1. Introduction
Reverse electrodialysis (RED) is one of the methods to convert the potential energy from salinity gradients into electrical energy. The main applications include: (i) primary energy generation from natural sources [
1,
2]; (ii) production of H
2 [
3,
4]; (iii) energy storage: the stack is used in the electrodialysis (ED) mode to store energy as a salinity gradient, and this energy can be recovered by operating the stack in the RED mode [
5,
6]; and (iv) the generating part of a heat-to-power system in combination with a thermally driven regeneration unit [
7,
8].
The requirements of the RED stack, the applied membranes, and the used salt are very different for each application. For primary energy generation, there is no free choice of the salts and the RED stack and membranes are designed for optimal power density and/or efficiency using feed water with natural ion composition. In energy storage systems, there is, in principle, a free choice of the electrolyte; important parameters are the total cycle efficiency, charge and discharge power density, energy density of the electrolyte, price of the electrolyte, and aspects concerning health, safety, and the environment. A special point in such systems is the water transport through the membranes as a source of imbalance during long-time operation. In heat-to-power systems, there are two methods of regeneration: distillation of the fluid phase (usually water) or of the salt (ammonium bicarbonate or ammonium carbonate). In the case of water distillation, there is much freedom in choosing an electrolyte and membranes; in contrast to energy storage systems, the amount of electrolyte is restricted and price is therefore less important than in storage systems.
Usually in ED and RED, organic membranes are applied polymer–based structures with exchanging fixed charges. The fixed charges are mostly sulfonate groups (~SO
3−) in the cation exchange membranes (CEMs) and quaternary ammonium groups (~N(CH
3)
3+) in the anion exchange membranes (AEMs). These polymeric membranes have some drawbacks: the permselectivity decreases at higher salt concentrations, and they facilitate water transport and deteriorate at high temperatures. In this paper, we launch a new membrane type based on reversible electrochemical reactions on both sides of the so-called pseudo-membrane. The adjective “pseudo” indicates that in most cases there is no real permeation of an ion through the membrane: they change ions at one side for ions at the other side. In fact, these pseudo-membranes are literally real exchange membranes, in contrast with normal “ion exchange membranes” that are based on ion transport.
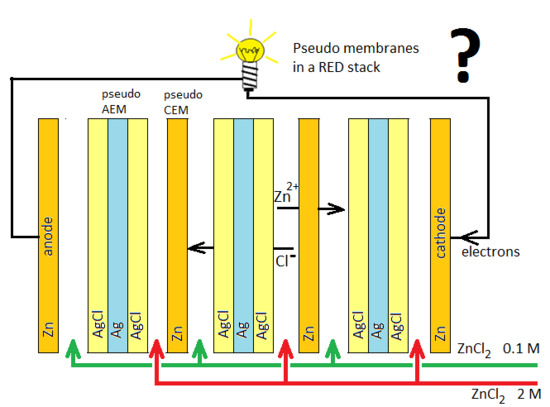
Figure 1 shows the principle: a RED stack is equipped with Zn pseudo-membranes, acting as cation-exchange membranes for Zn
2+ ions, and Ag/AgCl pseudo-membranes, acting as AEMs for Cl
−; the stack is fed with high- and low-concentration ZnCl
2 solutions.
The Zn pseudo-membrane functions as a CEM:
The AgCl/Ag/AgCl pseudo-membrane functions as an AEM:
The reactions on the end electrodes are:
cathode: Zn2+ + 2e → Zn;
anode: Zn → Zn2+ + 2e.
Obviously, a periodical flow switch should be applied; the switch period is especially dependent on the thickness of the AgCl layer. During the switch, the stack is not generating energy and this dead time is relative shorter with a longer switch period. Otherwise, a longer switch period requires a thicker AgCl layer, which introduces more electrical resistance. The thickness of the zinc electrodes is no particular problem: they can be overdimensioned without any serious consequence to ionic or electrical resistance.
The generated voltage over each pseudo-membrane is, in principle, the same as over a conventional ion exchange membrane. This can be seen as follows: a zinc rod in a solution of
H mol/L ZnCl
2 gets a potential
EH with respect to the solution:
where
R is the gas constant (8.3143 J/mol/K),
T is the temperature,
n is the number of involved charges, and
F is the Faraday constant (96,485 C/mol). Here, we have assumed an activity coefficient equal to 1. Thus the potential of the solution with respect to the zinc rod is −
EH.
Now we take a vessel divided by a zinc plate into two parts, both containing ZnCl
2, one with concentration
H (high) and the other with
L (low). Then the voltage difference between the two solutions is:
This result is the same equation as for the Donnan potential over a normal CEM. The same holds for the Ag/AgCl pseudo-membrane. Therefore, we can conclude that these new pseudo-membranes act as classical ion exchange membranes. They are very ion-specific, and co-ion transport is impossible, resulting in a permselectivity of 100%. Water transport through the Zn and Ag/AgCl pseudo-membranes is also unthinkable. Moreover, these inorganic structures are very resistant to high temperatures. We will introduce the terms pseudo CEM (pCEM) and pseudo AEM (pAEM), or, generally, pseudo ion exchange membrane (pIEM).
The combination of a pCEM with a normal AEM or a pAEM with a normal CEM seems not to be problematic. However, with two pseudo-membranes, there is the threat of redox reactions between them. In the system described above, these reactions are:
resulting in:
The reaction rate is dependent on the concentration of the dissolved Ag(I). In the first instance, we considered only the free Ag
+ ion with a very low concentration due to the small solubility product of AgCl (L
AgCl = 1.8 × 10
−10) and expected a rather low disintegration of the system. However, it turned out that much silver(I) is dissolved as chloro-complexes, limiting the lifespan of the system to a few hours. This complication is discussed in
Section 2.3.1.
In this paper, we present experiments with the Ag/AgCl and Zn pseudo-membranes and outline a new path for the application of pseudo-membranes.
2. Pseudo-Membranes, Systems, and Applications
In this chapter, we propose different modifications of pseudo-membranes and discuss possible combinations of pCEMs and pAEMs. Is should be emphasized that only Zn and Ag/AgCl pseudo-membranes were tested, individually with a classical ion exchange membrane and in combination, as depicted in
Figure 1. Important parameters for application of pseudo membranes are price (
Table 1), solubility (
Table 2) and standard reduction potentials (
Table 3).
2.1. Pseudo Cation Ion Exchange Membranes (pCEMs)
We will distinguish two classes of pCEMs: pure metals and intercalation compounds.
2.1.1. Pure Metal: Zn for Zn2+, Ni for Ni2+, Cu for Cu2+
The metal should be stable in water and not too expensive or harmful. Pseudo-membranes dissolve at one side and grow at the other side. This process is inverted frequently by current reversal; essential for a good operation is a smooth and homogeneous building of the metal layer.
Zn: The advantages are that zinc (and zinc salt) is rather cheap and has medium toxicity. These are important issues in energy storage systems, where huge quantities of electrolytes are stored. A disadvantage is that zinc has a rather low reduction potential, promoting the reduction of free Ag
+ if in combination with a pAEM (as discussed in
Section 2.3).
Ni: The reduction potential of Ni is much higher than of Zn [
12], which may be advantageous with respect to stability. Drawbacks are the high price [
9] and the toxicity [
10] of nickel salts, which make the application to energy storage systems impossible (
Table 1). However, in heat-to-power systems, nickel pCEMs may be considered.
Cu: Copper can exist in two oxidation states, which causes some complications. Therefore, copper cannot be used in systems containing CuCl
2. The reason is that the poorly soluble copper(I) chloride can be formed via an auto-redox reaction:
resulting in:
2.1.2. Intercalation Compounds: MnO2 for Na+; FePO4 for Li+; and Prussian Blue Analogues for Li+, Na+, and K+
These materials come from the battery world and contain transition metals with different oxidation states. Foreign ion can settle within the crystal structure, a process called intercalation. In a battery, there is a metallic- or carbon-based current collector where electrons are transferred from or to the redox material. In a pCEM, this metal or carbon/graphite is the substrate for the active redox layers on both sides. An (expensive) option is a platinized metal substrate. Intercalation pCEMs should be loaded on both sides and should be saturated with ions for 50% of the capacity in the start phase.
MnO
2/Na
2Mn
5O
10: The use of this manganese salt and oxide for a working sodium electrode is described by La Mantia et al. [
13] and Ye et al. [
14]:
Pan Xiong et al. [
15] studied the intercalation of the ions Li
+, Na
+, K
+, and Mg
2+ with MnO
2 and observed only minor structural changes in the two-dimensional layered structures.
LiFePO
4/FePO
4: This material is used in Li-ion batteries and many properties are known [
16,
17]. This mixture can absorb and release Li
+ ions due to the multivalent property of iron:
FeIII[FeIIIFeII(CN)6]3: This insoluble salt, called Prussian Blue, contains iron(III) and iron(II) ions; the blue color is ascribed to charge transfer between the ions with different oxidation states. The iron can be substituted by other transition metals. These Prussian Blue analogues (PBAs) have, in principle, the same structure but with vacancies of different dimensions, suitable for absorbing different alkali ions: Li+, Na+, and K+.
2.2. Pseudo Anion Ion Exchange Membranes (pAEMs)
In contrast to pCEMs, a pure elemental pAEM is generally impossible. A theoretical exception seems to be a sheet of solid iodine that might be pseudo-permeable for iodide ions via redox reactions on both sides; on the other hand, the I2 dissolves in an iodide-containing electrolyte, which in turn makes it difficult to apply. For more practical application, we encountered two classes: silver and copper halides and PbSO4.
2.2.1. Halide pAEMs: Ag/AgX and Cu/CuX for Cl−, Br−, and I−
Ag/AgX (X = Cl, Br, or I): A drawback is the resistance of the AgX layer: the electrochemical reactions take place at the Ag–AgX interface and the X− ion should diffuse through the AgX layer until the silver surface is reached.
Cu/CuX (X = Cl, Br, or I): In principle, a system with Zn pCEMs together with Cu/CuX and a ZnCl
2 electrolyte can be used for a short time. The solubility of the Cu(I)-halides is much larger than that of the silver analogues. This may shorten the lifespan of the membranes due to reactions such as:
Thus, the combination of a metallic pCEM with a copper(I)-halide is also unstable.
2.2.2. Lead(II) Sulfate for SO42−
Pb/PbSO4: The process of binding and releasing the sulfate ion is quite common in a lead-acid battery, and the combination of a Pb/PbSO4 pAEM with a normal CEM and an Na2SO4 electrolyte can be considered. Again, a combination of two pseudo-membranes, as in Zn–Pb/PbSO4–ZnSO4, is inherent unstable.
2.2.3. Intercalation Compounds: LDHs for Different Anions
Layered double hydroxides (LDHs) are structured like clays and are capable of absorbing hydroxyl ions [
18], chloride [
19], and inorganic anions [
20]. However, little is known about the application of LDHs as a chloride-absorbing electrode material, therefore we shall disregard this option.
2.3. Systems Based on Pseudo-Membranes
2.3.1. Combinations of pAEM with pCEM
Systems with pIEMs can, in principle, be equipped with classical electrode systems or with capacitive electrodes. However, a more simple solution is to construct the end electrodes of the same material as the pCEM, or eventually as the pAEM. We will denote the (unstable) system described in the introduction as Zn-Ag/AgCl–ZnCl2, indicating that Zn is the end electrode material and also the pCEM, Ag/AgCl is the pAEM, and ZnCl2 is the electrolyte; the same system with Ag/AgCl end electrodes will be denoted as Ag/AgCl–Zn-ZnCl2.
A special problem is the combination of an Ag/AgCl pAEM with a metal pCEM, e.g., Zn. The desired electrolyte in this case is a ZnCl
2 solution. Although the solubility product is rather low (L
AgCl = 1.8 × 10
−10) and the concentration of free silver ions is therefore also very low, it is known that a remarkable amount of AgCl is dissolved by complexation with chloride ions. From the work of Fritz [
21] we know that silver is present as Ag
+, AgCl, AgCl
2−, AgCl
32−, and AgCl
43− if silver chloride is in contact with a solution of HCl, NaCl, KCl, or NH
4Cl.
Table 4 shows the solubility of AgCl in different concentrations of NaCl and the distributions over the various compounds. It is remarkable that AgCl has much more solubility in KCl solutions. Moreover, the solubility is strongly temperature dependent.
If Ag/AgCl pAEMs are combined with classical CEMs, the rather high concentration of silver complexes seems not to introduce technical problems: the most abundant complex ion is double-negative charged and rejected by the CEM. However, a drawback for storage applications is the high price of the applied silver.
The combination of a Zn pCEM with an Ag/AgCl pAEM requires a ZnCl
2 electrolyte. The dissolved silver ions introduce an inherent unstable system due to
We found that this process occurred during one night, resulting in a black coating on the zinc plates and a damaged Ag/AgCl structure, making it impossible to repeat any RED or ED experiment the next day.
The system
Zn–Ag/AgCl–ZnCl
2 is exceptionally unstable by two effects: the high solubility of silver in the chloride solution and the large difference in reduction potential of silver and zinc. The silver concentration can be decreased by changing Cl for Br or I, because the solubility products of AgBr and Agl are much smaller than those of AgCl [
11], (
Table 2). However, the conclusion is that all combinations of a metallic pCEM with a silver halide pAEM are always unstable.
Within the system Cu-Pb/PbSO4–CuSO4, the Pb electrode is in direct contact with the Cu2+ ions, which may result in a fast redox reaction, Pb + Cu2+ + SO42− → PbSO4 + Cu, because copper is more noble than lead. Thus we wonder if changing Cu for Zn gives a more stable system.
In the system Zn-Pb/PbSO4–ZnSO4, the Pb2+ concentration is dictated by the solubility product of PbSO4 (LPbSO4 = 1.6 × 10−8). With a ZnSO4 concentration of about 1 M, the resulting Pb2+ concentration is about 10−8 M. Nevertheless, such a system is unstable due to the reaction Pb2+ + Zn → Pb + Zn2+.
The system Ni-Pb/PbSO4–NiSO4 is also unstable for the same reason as the zinc analogue. However, the difference between the reduction potentials of Zn2+ and Pb2+ is rather small, therefore we expect that the nickel system has a longer lifespan.
Not all combinations are possible, due to electrochemical considerations. We conclude that we are restricted to intercalation-based pCEMs together with Ag/AgX, Cu/CuX, or Pb/PbSO
4, provided that the substrate is resistant enough against oxidation.
Table 5 shows possible combinations of pCEMs with pAEMs with solubility achieved from [
22].
2.3.2. Hybrid Systems
Hybrid systems are constructed by combining a pseudo-membrane with a normal ion exchange membrane. Moreover, instead of using participating electrodes, noble metal electrodes (with a redox reaction in the electrolyte) or capacitive electrodes can be applied. Such hybrid stacks may be useful for the development of a whole pseudo system, because the pseudo-membranes can be tested separately. We used this method in an attempt to develop the Zn–Ag/AgCl–ZnCl2 system; first we tested a classical stack with Ag/AgCl pAEMs together with normal CEMs, then we built a stack with zinc pCEMs and normal AEMs, and finally we constructed the whole pIEM system.
2.4. Applications
(a) Heat-to-Power Systems
RED stacks with inorganic membranes can be applied in a heat-to-power generator, a closed-loop system where a regenerator is used to bring the concentrations of the outlet streams to their original values. This regenerator can be based on water distillation or evaporation of a volatile salt and is powered by waste heat. Osmosis through a classical membrane requires additional evaporation, and the low water transport through the inorganic membranes in such a heat-to-power system is therefore advantageous. Moreover, the thermal stability with respect to permselectivity and the small deterioration at elevated temperature make the application of inorganic membranes an interesting option. Because the volume of the closed-loop system is relative small, the price of salts is less important.
(b) Energy-Storage Systems
A RED/ED salinity gradient storage system consists, in the simplest form, of a RED/ED stack and two vessels with salt solutions. The solutions circulate through the stack in a closed system. During charge, a surplus of electrical energy is used to drive the stack in the electrodialysis (ED) mode, resulting in an increased salinity gradient between the two solutions. During discharge, this stack operates in the RED mode and the stored salinity gradient is converted to electrical energy. A drawback is that there is continuous water transport from the low- to high-concentration solution due to osmosis. Here, the low water permeability and high permselectivity of inorganic membranes are very advantageous. In contrast to the heat-to-power application, large amounts of electrolyte are needed and the price of the salt is important.
(c) End Membranes in ED or RED Stacks
End membranes are the outer membranes of a RED or ED stack and separate the electrode compartments from the feed compartments. In many applications, it is important that these membranes are a good barrier for unwanted transport from the electrode rinse solution to the feed water and vice versa. An example is the need to maintain low pH in the electrode rinse solution if the Fe
2+/Fe
3+ redox couple is used [
23,
24,
25,
26,
27]. In other applications, there is the danger of aggressive compounds in the electrode compartment that can damage the end membranes; chlorine is generated in an NaCl electrode rinse [
27]. In stacks for H
2 production, there may be a need for solid pressure-resistant walls around the electrodes [
3,
4]. The high specific permeability and mechanical robustness of pseudo-membranes can deal with these threats.
2.5. Preparing Ag/AgCl Pseudo-Membranes
2.5.1. Electrical Resistance of the AgCl Layer
The Ag/AgCl pseudo-membranes are made by anodizing silver plates between two inert cathodes with constant current in an HCl/NaCl solution. The involved reactions are:
The applied voltage
U(
t) is estimated by a constant value (
b) and a time-dependent part (
a·
t):
The constant
b is the starting potential caused by (i) Nernst potentials; (ii) overvoltages of the chemical redox reactions at the electrodes; and (iii) the ohmic drop in the electrolyte. The Nernst part has little meaning when silver plate is used as a pseudo-membrane during the RED or ED process, because during these processes, oxidation and reduction of silver cancel each other out on both sides of the silver plate. More interesting is the voltage increase
a due to the buildup of AgCl layer and the corresponding increase of resistance
dRL/
dt:
Thus the layer resistance
RL and the area resistance
RLa of the AgCl layer at time
t are:
If the Ag/AgCl plate is used in the RED/ED stack, RL and RLa are more or less constant because the AgCl sacrifice at one side is compensated by a growth at the other side, therefore the total thickness of the AgCl layers is constant.
The transported electrical charge per m
2 (
Qa(
t)) is:
To compare different experiments, we introduce the Faradic area resistance
RFa, the area resistance of an AgCl layer as produced by a charge of 1 Faraday per m
2:
where
F stands for the Faraday constant (1
F = 96,485 C/mol).
2.5.2. Thickness of the AgCl Layer
The thickness of the AgCl layer during the anodizing process is estimated from the applied charge per m2. After transport of 1 Faraday of electricity, 1 mol of AgCl is deposited on one side of the silver plate and 1 mol of Ag is converted to AgCl. From the densities (AgCl: 5.56; Ag: 10.50 g/cm3) and molar masses (AgCl: 143.32; Ag: 107.87 g/mol), it follows that this results in a decrease of 10.3 µm for Ag and an increase of 25.8 µm for AgCl. Therefore it is assumed that the AgCl layer is composed of pure crystalline AgCl without inclusion or pores.
In our experiments, the maximum anodizing time was 30 min with a current density of 60 A/m2, resulting in a transport of 1.12 Faraday, equivalent to a shrinkage of the silver plate of 11.5 µm on each side. Thus the 200 µm of material present is more than sufficient.
3. Experiment
3.1. Membranes and Pseudo-Membranes
The applied commercial membranes were Neosepta CMX and AMX (Tokuyama, Japan). Zinc pseudo-membranes and end electrodes were cut from 0.8 mm commercial galvanized iron plate for experiments 4.2 and 4.3 (Auke Rauwerda, Leeuwarden, The Netherlands) and from 0.8 mm zinc plate for experiment 4.4 (Rheinzink, Datteln, Germany). Ag/AgCl pseudo-membranes were made from 0.2 mm 99.9% pure silver plate (Drijfhout, Amsterdam, The Netherlands). Stacks were equipped with 500 µm woven spacers (Sefar, Lochem, The Netherlands). The dimensions were 14.5 × 14.5 cm2; the active membrane area in the stack was 10 × 10 cm2. After polishing, they were anodized between titanium mesh cathodes, coated with Ru–Ir mixed-metal oxide (MMO, MAGNETO Special Anodes B.V., Schiedam, The Netherlands). The current was applied with a Velleman LABPS3005D power supply (Conrad, Oldenzaal, The Netherlands); the distance between the silver plate and the Ti-electrodes was about 1 cm. During anodization, the applied voltage was measured. RED and ED experiments were performed with an Iviumstat potentiostat (Ivium Technologies bv, Eindhoven, The Netherlands).
3.2. Stacks
Four RED stacks (A, B, C, and D) were built, each with different (pseudo) ion exchange membranes, as shown in
Figure 2. All stacks were mounted in a cross-flow stack housing, resulting in an active cross-section of 10 × 10 cm
2 (REDstack bv, Sneek, The Netherlands).
Stack A was equipped with 4 cell pairs and an additional terminating membrane; each cell pair consisted of a Neosepta CMX membrane, an Ag/AgCl pAEM, and 2 spacers. End membranes were Neosepta CMX at both sides. The cell was equipped with Pt-coated titanium mesh electrodes with dimensions 10 × 10 cm2 (MAGNETO Special Anodes B.V., Schiedam, The Netherlands). The electrode rinse solution (flow rate 150 mL/min) contained the redox couple 0.2 M K3Fe(CN)6 with 0.2 M K4Fe(CN)6 in a 0.25 M NaCl bulk. First an RED test was performed and then an ED test.
Stack B was composed of 3 galvanized pCEMs with 4 normal AMX membranes. This stack contained galvanized end electrodes, the same as those used for the pseudo-membranes. No special electrode rinse was used.
Stack C, equipped solely with pseudo-membranes, was identical to stack B, except that it was provided with Ag/AgCl pAEMs instead of the classical AEMs.
Stack D was, in principle, the same as stack C. However, pure zinc was used for the pCEMs and electrodes and Ag/AgCl electrodes with a thinner AgCl layer were used.
Stacks B and C were equipped with galvanized iron with a zinc layer of about 20 µm. Each run lasted for 300 s with a maximum current of 20 mA. For oxidation at the anode and the anodic side of the pseudo-membranes, a zinc layer of 0.03 µm was dissolved, far below the available zinc amount.
3.3. Feed Water
Saltwater and freshwater streams (H = high concentration, L = low concentration) composed of dissolved NaCl (99.5% purity; ESCO, Harlingen, The Netherlands) or ZnCl2 (at least 98% purity, Boom, Meppel, The Netherlands) were fed into the RED stacks.
3.4. Electrochemical Measurements
Electrochemical measurements were performed with a potentiostat (Ivium Technologies, The Netherlands). Conductivity was measured with a Cond3310 conductivity meter equipped with a Tetracon 325 measuring cell (WTW, Weilheim, Germany). Power densities were calculated as power per area of total (pseudo) membrane. All stacks contained 4 cell pairs with a cross-section of 10 × 10 cm2, resulting in a total membrane area of 0.08 m2.
3.5. Stack Operation
During each lab test, a restricted number of RED runs was performed. After each run, the feed water was switched together with a polarity reversal. Then, after a rest time, the next run started, and so on.