Femtosecond Laser Processing of Thick Film Cathodes and Its Impact on Lithium-Ion Diffusion Kinetics
Abstract
:1. Introduction
2. Materials and Methods
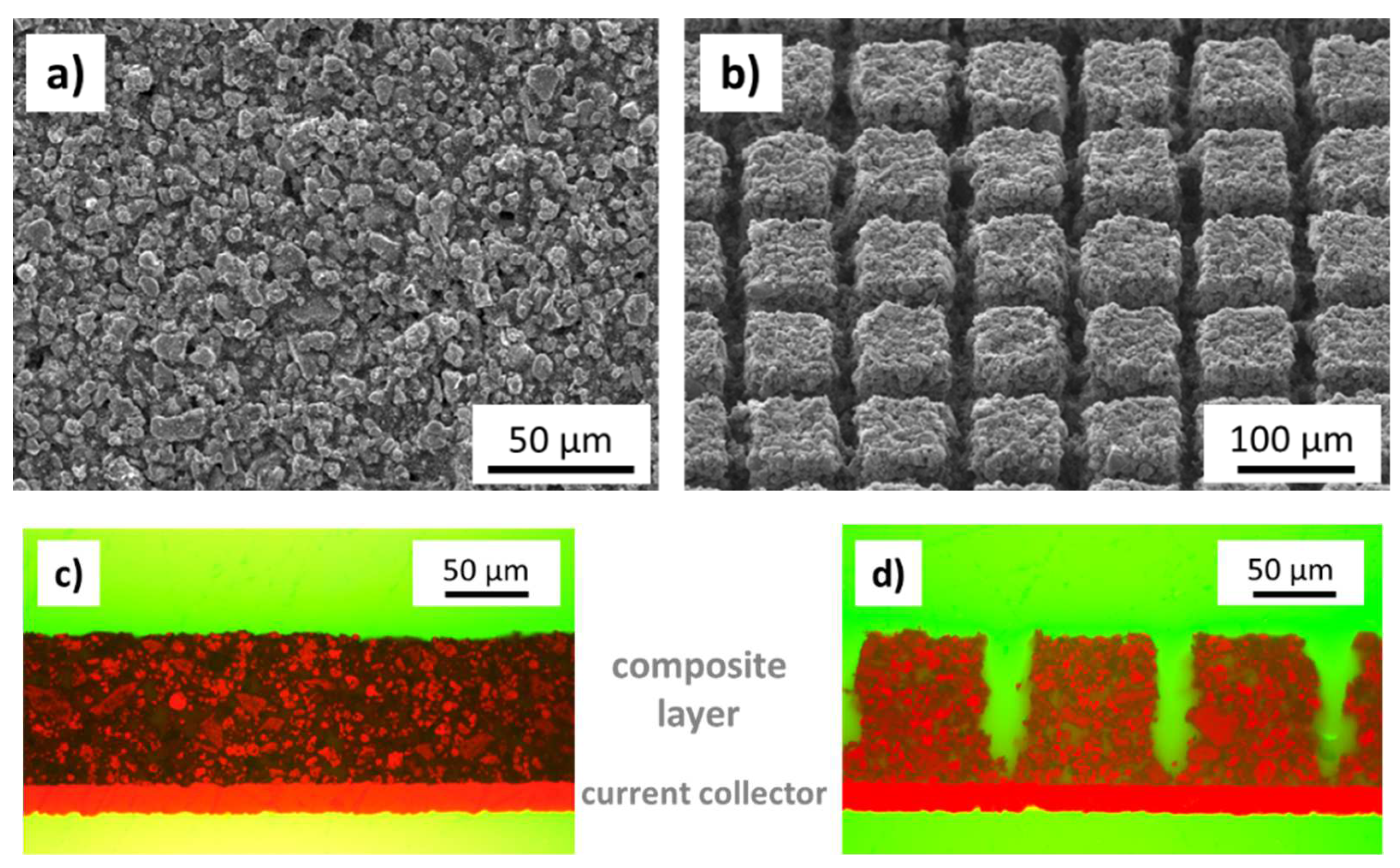
2.1. Cathode Material and Electrodes
2.2. Laser Structuring
2.3. Electrochemical Analysis
2.3.1. Galvanostatic Measurements
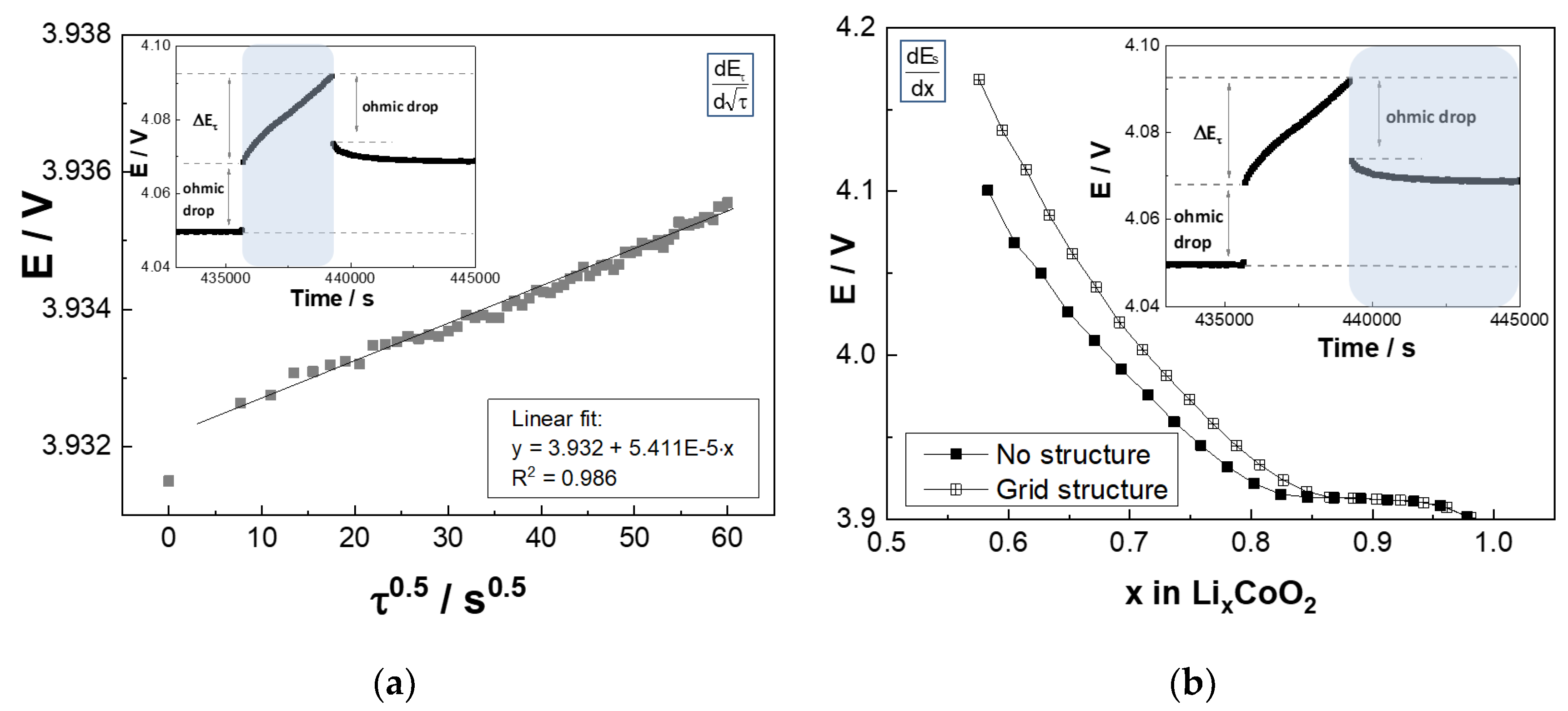
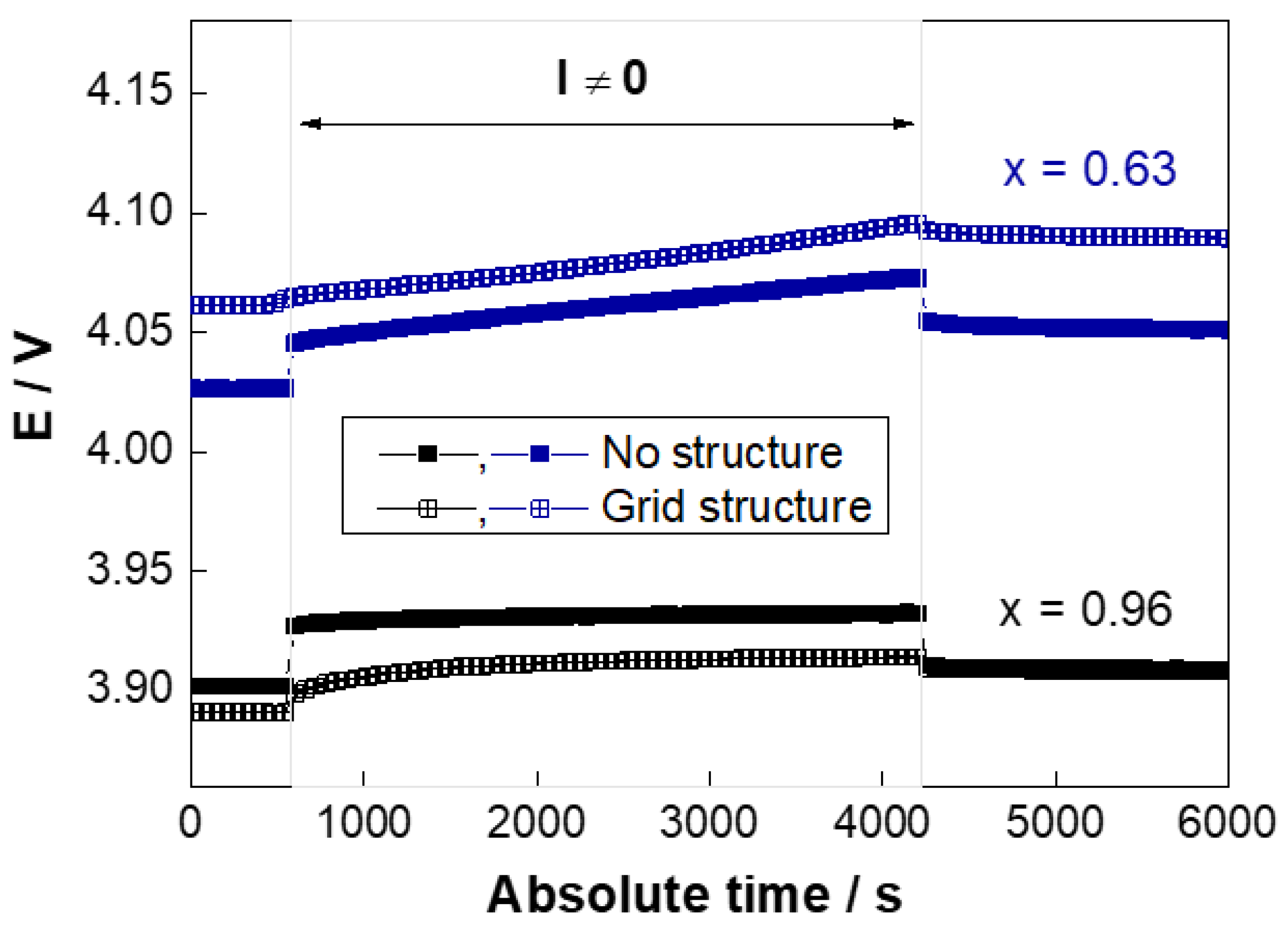
2.3.2. Galvanostatic Intermittent Titration Technique
3. Results and Discussion
3.1. Galvanostatic Cycling—Capacity Retention
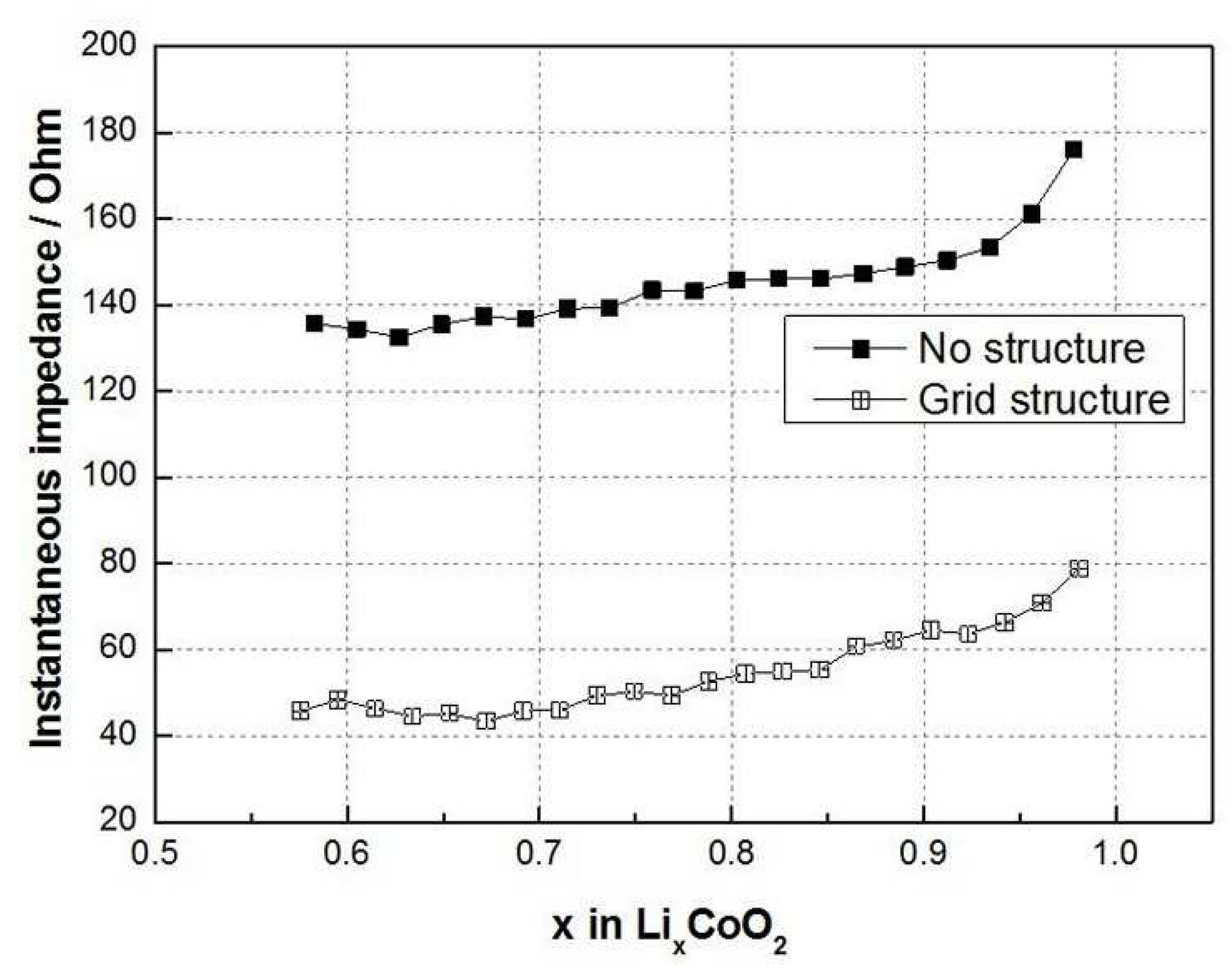
3.2. GITT—Chemical Diffusion Coefficient
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goodenough, J.B.; Kim, Y. Challenges for rechargeable Li batteries. Chem. Mater. 2010, 22, 587–603. [Google Scholar] [CrossRef]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Amatucci, G.; Du Pasquier, A.; Blyr, A.; Zheng, T.; Tarascon, J.M. The elevated temperature performance of the LiMn2O4/C system: Failure and solutions. Electrochim. Acta 1999, 45, 255–271. [Google Scholar] [CrossRef]
- Sakti, A. Quantification of Performance and Cost Trajectory of Li-ion Battery Designs for Personal Vehicle Electrification in the Near Future. Ph.D. Thesis, Carnegie Mellon University, Pittsburgh, PA, USA, 2013. [Google Scholar]
- Andre, D.; Kim, S.-J.; Lamp, P.; Lux, S.F.; Maglia, F.; Paschos, O.; Stiaszny, B. Future generations of cathode materials: An automotive industry perspective. J. Mater. Chem. A 2015, 3, 6709–6732. [Google Scholar] [CrossRef]
- Rakebrandt, J.-H.; Smyrek, P.; Zheng, Y.; Seifert, H.J.; Pfleging, W. Laser processing of thick Li(NiMnCo)O2 electrodes for lithium-ion batteries. In Laser-Based Micro-and Nanoprocessing XI; International Society for Optics and Photonics: Bellingham, WA, USA, 2017; Volume 10092, p. 100920M. [Google Scholar]
- Zheng, Y.; Seifert, H.J.; Shi, H.; Zhang, Y.; Kübel, C.; Pfleging, W. 3D silicon/graphite composite electrodes for high-energy lithium-ion batteries. Electrochim. Acta 2019, 317, 502–508. [Google Scholar] [CrossRef]
- Long, J.W.; Dunn, B.; Rolison, D.R.; White, H.S. Three-dimensional battery architectures. Chem. Rev. 2004, 104, 4463–4492. [Google Scholar] [CrossRef] [PubMed]
- Oudenhoven, J.F.M.; Baggetto, L.; Notten, P.H.L. All-Solid-State Lithium-Ion Microbatteries: A Review of Various Three-Dimensional Concepts. Adv. Energy Mater. 2011, 1, 10–33. [Google Scholar] [CrossRef]
- Kohler, R.; Besser, H.; Hagen, M.; Ye, J.; Ziebert, C.; Ulrich, S.; Pröll, J.; Pfleging, W. Laser micro-structuring of magnetron-sputtered SnOx thin films as anode material for lithium ion batteries. Microsyst. Technol. 2011, 17, 225–232. [Google Scholar] [CrossRef]
- Shi, H.; Liu, X.; Wu, R.; Zheng, Y.; Li, Y.; Cheng, X.; Pfleging, W.; Zhang, Y. In Situ SEM Observation of Structured Si/C Anodes Reactions in an Ionic-Liquid-Based Lithium-Ion Battery. Appl. Sci. 2019, 9, 956. [Google Scholar] [CrossRef]
- Xia, H.; Wan, Y.H.; Assenmacher, W.; Mader, W.; Yuan, G.L.; Lu, L. Facile synthesis of chain-like LiCoO2 nanowire arrays as three-dimensional cathode for microbatteries. NPG Asia Mater. 2014, 6, e126. [Google Scholar] [CrossRef]
- Xiong, W.; Xia, Q.Y.; Xia, H. Three-dimensional self-supported metal oxides as cathodes for microbatteries. Funct. Mater. Lett. 2014, 7, 1430003. [Google Scholar] [CrossRef]
- Ferrari, S.; Loveridge, M.; Beattie, S.D.; Jahn, M.; Dashwood, R.J.; Bhagat, R. Latest advances in the manufacturing of 3D rechargeable lithium microbatteries. J. Power Sources 2015, 286, 25–46. [Google Scholar] [CrossRef] [Green Version]
- Pfleging, W.; Pröll, J. A new approach for rapid electrolyte wetting in tape cast electrodes for lithium-ion batteries. J. Mater. Chem. A 2014, 2, 14918–14926. [Google Scholar] [CrossRef]
- Lim, D.G.; Chung, D.W.; Kohler, R.; Pröll, J.; Scherr, C.; Pfleging, W.; Garcia, R.E. Designing 3D Conical-Shaped Lithium-Ion Microelectrodes. J. Electrochem. Soc. 2014, 161, A302–A307. [Google Scholar] [CrossRef]
- Pikul, J.H.; Zhang, H.G.; Cho, J.; Braun, P.V.; King, W.P. High-power lithium ion microbatteries from interdigitated three-dimensional bicontinuous nanoporous electrodes. Nat. Commun. 2013, 4, 1732. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.G.; Yu, X.D.; Braun, P.V. Three-dimensional bicontinuous ultrafast-charge and -discharge bulk battery electrodes. Nat. Nanotechnol. 2011, 6, 277–281. [Google Scholar] [CrossRef] [PubMed]
- Pröll, J.; Kim, H.; Piqué, A.; Seifert, H.J.; Pfleging, W. Laser-printing and femtosecond-laser structuring of LiMn2O4 composite cathodes for Li-ion microbatteries. J. Power Sources 2014, 255, 116–124. [Google Scholar] [CrossRef]
- Kohler, R.; Pröll, J.; Bruns, M.; Ulrich, S.; Seifert, H.J.; Pfleging, W. Conical surface structures on model thin-film electrodes and tape-cast electrode materials for lithium-ion batteries. Appl. Phys. A 2013, 112, 77–85. [Google Scholar] [CrossRef]
- Kim, H.; Pröll, J.; Kohler, R.; Pfleging, W.; Pique, A. Laser-Printed and Processed LiCoO2 CathodeThick Films for Li-Ion Microbatteries. J. Laser Micro Nanoeng. 2012, 7, 320–325. [Google Scholar] [CrossRef]
- Kim, J.S.; Pfleging, W.; Kohler, R.; Seifert, H.J.; Kim, T.Y.; Byun, D.; Jung, H.G.; Choi, W.C.; Lee, J.K. Three-dimensional silicon/carbon core-shell electrode as an anode material for lithium-ion batteries. J. Power Sources 2015, 279, 13–20. [Google Scholar] [CrossRef]
- Pfleging, W. A review of laser electrode processing for development and manufacturing of lithium-ion batteries. Nanophotonics 2018, 7, 549–573. [Google Scholar] [CrossRef]
- Tang, X.-X.; Liu, W.; Ye, B.-Y.; Tang, Y. Preparation of current collector with blind holes and enhanced cycle performance of silicon-based anode. Trans. Nonferrous Met. Soc. China 2013, 23, 1723–1727. [Google Scholar] [CrossRef]
- Zhang, N.; Zheng, Y.; Trifonova, A.; Pfleging, W. Laser structured Cu foil for high-performance lithium-ion battery anodes. J. Appl. Electrochem. 2017, 47, 829–837. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; An, Z.; Smyrek, P.; Seifert, H.J.; Kunze, T.; Lang, V.; Lasagni, A.F.; Pfleging, W. Direct laser interference patterning and ultrafast laser-induced micro/nano structuring of current collectors for lithium-ion batteries. In Laser-based Micro-and Nanoprocessing X; International Society for Optics and Photonics: Bellingham, WA, USA, 2016; Volume 9736, p. 97361B. [Google Scholar]
- Notten, P.H.L.; Roozeboom, F.; Niessen, R.A.H.; Baggetto, L. 3-D integrated all-solid-state rechargeable batteries. Adv. Mater. 2007, 19, 4564–4567. [Google Scholar] [CrossRef]
- Kohler, R.; Smyrek, P.; Ulrich, S.; Bruns, M.; Trouillet, V.; Pfleging, W. Patterning and annealing of nanocrystalline LiCoO2 thin films. J. Optoelectron. Adv. Mater. 2010, 12, 547–552. [Google Scholar]
- Pröll, J.; Kohler, R.; Torge, M.; Ulrich, S.; Ziebert, C.; Bruns, M.; Seifert, H.J.; Pfleging, W. Laser microstructuring and annealing processes for lithium manganese oxide cathodes. Appl. Surf. Sci. 2011, 257, 9968–9976. [Google Scholar] [CrossRef]
- Pröll, J.; Weidler, P.G.; Kohler, R.; Mangang, A.; Heissler, S.; Seifert, H.J.; Pfleging, W. Comparative studies of laser annealing technique and furnace annealing by X-ray diffraction and Raman analysis of lithium manganese oxide thin films for lithium-ion batteries. Thin Solid Films 2013, 531, 160–171. [Google Scholar] [CrossRef]
- Gotcu, P.; Seifert, H.J. Thermophysical properties of LiCoO2-LiMn2O4 blended electrode materials for Li-ion batteries. Phys. Chem. Chem. Phys. 2016, 18, 10550–10562. [Google Scholar] [CrossRef]
- Manthiram, A.; Muraliganth, T. Lithium Intercalation Cathode Materials for Lithium-Ion Batteries. In Handbook of Battery Material; Daniel, C., Besenhar, J.O., Eds.; Wiley-VCH: Weinheim, Germany, 2011; pp. 343–375. [Google Scholar]
- Mangang, M.; Seifert, H.J.; Pfleging, W. Influence of laser pulse duration on the electrochemical performance of laser structured LiFePO4 composite electrodes. J. Power Sources 2016, 304, 24–32. [Google Scholar] [CrossRef]
- Smyrek, P.; Pröll, J.; Seifert, H.J.; Pfleging, W. Laser-Induced Breakdown Spectroscopy of Laser-Structured Li(NiMnCo)O2 Electrodes for Lithium-Ion Batteries. J. Electrochem. Soc. 2016, 163, A19–A26. [Google Scholar] [CrossRef]
- Gotcu, P.; Pfleging, W.; Smyrek, P.; Seifert, H.J. Thermal behaviour of LixMeO2 (Me = Co or Ni + Mn + Co) cathode materials. Phys. Chem. Chem. Phys. 2017, 19, 11920–11930. [Google Scholar] [CrossRef] [PubMed]
- Weppner, W.; Huggins, R.A. Determination of the Kinetic Parameters of Mixed-Conducting Electrodes and Application to the System Li3Sb. J. Electrochem. Soc. 1977, 124, 1569–1578. [Google Scholar] [CrossRef]
- Smyrek, P.; Bergfeldt, T.; Seifert, H.J.; Pfleging, W. Laser-induced breakdown spectroscopy for the quantitative measurement of lithium concentration profiles in structured and unstructured electrodes. J. Mater. Chem. A 2019, 7, 5656–5665. [Google Scholar] [CrossRef] [Green Version]
- Reimers, J.N.; Dahn, J.R. Electrochemical and Insitu X-Ray-Diffraction Studies of Lithium Intercalation in LixCoO2. J. Electrochem. Soc. 1992, 139, 2091–2097. [Google Scholar] [CrossRef]
- Chen, Z.H.; Dahn, J.R. Methods to obtain excellent capacity retention in LiCoO2 cycled to 4.5 V. Electrochim. Acta 2004, 49, 1079–1090. [Google Scholar] [CrossRef]
- Reynier, Y.; Graetz, J.; Swan-Wood, T.; Rez, P.; Yazami, R.; Fultz, B. Entropy of Li intercalation in LixCoO2. Phys. Rev. B 2004, 70, 174304. [Google Scholar] [CrossRef]
- Reimers, J.N.; Dahn, J.R.; Vonsacken, U. Effects of Impurities on the Electrochemical Properties of LiCoO2. J. Electrochem. Soc. 1993, 140, 2752–2754. [Google Scholar] [CrossRef]
- Chang, K.; Hallstedt, B.; Music, D.; Fischer, J.; Ziebert, C.; Ulrich, S.; Seifert, H.J. Thermodynamic description of the layered O3 and O2 structural LiCoO2–CoO2 pseudo-binary systems. Calphad 2013, 41, 6–15. [Google Scholar] [CrossRef]
- Hong, J.S.; Selman, J.R. Relationship between calorimetric and structural characteristics of lithium-ion cells—II. Determination of Li transport properties. J. Electrochem. Soc. 2000, 147, 3190–3194. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfleging, W.; Gotcu, P. Femtosecond Laser Processing of Thick Film Cathodes and Its Impact on Lithium-Ion Diffusion Kinetics. Appl. Sci. 2019, 9, 3588. https://doi.org/10.3390/app9173588
Pfleging W, Gotcu P. Femtosecond Laser Processing of Thick Film Cathodes and Its Impact on Lithium-Ion Diffusion Kinetics. Applied Sciences. 2019; 9(17):3588. https://doi.org/10.3390/app9173588
Chicago/Turabian StylePfleging, Wilhelm, and Petronela Gotcu. 2019. "Femtosecond Laser Processing of Thick Film Cathodes and Its Impact on Lithium-Ion Diffusion Kinetics" Applied Sciences 9, no. 17: 3588. https://doi.org/10.3390/app9173588





