V.D. Testing of plasmochemical unit Orenburg’s bench for H2S-containing gases treatment. VANT 1991, 2, 5–7. [Google Scholar]
- Naman, S.A.; Ture, I.E.; Veziroglu, T.N. Industrial extraction pilot plant for stripping H2S gas from Black Sea water. Int. J. Hydrogen Energy 2008, 33, 6577–6585. [Google Scholar] [CrossRef]
- Ipsakis, D.; Kraia, T.; Marnellos, G.E.; Ouzounidou, M.; Voutetakis, S.; Dittmeyer, R.; Dubbe, A.; Haas-Santo, K.; Konsolakis, M.; Figen, H.E.; et al. An electrocatalytic membrane-assisted process for hydrogen production from H2S in Black Sea: Preliminary results. Int. J. Hydrogen Energy 2015, 40, 7530–7538. [Google Scholar] [CrossRef]
- Kirk, T.J.; Winnick, J. A hydrogen sulfide solid-oxide fuel cell using ceria-based electrolytes. J. Electrochem. Soc. 1993, 140, 3494–3496. [Google Scholar] [CrossRef]
- Aguilar, L.; Zha, S.; Cheng, Z.; Winnick, J.; Liu, M. A solid oxide fuel cell operating on hydrogen sulfide (H2S) and sulfur-containing fuels. J. Power Sources 2004, 135, 17–24. [Google Scholar] [CrossRef]
- Chuang, K.T.; Luo, J.; Sanger, A.R. Evolution of fuel cells powered by H2S-containing gases. Chem. Ind. Chem. Eng. Q. 2008, 14, 69–76. [Google Scholar] [CrossRef]
- Beschkov, V.; Hristov, V.; Petkov, P. Energy Yield from Sulfide Containing Marine Water. B.G. Patent No. 1775/25.11.2013, Ref. No. 2352/14.02.2013.
- Beschkov, V.; Razkazova-Velkova, E.; Vlaev, S.D.; Martinov, M.; Petkov, P.; Raicheff, R. Method of Oxidation of Sulfide Ions in Fuel Cells. B.G. Patent Application Ref. No. 111367/17.12.2012.
- U.S. Energy Information Administration. Capital Cost Estimates for Utility Scale Electricity Generating Plants; U.S. Energy Information Administration: Washington, DC, USA, 2016; pp. 7–8. Available online: https://www.eia.gov/analysis/studies/powerplants/capitalcost/pdf/capcost_assumption.pdf (accessed on 29 August 2018).
- Tarjanne, R.; Kivistö, A. Comparison of Electricity Generation Costs; Lappeenranta University of Technology: Lappeenranta, Finland, 2008; pp. 8–9. [Google Scholar]
- Dutta, P.K.; Rabaey, K.; Yuan, Z.; Keller, J. Spontaneous electrochemical removal of aqueous sulfide. Water Res. 2008, 42, 4965–4975. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.-F.; Song, W.; Tong, Z.-H.; Sun, M. A fuel-cell-assisted iron redox process for simultaneous sulfur recovery and electricity production from synthetic sulfide wastewater. J. Hazard. Mater. 2012, 243, 350–356. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Han, J.-I. Performance of direct alkaline sulfide fuel cell without sulfur deposition of anode. Int. J. Hydrogen Energy 2014, 39, 7142–7146. [Google Scholar] [CrossRef]
- Iordache, S.M.; Ciocanea, A.; Balan, A.; Stamatin, I.; Budea, S.; Ceaus, C.; Trefilov, A.M.I.; Voinea, S. Recovering hydrogen sulfide from sulfurous waters with PEM fuel cells. Energy Procedia 2016, 85, 273–278. [Google Scholar] [CrossRef]
- Petrov, K.; Baykara, S.Z.; Ebrasu, D.; Gulin, M.; Veziroglu, A. An assessment of electrolytic hydrogen production from H2S in Black Sea waters. Int. J. Hydrogen Energy 2011, 36, 8936–8942. [Google Scholar] [CrossRef]
- Fukuzumi, S.; Lee, Y.-M.; Nam, W. Fuel production from seawater and fuel cells using seawater. ChemSusChem 2017, 10, 4264–4276. [Google Scholar] [CrossRef] [PubMed]
- Suhotin, A.M. Guidebook on Electrochemistry (in Russian); Himia: Leningrad, Russia, 1981. [Google Scholar]
- Romero Cotrino, A. Control of Hydrogen Sulfide from Groundwater Using Packed-Bed Anion Exchange and Other Technologies. Master’s Thesis, University of South Florida, Tampa, FL, USA, 2006; p. 8. [Google Scholar]
- Chakraborty, U.K. Reversible and irreversible potentials and an inaccuracy in popular models in the fuel cell literature. Energies 2018, 11, 1851. [Google Scholar] [CrossRef]
- O’Hayre, R.P.; Cha, S.-W.; Colella, W.G.; Prinz, F.B. Fuel Cell Fundamentals, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 59–64. ISBN 978-0-470-25843-9. [Google Scholar]
- Merle, G.; Wessling, M.; Nijmeijer, K. Anion exchange membranes for alkaline fuel cells: A review. J. Membr. Sci. 2011, 377, 1–35. [Google Scholar] [CrossRef]
- Rees, T.D.; Gyllenpetz, A.B.; Docherty, A.C. The determination of trace amounts of sulphide in condensed steam with N-diethyl-P-phenylenediamine. Analyst 1971, 96, 201–208. [Google Scholar] [CrossRef]
- Dobos, D. Electrochemical Data (a Russian edition); Mir: Moscow, Russia, 1980; p. 34. [Google Scholar]



| Fuel | Energy Capacity, GJ/t |
|---|---|
| Oil equivalent | 41.86 |
| Hydrogen | 143.02 |
| Methane | 56.46 |
| Ethanol | 26.8 |
| Methanol | 19.9 |
| Hydrogen sulfide | 24.52 |
| Energy Source | Features | Features/Sulfide-Driven Fuel Cell |
|---|---|---|
| Heat power stations (oil, gas, coal) | Carbon emissions; expensive production; heavy operation; waste handling Capital costs, $500–5000/kW Operational costs, 6–15 €/MWh | Carbon free; less operational costs; easy switch-on/off; no waste Capital costs, ~$2000/kW Operational costs, ~10 €/MWh |
| Nuclear fuel | Expensive fuel production; heavy and hazardous operation; expensive and dangerous waste storage. Capital costs, ~$6000/kW Operational costs, ~15 €/MWh | Less operational costs; easy switch-on/off; no waste |
| Wind | Weather dependent; impact on environment Capital costs, ~$1900/kW Operational costs, 10 €/MWh | Weather independent, positive environmental impact |
| Solar | Weather dependent; environmental hazards (soil and biodiversity degradation) Capital costs, ~$2600/kW Operational costs, 10 €/MWh | Weather independent, positive environmental impact |
| Reversible Redox Anode Reactions (Short Excerpt) | Number of Exchanged Electrons | Standard Electrode Potential [V], 25 °C |
|---|---|---|
| SO42− + H2O + 2e = SO32− + 2OH− | 2 | −0.93 |
| SO32− + 3H2O + 6e = S2− + 6OH− | 6 | −0.66 |
| S22− + 2e = 2S2− | 1 | −0.524 |
| S + 2e = S2− | 2 | −0.48 |
| 2SO42− + 4H++ 2e = S2O62− + 2H2O | 2 | −0.22 |
| S + H+ + 2e = HS− | 2 | −0.065 |
| S2O32− + 6H+ +8e = 2S2− + 3H2O | 4 | −0.006 |
| HSO3− + 5H+ + 4e = S +3H2O | 4 | 0 |
| SO42− + 8H+ + 8e = S2− + 4H2O | 8 | 0.149 |
| S2O32− + 8H+ + 8e = 2HS− + 3H2O | 4 | 0,2 |
| Sulfide Concentration, g dm−3 | Supporting Electrolyte | Maximum Current Density, A/m2 | Maximum Power Density, W/m2 | Cell Efficiency, % | Reference |
|---|---|---|---|---|---|
| 0.241 | 0.27M NaCl | 8 | 2.6 | 30.5 | This work |
| 0.320 | 3M NaOH | 16 | 15 | 50 | Ref. [23] |
| 70 | Water (?) | 0.075 | 0.025 | 40 | Ref. [24] |
| 150 | Water (?) | 0.5 | 0.031 | 30 | Ref. [24] |
| Cell Type | Area cm2 | Inlet Sulfide Concentration mg/dm−3 | OCP, V | Voltage Cell Efficiency, % | Cell Resistivity, Ohms | Current Density, A/m2 | Power Density, W/m2 | Over-Potential, mV | Sulfide Conversion % |
|---|---|---|---|---|---|---|---|---|---|
| Cylindrical cell. Feed 0.2 dm−3/h | 7 | 241 | 0.49 | 54.5 | 45 | 5.5 | 10. | 0.1 | 95.3 |
| Rectangular single cell, direct aeration. Feed 0.4 dm−3/h | 650 | 240 | 0.52 | 49.5 | 1.0 | 4.6 | 1.9 | 0.1 | 57.2 |
| Rectangular single cell, ejector. Feed 0.4 dm−3/h | 650 | 260 | 0.38 | 58 | 1.8 | 2.2 | 0.6 | 0.7 | 37 |
| Rectangular stack, 2 cells; direct aeration. Feed 2 × 0.4 dm−3/h | 650 | 240 | 1.42 | 78 | 11.7 | 1.2 | 0.8 | 0.2 | 76 |
| 2 Rectangular cells, cascade. Feed 0.4 dm−3/h | 650 | 236 | 1.2 | 36 | 11.3 | 1.3 | 0.57 | -3 | 30–100 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beschkov, V.; Razkazova-Velkova, E.; Martinov, M.; Stefanov, S. Electricity Production from Marine Water by Sulfide-Driven Fuel Cell. Appl. Sci. 2018, 8, 1926. https://doi.org/10.3390/app8101926
Beschkov V, Razkazova-Velkova E, Martinov M, Stefanov S. Electricity Production from Marine Water by Sulfide-Driven Fuel Cell. Applied Sciences. 2018; 8(10):1926. https://doi.org/10.3390/app8101926
Chicago/Turabian StyleBeschkov, Venko, Elena Razkazova-Velkova, Martin Martinov, and Stefan Stefanov. 2018. "Electricity Production from Marine Water by Sulfide-Driven Fuel Cell" Applied Sciences 8, no. 10: 1926. https://doi.org/10.3390/app8101926