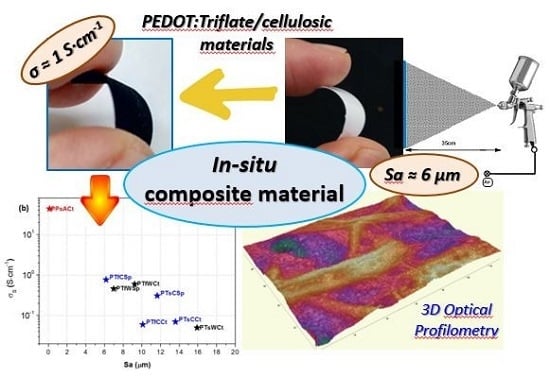
In-Situ Approaches for the Preparation of Polythiophene-Derivative Cellulose Composites with High Flexibility and Conductivity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Cellulose-Polythiophene Materials
2.2.1. Cellulosic Substrates
2.2.2. Synthesis of Monomers and Polymerization
2.2.3. Polythiophenes-Derivative/Cellulose Composite Materials: In-Situ Polymerization
2.2.4. Polythiophenes-Derivative/Cellulose Composite Materials: Conjugated Polymers Solutions
2.3. Characterization
3. Results and Discussions
3.1. Polymerization
3.2. Synthesis of PDBProDOT
3.3. Preparation of Polythiophene-Derivative/Cellulose Composite Materials
3.4. Characterization of Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Shirakawa, H.; Louis, E.J.; MacDiarmid, A.G.; Chiang, C.K.; Heeger, A.J. Synthesis of electrically conducting organic polymers: Halogen derivatives of polyacetylene (CH)x. J. Chem. Soc. Chem. Commun. 1977, 16, 578–580. [Google Scholar] [CrossRef]
- Elschner, A.; Kirchmeyer, S.; Lovenich, W.; Merker, U.; Reuter, K. PEDOT: Principles and Applications of an Intrinsically Conductive Polymer; CRC Press: Boca Raton, FL, USA, 2010; pp. 167–244. ISBN 9781420069112. [Google Scholar]
- Snook, G.A.; Kao, P.; Best, A.S. Conducting-polymer-based supercapacitor devices and electrodes. J. Power Sources 2011, 196, 1–12. [Google Scholar] [CrossRef]
- Ghosh, S.; Inganas, O. Conducting polymer hydrogels as 3D electrodes: Applications for supercapacitors. Adv. Mater. 1999, 11, 1214–1218. [Google Scholar] [CrossRef]
- Nyholm, L.; Nyström, G.; Mihranyan, A.; Stromme, M. Toward flexible polymer and paper-based energy storage devices. Adv. Mater. 2011, 23, 3751–3769. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Shin, M.K.; Kim, S.H.; Cho, H.U.; Spinks, G.M.; Wallace, G.G.; Lima, M.D.; Lepró, X.; Kozlov, M.E.; Baughman, R.H.; et al. Ultrafast charge and discharge biscrolled yarn supercapacitors for textiles and microdevices. Nat. Commun. 2013, 4, 1–8. [Google Scholar] [CrossRef]
- Carlberg, J.C.; Inganas, O. Fast optical spectroscopy of the electrochemical doping of poly(3,4-ethylenedioxythiophene). J. Electrochem. Soc. 1998, 145, 3810–3814. [Google Scholar] [CrossRef]
- Otero, T.F.; Caballero-Romero, M. Conformational energy from the oxidation kinetics of poly(3,4-ethylenedioxythiophene) films. Polym. Int. 2010, 59, 329–336. [Google Scholar] [CrossRef]
- D’Arcy, J.M.; El-Kady, M.F.; Khine, P.P.; Zhang, L.; Lee, S.H.; Davis, N.R.; Liu, D.S.; Yeung, M.T.; Kim, S.Y.; Turner, C.L.; et al. Vapor-phase polymerization of nanofibrillar poly(3,4-ethylenedioxythiophene) for supercapacitors. ACS Nano 2014, 8, 1500–1510. [Google Scholar] [CrossRef]
- Aharoni, S.M. Rigid backbone polymers XXI: Stress-strain behaviour of uncrosslinked and of crosslinked rodlike polyisocyanates. Polymer 1981, 22, 418–419. [Google Scholar] [CrossRef]
- Heeger, A.J. Semiconducting and metallic polymers: The fourth generation of polymeric materials. Angew. Chem. Int. Ed. 2001, 40, 2591–2611. [Google Scholar] [CrossRef]
- Tokito, S.; Smith, P.; Heeger, A.J. Mechanical and electrical properties of poly-(2,5-thienylene vinylene) fibers. Synth. Met. 1990, 36, 183–194. [Google Scholar] [CrossRef]
- Bruner, C.; Dauskardt, R. Role of molecular weight on the mechanical device properties of organic polymer solar cells. Macromolecules 2014, 47, 1117–1121. [Google Scholar] [CrossRef]
- Zhang, Y.Z.; Wang, Y.; Cheng, T.; Lai, W.Y.; Pang, H.; Huang, W. Flexible supercapacitors based on paper substrates: A new paradigm for low-cost energy storage. Chem. Soc. Rev. 2015, 44, 5181–5199. [Google Scholar] [CrossRef] [PubMed]
- Kurra, N.; Park, J.; Alshareef, H.N. A conducting polymer nucleation scheme for efficient solid-state supercapacitors on paper. J. Mater. Chem. A 2014, 2, 17058–17065. [Google Scholar] [CrossRef]
- Wang, Z.; Tammela, P.; Huo, J.; Zhang, P.; Strømme, M.; Nyholm, L. Solution-processed poly(3,4-ethylenedioxythiophene) nanocomposite paper electrodes for high-capacitance flexible supercapacitors. J. Mater. Chem. A 2016, 4, 1714–1722. [Google Scholar] [CrossRef]
- Wang, Z.; Tammela, P.; Strømme, M.; Nyholm, L. Cellulose-based supercapacitors: Material and performance considerations. Adv. Energy Mater. 2017, 7, 1700130. [Google Scholar] [CrossRef]
- Shi, X.; Hu, Y.; Li, M.; Duan, Y.Y.; Wang, Y.; Chen, L.; Zhang, L. Highly specific capacitance materials constructed via in situ synthesis of polyaniline in a cellulose matrix for supercapacitors. Cellulose 2014, 21, 2337–2347. [Google Scholar] [CrossRef]
- Agarwal, M.; Lvov, Y.; Varahramyan, K. Conductive wood microfibres for smart paper through layer-by-layer nanocoating. Nanotechnology 2006, 17, 5319–5325. [Google Scholar] [CrossRef]
- De Francisco, R.; Hoyos, M.; García, N.; Tiemblo, P. Superhydrophobic and highly luminescent polyfluorene/silica hybrid coatings deposited onto glass and cellulose-based substrates. Langmuir 2015, 31, 3718–3726. [Google Scholar] [CrossRef]
- De Francisco, R.; Tiemblo, P.; Hoyos, M.; González-Arellano, C.; García, N.; Berglund, L.; Synytska, A. Multipurpose ultra and superhydrophobic surfaces based on oligodimethylsiloxane-modified nanosilica. ACS Appl. Mater. Interfaces 2014, 6, 18998–19010. [Google Scholar] [CrossRef]
- De Leeuw, D.M.; Kraakman, P.A.; Bongaerts, P.F.G.; Mutsaers, C.M.J.; Klaassen, D.B.M. Electroplating of conductive polymers for the metallization of insulators. Synth. Met. 1994, 66, 263–273. [Google Scholar] [CrossRef]
- Gueye, M.N.; Carella, A.; Massonnet, N.; Yvenou, E.; Brenet, S.; Faure-Vincent, J.; Pouget, S.; Rieutord, F.; Okuno, H.; Benayad, A.; et al. Structure and dopant engineering in PEDOT thin films: Practical tools for a dramatic conductivity enhancement. Chem. Mater. 2016, 28, 3462–3468. [Google Scholar] [CrossRef]
- Welsh, D.M.; Kloeppner, L.J.; Madrigal, L.; Pinto, M.R.; Thompson, B.C.; Schanze, K.S.; Abboud, K.A.; Powell, D.; Reynolds, J.R. Regiosymmetric dibutyl-substituted poly(3,4-propylenedioxythiophene)s as highly electron-rich electroactive and luminescent polymers. Macromolecules 2002, 35, 6517–6525. [Google Scholar] [CrossRef]
- Reeves, B.D.; Grenier, C.R.G.; Argun, A.A.; Cirpan, A.; McCarley, T.D.; Reynolds, J.R. Spray coatable electrochromic dioxythiophene polymers with high coloration efficiencies. Macromolecules 2004, 37, 7559–7569. [Google Scholar] [CrossRef]
- Smits, F.M. Measurement of sheet resistivities with 4-point probe. Bell Syst. Tech. J. 1958, 37, 711–718. [Google Scholar] [CrossRef]
- Kim, T.Y.; Kim, J.E.; Suh, K.S. Effects of alcoholic solvents on the conductivity of tosylate-doped poly(3,4-ethylenedioxythiophene) (PEDOT-OTs). Polym. Int. 2006, 55, 80–86. [Google Scholar] [CrossRef]
- Ha, Y.H.; Nikolov, N.; Pollack, S.K.; Mastrangelo, J.; Martin, B.D.; Shashidhar, R. Towards a transparent, highly conductive poly (3,4-ethylenedioxythiophene). Adv. Funct. Mater. 2004, 14, 615–622. [Google Scholar] [CrossRef]
- Baluja, S.; Abdullah, E.; Alnayab, M.; Hirapara, A. Thermodynamic Models for Determination of solubility of cellulose acetate in various solvents at different temperatures. Acta Chim. Pharm. Indica 2017, 7, 1–10. [Google Scholar]
- Bicchieri, M.; Pepa, S. The degradation of cellulose with ferric and cupric ions in a low-acid medium. Restaurator 1996, 17, 165–183. [Google Scholar] [CrossRef]
- Tsuboi, M. Infrared spectrum and crystal structure of cellulose. J. Polym. Sci. 1957, 25, 159–171. [Google Scholar] [CrossRef]
- Zavastin, D.; Cretescu, I.; Bezdadea, M.; Bourceanu, M.; Drǎgan, M.; Lisa, G.; Mangalagiu, I.; Vasić, V.; Savić, J. Preparation, characterization and applicability of cellulose acetate-polyurethane blend membrane in separation techniques. Colloids Surfaces A Physicochem. Eng. Asp. 2010, 370, 120–128. [Google Scholar] [CrossRef]
- Chen, Y.; Qian, X.; An, X. Preparation and characterization of conductive paper via in situ polymerization of 3, 4-ethylenedioxythiophene. BioResources 2011, 6, 3410–3423. [Google Scholar]
- Lee, S.; Gleason, K.K. Enhanced optical property with tunable band gap of cross-linked PEDOT copolymers via oxidative chemical vapor deposition. Adv. Funct. Mater. 2015, 25, 85–93. [Google Scholar] [CrossRef]
- Damlin, P.; Kvarnström, C.; Ivaska, A. Electrochemical synthesis and in situ spectroelectrochemical characterization of poly(3,4-ethylenedioxythiophene) (PEDOT) in room temperature ionic liquids. J. Electroanal. Chem. 2004, 570, 113–122. [Google Scholar] [CrossRef]
- Sezai Sarac, A.; Gencturk, A.; Schulz, B.; Gilsing, H.D.; Serantoni, M. Nanoscale surface morphology and monomer concentration dependence on impedance of electrocoated 2,2-dimethyl-3,4-propylene-dioxythiophene on carbon fiber microelectrode. Nanosci. Nanotechnol. 2007, 7, 3543–3552. [Google Scholar] [CrossRef]
- Wang, X.; Grimoldi, A.; Håkansson, K.; Fall, A.; Granberg, H.; Mengistie, D.; Edberg, J.; Engquist, I.; Nilsson, D.; Berggren, M.; et al. Anisotropic conductivity of Cellulose-PEDOT:PSS composite materials studied with a generic 3D four-point probe tool. Org. Electron. Phys. Mater. Appl. 2019, 66, 258–264. [Google Scholar] [CrossRef]
- Logakis, E.; Pandis, C.H.; Pissis, P.; Pionteck, J.; Pötschke, P. Highly conducting poly(methyl methacrylate)/carbon nanotubes composites: Investigation on their thermal, dynamic-mechanical, electrical and dielectric properties. Compos. Sci. Technol. 2011, 71, 854–862. [Google Scholar] [CrossRef] [Green Version]
- Massonnet, N.; Carella, A.; De Geyer, A.; Faure-Vincent, J.; Simonato, J.P. Metallic behaviour of acid doped highly conductive polymers. Chem. Sci. 2015, 6, 412–417. [Google Scholar] [CrossRef]
- Morais, R.M.; Klem, M.S.; Ozório, M.S.; Gomes, T.C.; Alves, N. Roughness influence on the sheet resistance of the PEDOT:PSS printed on paper. Curr. Appl. Phys. 2018, 18, 254–260. [Google Scholar] [CrossRef] [Green Version]
- Takano, T.; Masunaga, H.; Fujiwara, A.; Okuzaki, H.; Sasaki, T. PEDOT nanocrystal in highly conductive PEDOT:PSS polymer films. Macromolecules 2012, 45, 3859–3865. [Google Scholar] [CrossRef]







| In-Situ Polymerization | |||
|---|---|---|---|
| Sample | Substrate | Proc. Technique | Oxidizing Agent |
| PTsWCt | W | Casting (Ct) | Fe(Tos)3 |
| PTsACt | A | ||
| PTsCCt | C | ||
| PTsWSp | W | Spray (Sp) | |
| PTsASp | A | ||
| PTsCSp | C | ||
| PTfWCt | W | Casting (Ct) | Fe(Trif)3 |
| PTfACt | A | ||
| PTfCCt | C | ||
| PTfWSp | W | Spray (Sp) | |
| PTfASp | A | ||
| PTfCSp | C | ||
| Conjugated Polymers Solution | |||
|---|---|---|---|
| Sample | Substrate | Proc. Technique | CPs * |
| PPsWCt | W | Casting (Ct) | PEDOT:PSS |
| PPsACt | A | ||
| PPsCCt | C | ||
| PPsWSp | W | Spray (Sp) | PEDOT:PSS |
| PPsASp | A | ||
| PPsCSp | C | ||
| PPDWCt | W | Casting (Ct) | PDBProDOT |
| PPDACt | A | ||
| PPDCCt | C | ||
| PPDWSp | W | Spray (Sp) | |
| PPDASp | A | ||
| PPDCSp | C | ||
| Pure Polymer Samples | σ (S∙cm−1) | σs (S∙cm−1) | Polymer content (wt.%) |
|---|---|---|---|
| PEDOT:Tosylate | 2.5 × 10−3 | 8.4 | - |
| PEDOT:Triflate | 4.9 × 10−1 | 6.6 | - |
| PEDOT:PSS | 4.5 × 10−4 | 49 | - |
| PDBProDOT | 1.0 × 10−3 | * | - |
| In-Situ Polymerization | |||
| PTsWCt | 4.2 × 10−5 | 0.05 | 43 |
| PTsCCt | 2.3 × 10−4 | 0.07 | 34 |
| PTsWSp | 6.3 × 10−6 | * | 29 |
| PTsCSp | 2.8 × 10−7 | 0.31 | 45 |
| PTfWCt | 3.6 × 10−4 | 0.60 | 80 |
| PTfCCt | 2.1 × 10−4 | 0.06 | 76 |
| PTfWSp | 6.7 × 10−5 | 0.46 | 24 |
| PTfCSp | 2.0 × 10−4 | 0.77 | 38 |
| Soluble CPs | |||
| PPsWCt | 6.0 × 10−7 | * | 80 |
| PPsACt | 3.0 × 10−5 | 43.67 | 68 |
| PPsWSp | 4.3 × 10−8 | * | 7 |
| PPsASp | 1.3 × 10−8 | * | 11 |
| PPsCSp | 2.5 × 10−4 | * | 24 |
| PPDWCt | 5.1 × 10−11 | * | 6 |
| PPDCCt | 4.2 × 10−10 | * | 6 |
| PPDWSp | 1.3 × 10−9 | * | * |
| PPDASp | 5.6 × 10−13 | * | 12 |
| PPDCSp | 4.9× 10−11 | * | 4 |
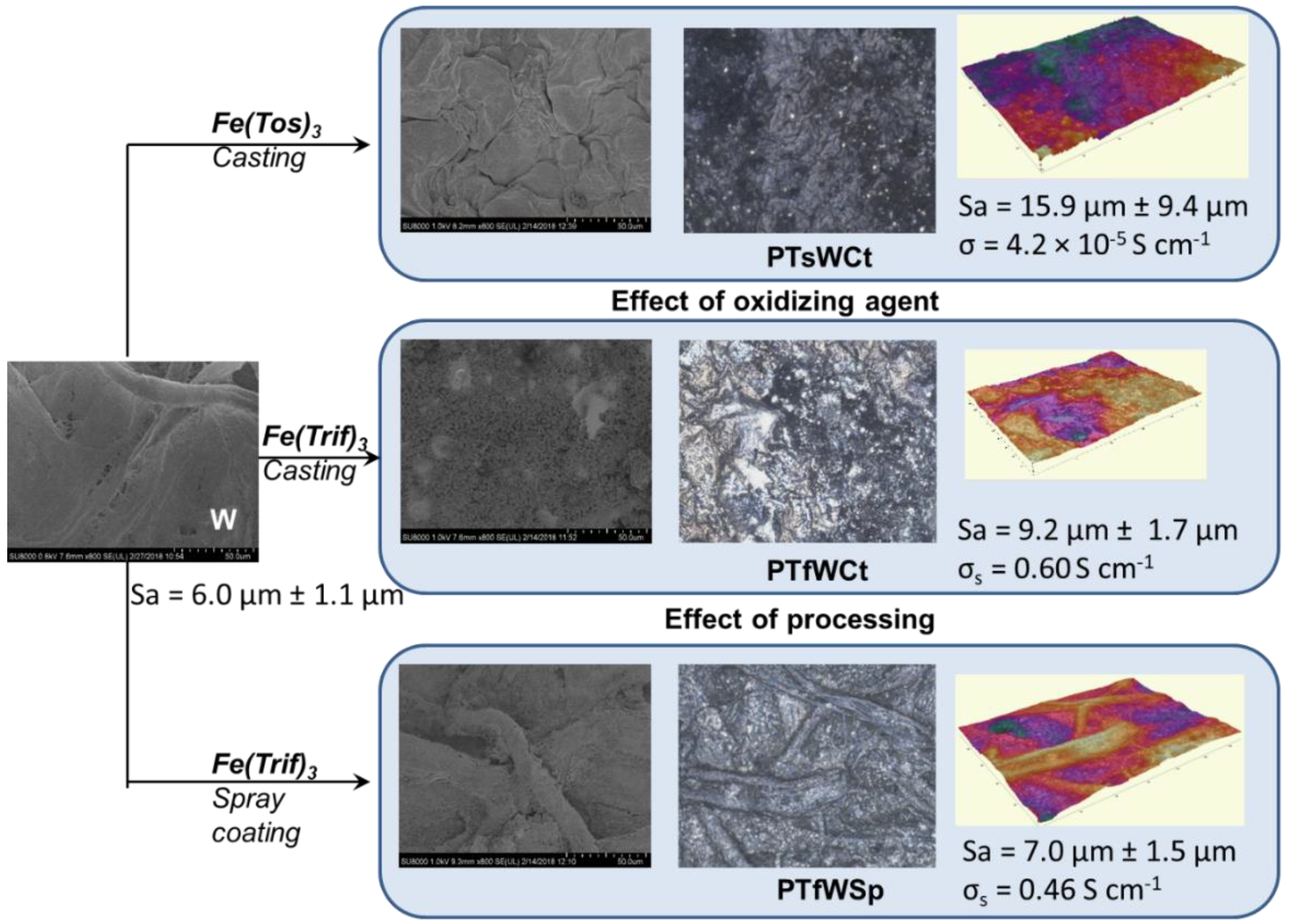
| Samples | σs (S∙cm−1) | PEDOT Content (wt.%) | PEDOT Mass Loading (mg∙cm−2) | Sa (μm) |
|---|---|---|---|---|
| PTsWCt | 0.05 | 43 | 7.4 | 15.9 ± 9.5 |
| PTsCCt | 0.07 | 34 | 5.6 | 13.6 ± 3.9 |
| PTsCSp | 0.31 | 45 | 8.8 | 11.6 ± 3.1 |
| PTfWCt | 0.60 | 80 | 36.4 | 9.2 ± 1.7 |
| PTfWSp | 0.46 | 24 | 2.7 | 7.0 ± 1.5 |
| PTfCSp | 0.77 | 38 | 6.7 | 6.2 ± 0.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
González, F.; Tiemblo, P.; Hoyos, M. In-Situ Approaches for the Preparation of Polythiophene-Derivative Cellulose Composites with High Flexibility and Conductivity. Appl. Sci. 2019, 9, 3371. https://doi.org/10.3390/app9163371
González F, Tiemblo P, Hoyos M. In-Situ Approaches for the Preparation of Polythiophene-Derivative Cellulose Composites with High Flexibility and Conductivity. Applied Sciences. 2019; 9(16):3371. https://doi.org/10.3390/app9163371
Chicago/Turabian StyleGonzález, Francisco, Pilar Tiemblo, and Mario Hoyos. 2019. "In-Situ Approaches for the Preparation of Polythiophene-Derivative Cellulose Composites with High Flexibility and Conductivity" Applied Sciences 9, no. 16: 3371. https://doi.org/10.3390/app9163371