Characterization of Different Chemical Blowing Agents and Their Applicability to Produce Poly(Lactic Acid) Foams by Extrusion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Testing Methods
2.2.1. Gel Permeation Chromatography (GPC)
2.2.2. Differential Scanning Calorimetry (DSC) for Chemical Blowing Agents
2.2.3. Differential Scanning Calorimetry (DSC) for Biopolymer Foams
2.2.4. Thermogravimetric Analysis-Fourier-Transform Infrared Spectrometry (TGA-FTIR)
2.2.5. Thermogravimetric Analysis Performed in Isothermal Conditions
2.2.6. Scanning Electron Microscopy (SEM)
2.2.7. Foam Characterisation
2.2.8. Measuring Foam Strength
3. Results of the Investigation of the Foaming Agents
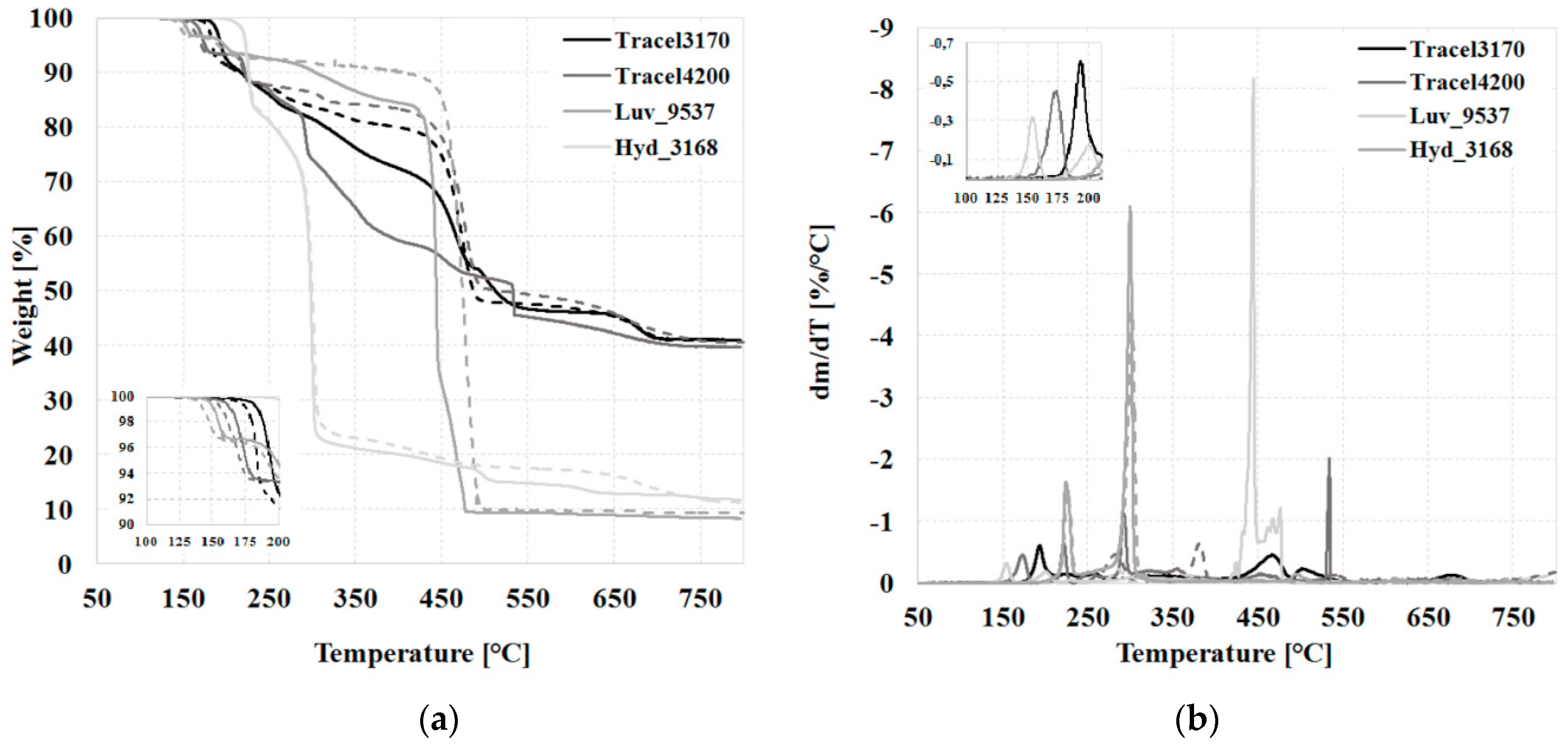
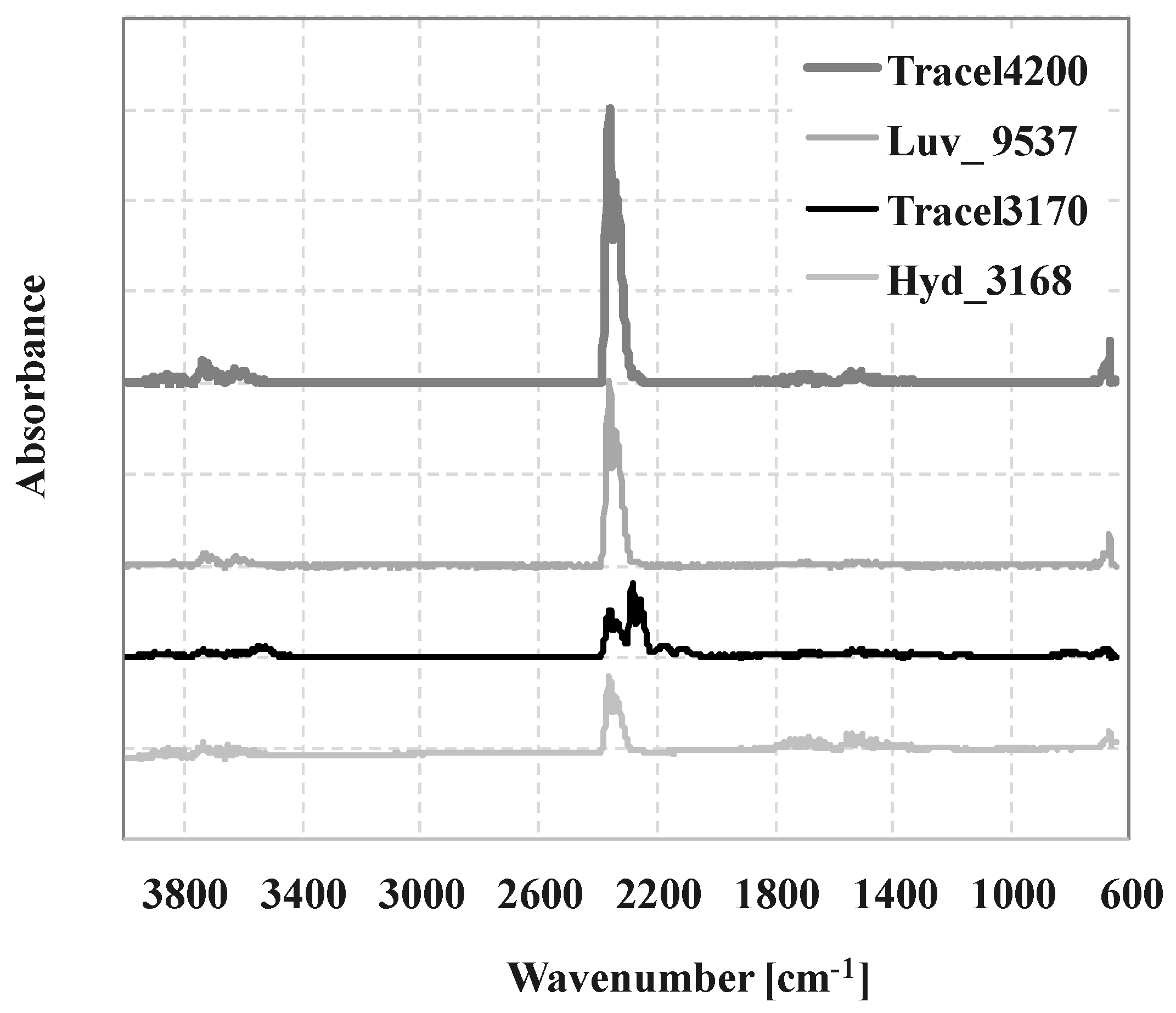
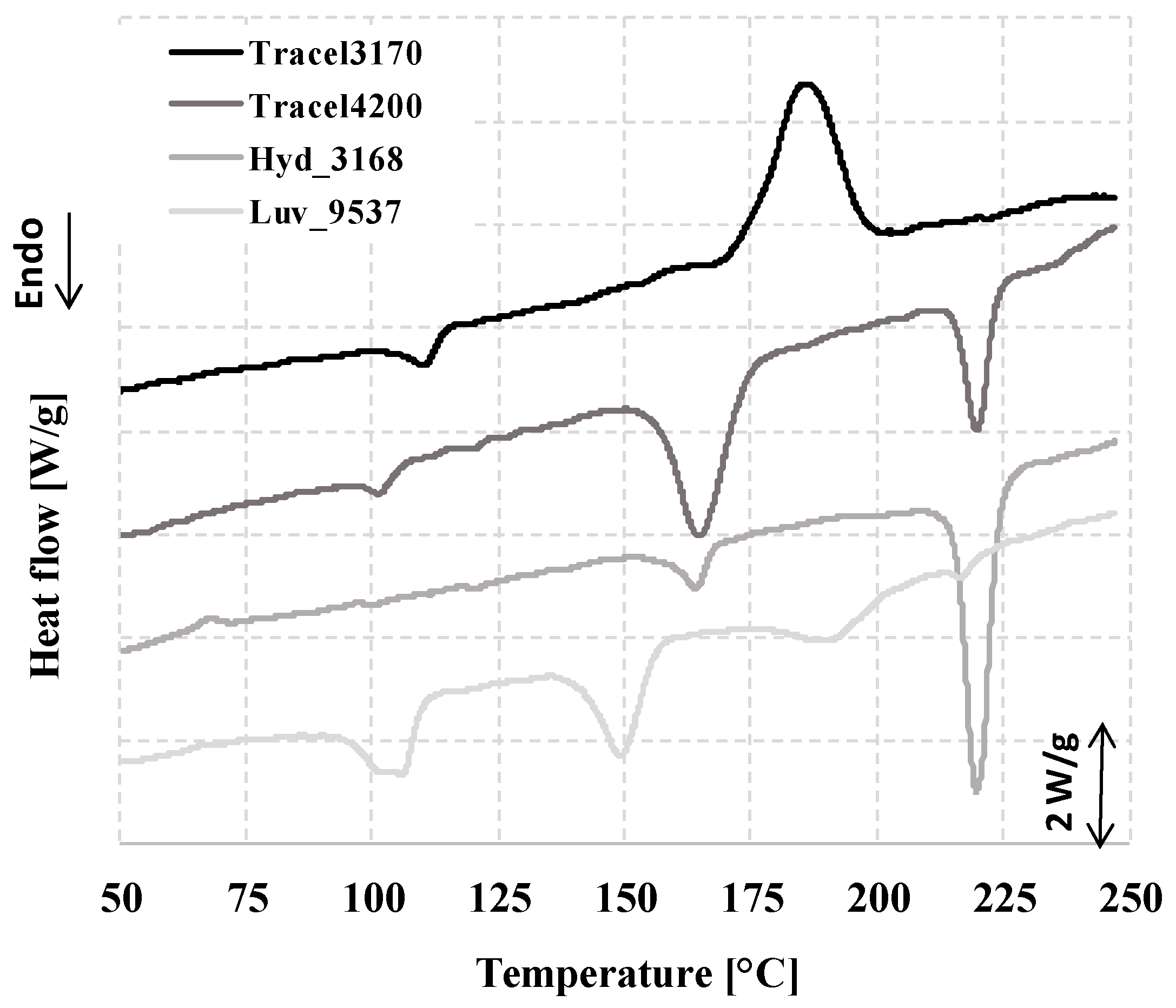
3.1. The Thermal and Morphological Properties of CBAs, TGA, TGA-FTIR, DSC
3.2. Isothermal Thermogravimetric Analysis
4. Chemical Foaming of Poly(Lactic Acid)
Foaming, Processing
5. Results of the Foamed Poly(Lactic Acid)
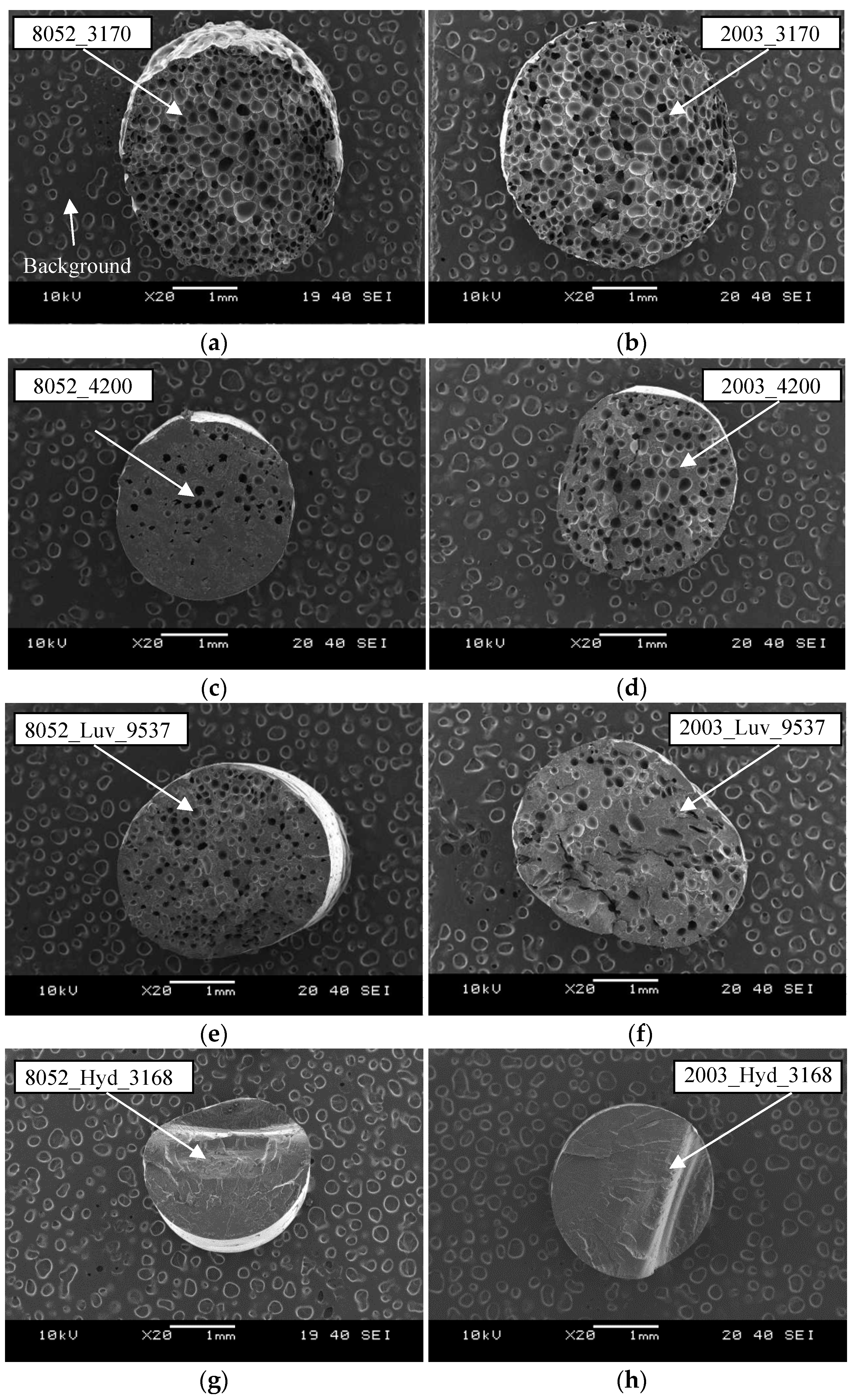
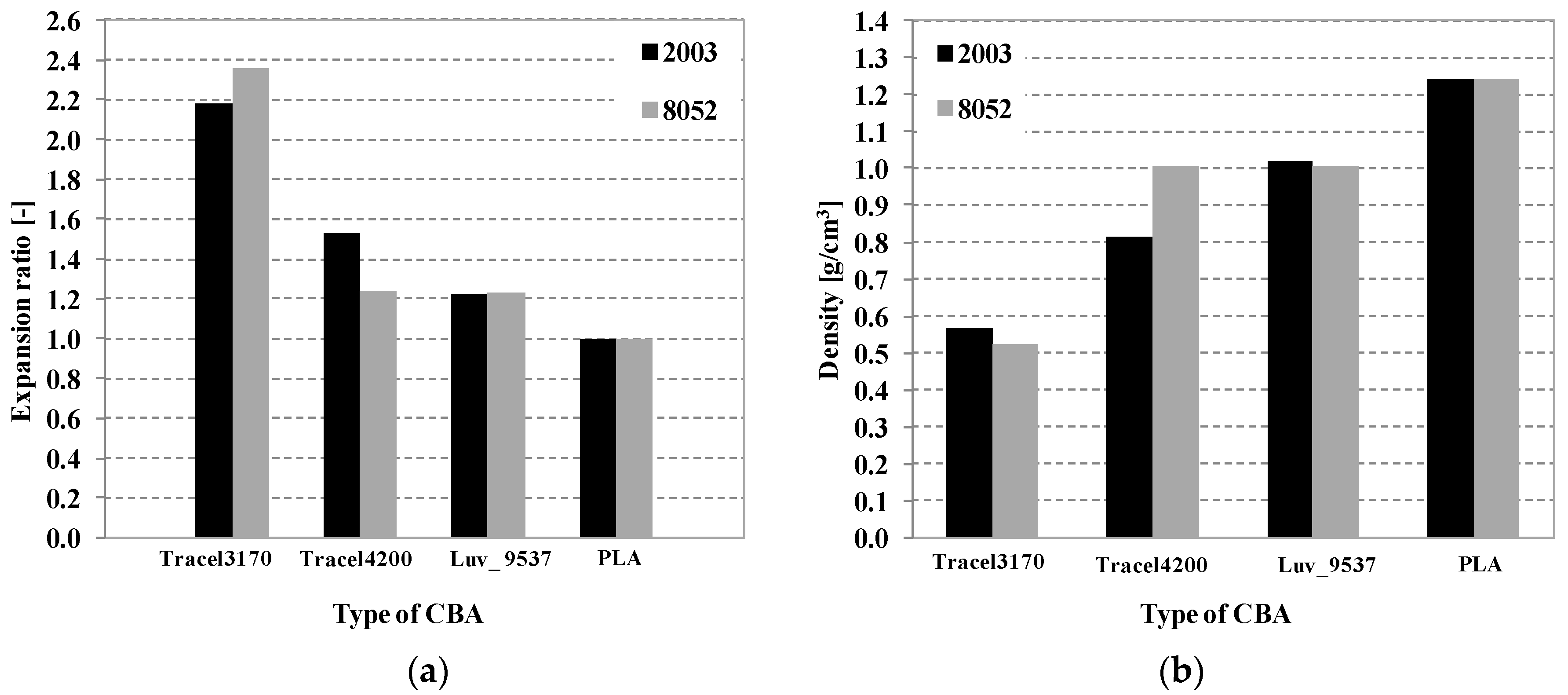
5.1. Scanning Electron Microscopy
5.2. The Morphology of Foam Structures
5.3. Differential Scanning Calorimetry
5.4. Foam Strength
6. Conclusions
Author Contributions
Funding
 Supported by the ÚNKP-17-4-III New National Excellence Program of the Ministry of Human Capacities. This research was also supported by The National Research, Development and Innovation Office (NVKP_16-1-2016-0012).
Supported by the ÚNKP-17-4-III New National Excellence Program of the Ministry of Human Capacities. This research was also supported by The National Research, Development and Innovation Office (NVKP_16-1-2016-0012).Acknowledgments
Conflicts of Interest
References
- Jost, V. Packaging related properties of commercially available biopolymers—An overview of the status quo. Express Polym. Lett. 2018, 12, 429–435. [Google Scholar] [CrossRef]
- Aeschelmann, F.; Carus, M. Biobased Building Blocks and Polymers in the World: Capacities, Production, and Applications–Status Quo and Trends Towards 2020. In Industrial Biotechnology; nova-Institut GmbH: Huerth, Germany, 2015; p. 154. [Google Scholar]
- Di Maio, E.; Kiran, E. Foaming of polymers with supercritical fluids and perspectives on the current knowledge gaps and challenges. J. Supercrit. Fluids 2018, 134, 157–166. [Google Scholar] [CrossRef]
- Wypych, G. Handbook of Foaming and Blowing Agents; ChemTec Publishing: Toronto, ON, Canada, 2017; Volume 1. [Google Scholar]
- Raps, D.; Hossieny, N.; Park, C.B.; Altstädt, V. Past and present developments in polymer bead foams and bead foaming technology. Polymer 2015, 56, 5–19. [Google Scholar] [CrossRef]
- Orbulov, I.; Katona, B. Structural Damages in Syntactic Metal Foams Caused by Monotone or Cyclic Compression. Periodica Polytech. Mech. Eng. 2017, 61, 146–152. [Google Scholar]
- Okolieocha, C.; Raps, D.; Subramaniam, K.; Altstädt, V. Microcellular to nanocellular polymer foams: Progress (2004–2015) and future directions—A review. Eur. Polym. J. 2015, 73, 500–519. [Google Scholar] [CrossRef]
- Lee, S.-T.; Park, C.B.; Ramesh, N.S. Polymeric Foams: Science and Technology; Taylor and Francis Groupe: Boca Raton, FL, USA, 2007. [Google Scholar]
- Lee, S.-T. Foam Extrusion, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Lee, S.-T. Polymer Foams Innovations in Processes, Technologies, and Products; CRC Press: Boca Raton, FL, USA, 2017. [Google Scholar]
- Khemani, K.C. Polymeric Foams Science and Technology; American Chemical Society: Washington, DC, USA, 1997. [Google Scholar]
- Eaves, D. Polymer Foams Trends in Use and Technology; Rapra Technology Limited: Shawbury, UK, 2001. [Google Scholar]
- Ludwiczak, J.; Kozlowski, M. Foaming of Polylactide in the Presence of Chain Extender. J. Polym. Environ. 2014, 23, 137. [Google Scholar] [CrossRef]
- Julien, J.M.; Quantin, J.-C.; Bénézet, J.-C.; Bergeret, A.; Lacrampe, M.F.; Krawczak, P. Chemical foaming extrusion of poly(lactic acid) with chain-extenders: Physical and morphological characterizations. Eur. Polym. J. 2015, 67, 40–49. [Google Scholar] [CrossRef]
- Julien, J.-M.; Bénézet, J.-C.; Lafranche, E.; Quantin, J.-C.; Bergeret, A.; Lacrampe, M.-F.; Krawczak, P. Development of poly(lactic acid) cellular materials: Physical and morphological characterizations. Polymer 2012, 53, 5885–5895. [Google Scholar] [CrossRef]
- Matuana, M.L.; Faruk, O.; Diaz, C.A. Cell morphology of extrusion foamed poly(lactic acid) using endothermic chemical foaming agent. Bioresour. Technol. 2009, 100, 5947–5954. [Google Scholar] [CrossRef] [PubMed]
- Tramaco Vertrieb und Verarbeitung von Chemieprodukten GmbH; Chemical Foaming Agents: Pinneberg, Germany, 2014.
- Yuan, H.; Liu, Z.; Ren, J. Preparation, Characterization, and Foaming Behavior of Poly(lactic acid)/Poly(butylene adipate-co-butylene terephthalate) Blend. Polym. Eng. Sci. 2009, 49, 1004–1012. [Google Scholar] [CrossRef]
- Niaounakis, M. Biopolymers: Applications and Trends; PDL Handbook Series; William Andrew: Chadds Ford, PA, USA, 2015. [Google Scholar]
- Nofar, M.; Park, C.B. Poly (lactic acid) foaming. Prog. Polym. Sci. 2014, 39, 1721–1741. [Google Scholar] [CrossRef]
- Bocz, K.; Tábi, T.; Vadas, D.; Sauceau, M.; Fages, J.; Marosi, G. Characterisation of natural fibre reinforced PLA foams prepared by supercritical CO2 assisted extrusion. Express Polym. Lett. 2016, 10, 3144. [Google Scholar] [CrossRef]
- Wypych, G. Databook of Blowing and Auxiliary Agent; ChemTech Publishing: Toronto, ON, Canada, 2016. [Google Scholar]
- Tábi, T.; Hajba, S.; Kovács, J.G. Effect of crystalline forms (α′ and α) of poly(lactic acid) on its mechanical, thermo-mechanical, heat deflection temperature and creep properties. Eur. Polym. J. 2016, 82, 232–243. [Google Scholar] [CrossRef]
- Xu, X.; Park, C.B.; Xu, D.; Pop-Iliev, R. Effects of Die Geometry on Cell Nucleation of PS Foams Blown With CO2. Polym. Eng. Sci. 2003, 43, 1378–1390. [Google Scholar] [CrossRef]
- Li, G.; Li, H.; Turng, L.S.; Gong, S.; Zhang, C. Measurement of gas solubility and diffusivity in polylactide. Fluid Phase Equilib. 2006, 246, 158–166. [Google Scholar] [CrossRef]
- National Institute of Standards and Technology IR Database. Available online: https://webbook.nist.gov/chemistry (accessed on 15 December 2017).
- Frackowiak, S.; Ludwiczak, J.; Leluk, K.; Orzechowski, K.; Kozlowski, M. Foamed poly(lactic acid) composites with carbonaceous fillers for electromagnetic shielding. Mater. Des. 2015, 65, 749–756. [Google Scholar] [CrossRef]







| CBA Trade Name | Type of CBA | Blowing Agent * | Effective Gases * | Other Information * | Abbreviation |
|---|---|---|---|---|---|
| Tracel IM 3170 MS | exothermic | azodicarbonamide | N2, CO, CO2, NH3 | - | Tracel3170 |
| Tracel IMC 4200 | endothermic | citric acid and baking soda | CO2, H2O | water is formed, it contains a nucleating agent | Tracel4200 |
| Hydrocerol CT 3168 | endothermic | n.d. | n.d. | recommended for PLA | Hyd_3168 |
| Luvobatch PE BA 9537 | endothermic | n.d. | n.d. | carrier PE | Luv_9537 |
| Sample | dTG Range N2 | Mass Reduction at 190 °C N2 | dTG Range Air | Mass Reduction at 190 °C Air |
|---|---|---|---|---|
| [-] | [°C] | [%] | [°C] | [%] |
| Tracel3170 | 145–239 | 7.5 | 147–212 | 2.8 |
| Tracel4200 | 145–188, 204–245 | 6.9 | 147–187, 196–232 | 6.5 |
| Hyd_3168 | 186–237 | 0.1 | 193–237 | 0.1 |
| Luv_9537 | 136–168, 168–225 | 4.8 | 137–165, 174–214 | 3.9 |
| Foaming Agent | Absorbance Maximums [-] | Type of Gas Generated | |
|---|---|---|---|
| Wave Number [cm−1] | Temperature [°C] | ||
| Tracel3170 | 600–750, 2250–2350, 3600–3750 | 183 | CO2 |
| 2050–2250 | 183 | CO | |
| 750-1250, 1450–1800, 3200–3500 | 183 | NH3 | |
| Tracel4200 | 600–750, 2250–2350, 3600–3750 | 168 | CO2 |
| 1600–1700, 3500–3700 | 168 | H2O | |
| Luv_9537 | 2050–2250 | 147 | CO |
| 600–750, 2250–2350, 3600–3750 | 147 | CO2 | |
| Hyd_3168 | 2050–2250 | 225 | CO |
| 600–750, 2250–2350, 3600–3750 | 225 | CO2 | |
| Sample | Decomposition Range According to dTG * N2 | Decomposition Range According to dTG * Air | State Change ** N2 |
|---|---|---|---|
| [-] | [°C] | [°C] | [°C] |
| Tracel3170 | 145–239 | 147–212 | 165–200 |
| Tracel4200 | 145–188, 204–245 | 147–187, 196–232 | 150–180, 210–225 |
| Hyd_3168 | 186–237 | 193–237 | 153–169, 208–229 |
| Luv_9537 | 136–168, 168–225 | 137–165, 174–214 | 136–160, 175–200 |
| Sample | Mass Reduction (10 °C/min, 190 °C) Air | Mass Reduction (Isothermal, 190 °C, 330 s) Air |
|---|---|---|
| [-] | [%] | [%] |
| Tracel3170 | 2.8 | 4.9 |
| Tracel4200 | 6.5 | 6.8 |
| Luv_9537 | 3.9 | 5.4 |
| Hyd_3168 | 0.1 | 0.9 |
| Sample Code | PLA Used | CBA Used | CBA Dosing | Note |
|---|---|---|---|---|
| [-] | [Type] | [Type] | [wt%] | [-] |
| 2003_ref | 2003D | - | 0 | extruded once |
| 8052_ref | 8052D | - | 0 | extruded once |
| 2003_3170 | 2003D | Tracel3170 | 2 | - |
| 8052_3170 | 8052D | Tracel3170 | 2 | - |
| 2003_4200 | 2003D | Tracel4200 | 2 | - |
| 8052_4200 | 8052D | Tracel4200 | 2 | - |
| 2003_Luv_9537 | 2003D | Luv_9537 | 2 | - |
| 8052_Luv_9537 | 8052D | Luv_9537 | 2 | - |
| 2003_Hyd_3168 | 2003D | Hyd_3168 | 2 | - |
| 8052_Hyd_3168 | 8052D | Hyd_3168 | 2 | - |
| Sample Code | Tmelt | Pressure Drop | |
|---|---|---|---|
| - | °C | bar | MPa |
| 2003_ref | 196 | 74 | 7.3 |
| 8052_ref | 196 | 43 | 4.2 |
| 2003_3170 | 196 | 32 | 3.2 |
| 8052_3170 | 195 | 21 | 2.0 |
| 2003_4200 | 196 | 14 | 1.3 |
| 8052_4200 | 196 | 13 | 1.2 |
| 2003_Luv_9537 | 196 | 18 | 1.7 |
| 8052_Luv_9537 | 196 | 34 | 3.3 |
| 2003_Hyd_3168 | 197 | 56 | 5.5 |
| 8052_Hyd_3168 | 196 | 46 | 4.5 |
| Type of Sample | Tg | Tc1 | Tm1 | Tm2 | ||||
|---|---|---|---|---|---|---|---|---|
| [-] | [°C] | [°C] | [J/g] | [°C] | [°C] | [J/g] | [%] | [%] |
| 2003_ref | 59.6 | 116.7 | 23.8 | 151.1 | - | 26.4 | 28.4 | 6.9 |
| 8052_ref | 59.2 | 115.9 | 25.2 | 150.3 | - | 28.8 | 31 | 3.1 |
| 2003_3170 | 57.5 | 108.4 | 26.5 | 147.3 | 153.8 | 28.1 | 30.2 | 1.7 |
| 8052_3170 | 59.6 | 107.5 | 27.4 | 147.6 | 154.8 | 31.4 | 33.8 | 4.3 |
| 2003_4200 | 59.3 | 114.5 | 29.5 | 147.2 | 154.2 | 32.2 | 34.6 | 2.9 |
| 8052_4200 | 59.6 | 107.9 | 29.3 | 143.7 | 154.9 | 30.5 | 32.8 | 1.3 |
| 2003_Luv_9537 | 57.2 | 105.6 | 28.8 | 146.4 | 154.4 | 30.1 | 32.4 | 1.4 |
| 8052_Luv_9537 | 55.4 | 105.4 | 33.7 | 147.1 | 154.8 | 34.5 | 37.1 | 0.9 |
| 2003_Hyd_3168 | 56.2 | 112.3 | 29.6 | 148.5 | 155.2 | 31 | 33.3 | 1.5 |
| 8052_Hyd_3168 | 57.5 | 108.3 | 27.4 | 148.5 | 155.5 | 28.3 | 30.4 | 1.0 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kmetty, Á.; Litauszki, K.; Réti, D. Characterization of Different Chemical Blowing Agents and Their Applicability to Produce Poly(Lactic Acid) Foams by Extrusion. Appl. Sci. 2018, 8, 1960. https://doi.org/10.3390/app8101960
Kmetty Á, Litauszki K, Réti D. Characterization of Different Chemical Blowing Agents and Their Applicability to Produce Poly(Lactic Acid) Foams by Extrusion. Applied Sciences. 2018; 8(10):1960. https://doi.org/10.3390/app8101960
Chicago/Turabian StyleKmetty, Ákos, Katalin Litauszki, and Dániel Réti. 2018. "Characterization of Different Chemical Blowing Agents and Their Applicability to Produce Poly(Lactic Acid) Foams by Extrusion" Applied Sciences 8, no. 10: 1960. https://doi.org/10.3390/app8101960





