Ion Channel Properties of a Cation Channelrhodopsin, Gt_CCR4
Abstract
:1. Introduction
2. Materials and Methods
2.1. Expression Plasmids
2.2. Cell Culture
2.3. Electrophysiology
2.4. Optics
2.5. Confocal Images
2.6. Statistical Analysis
3. Results
3.1. Basic Characterization of Photocurrent
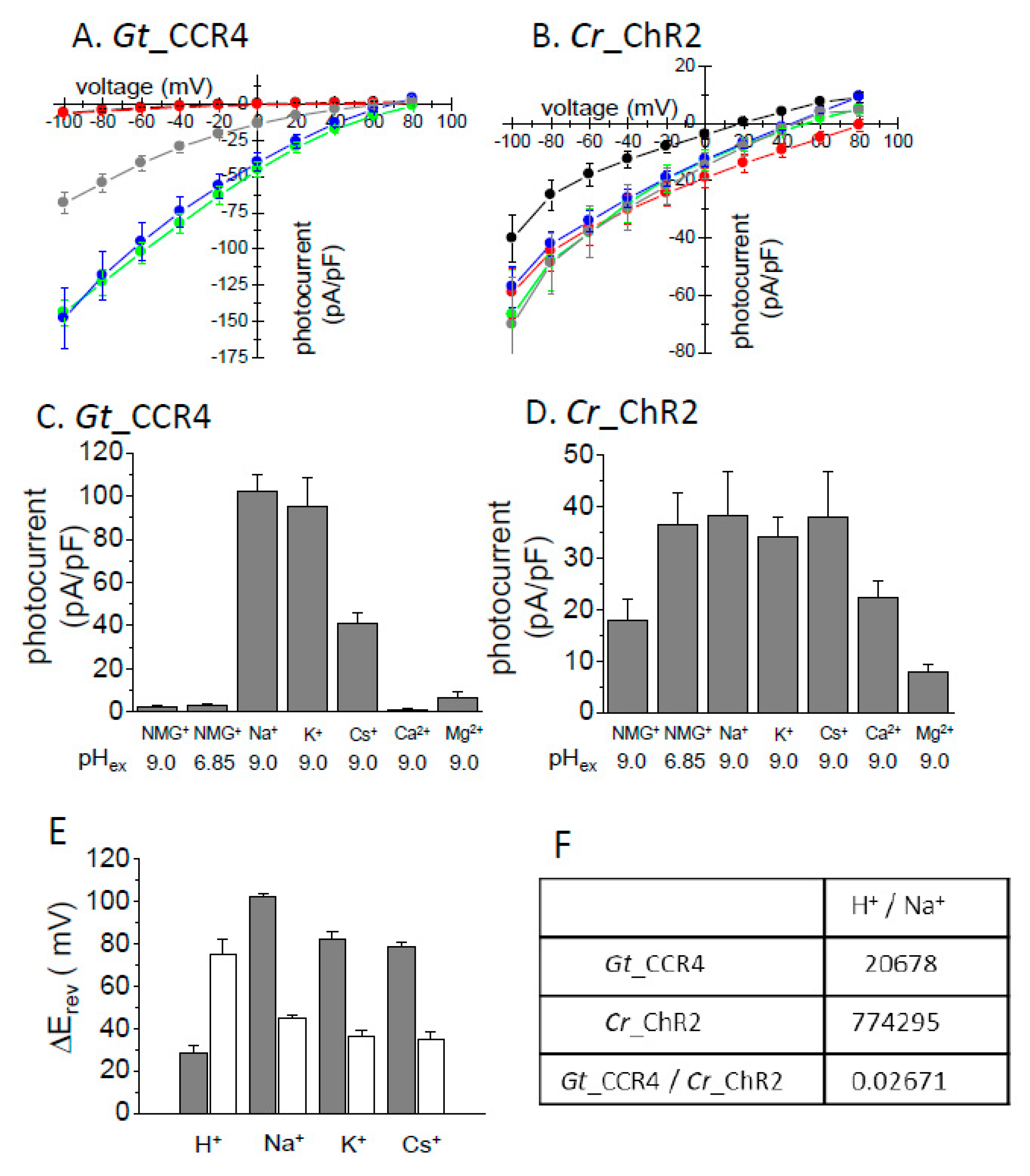
3.2. Ion Selectivity
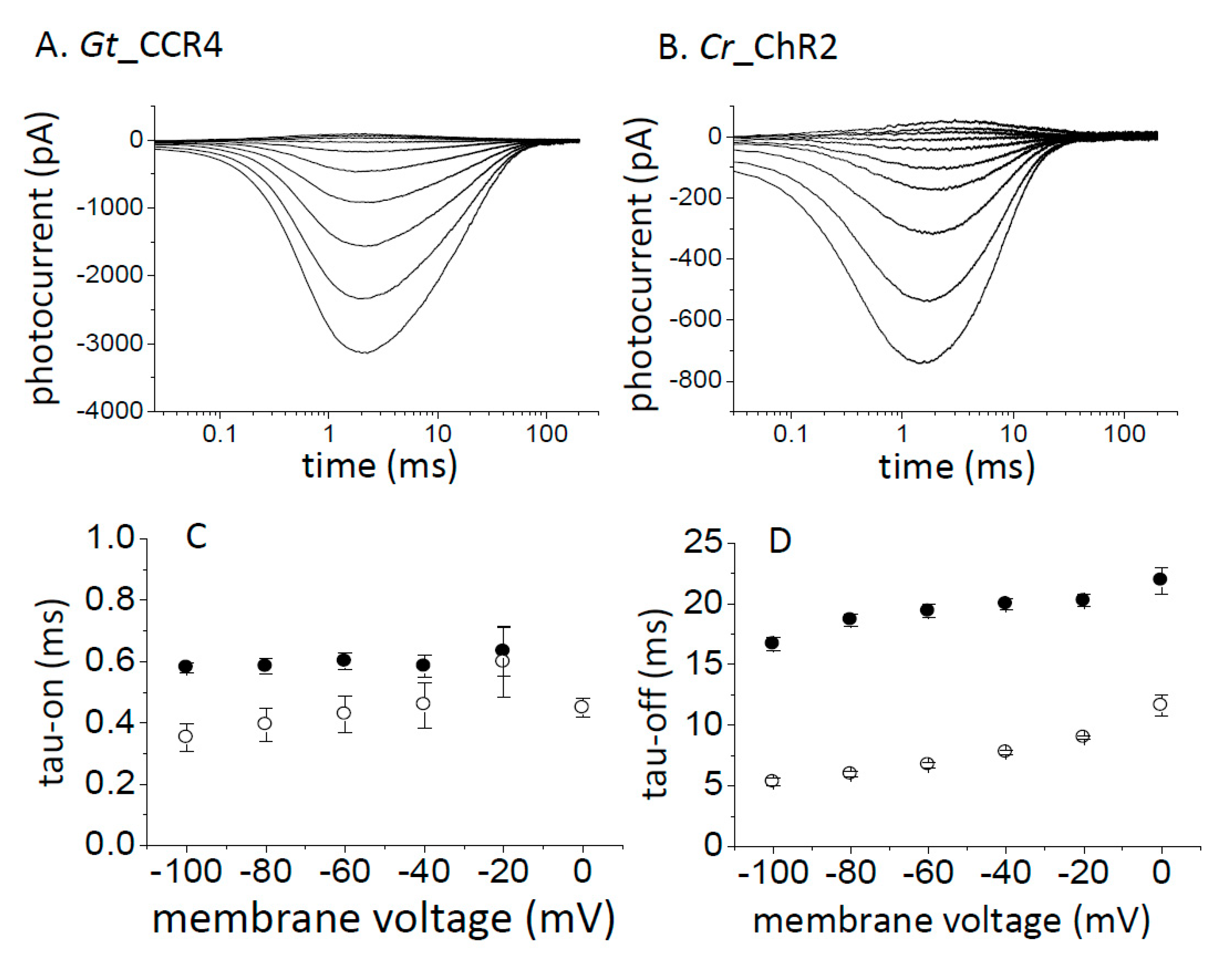
3.3. Flash Laser Electrophysiology
3.4. High Frequency Stimulation
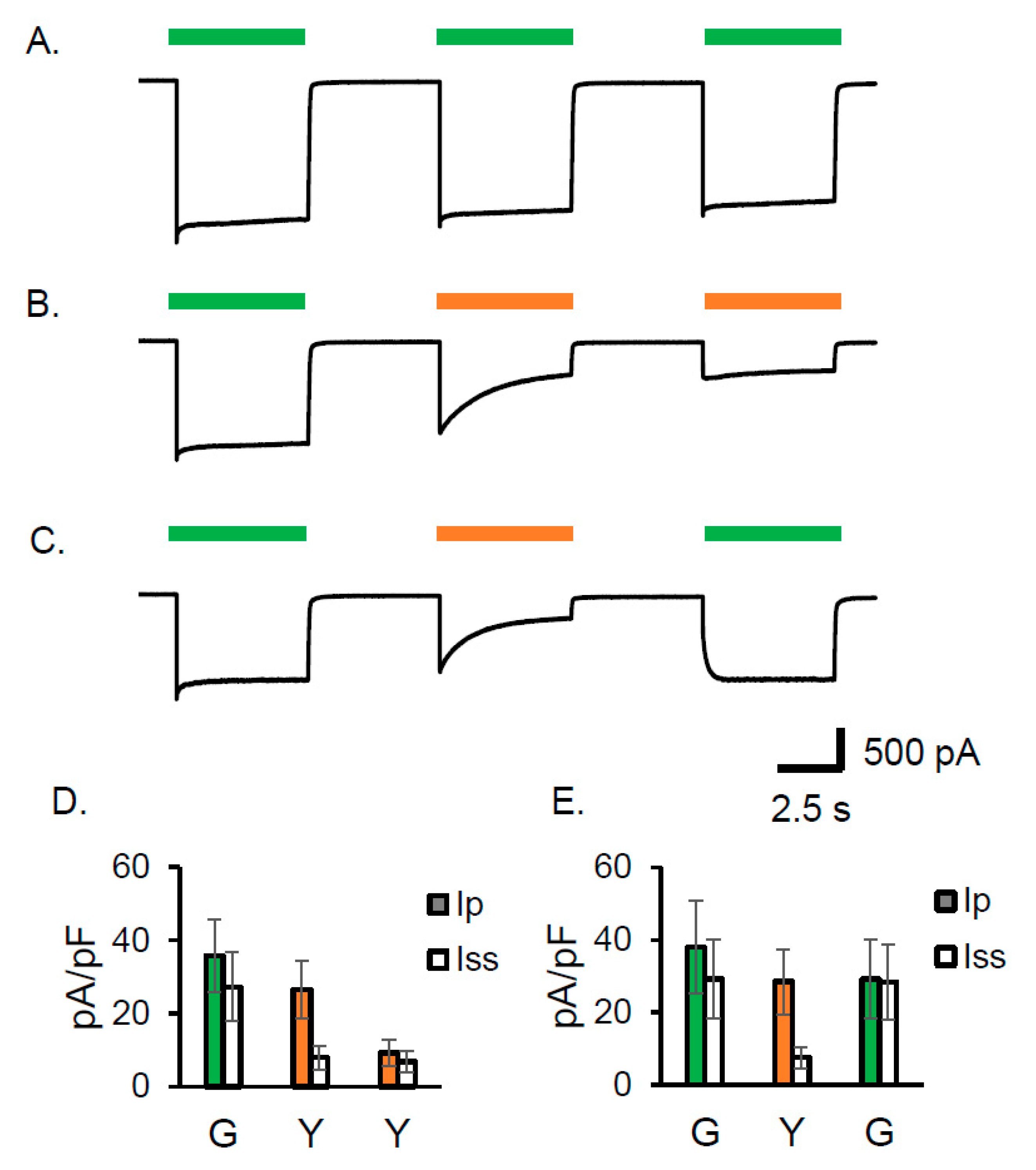
3.5. Gt_CCR4 Is Inactivated by 590 nm Light But Fully Reactivated by Blue Light
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ernst, O.P.; Lodowski, D.T.; Elstner, M.; Hegemann, P.; Brown, L.S.; Kandori, H. Microbial and animal rhodopsins: Structures, functions, and molecular mechanisms. Chem. Rev. 2014, 114, 126–163. [Google Scholar] [CrossRef] [PubMed]
- Oesterhelt, D.; Stoeckenius, W. Rhodopsin-like protein from the purple membrane of Halobacterium halobium. Nat. New Biol. 1971, 233, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Matsuno-Yagi, A.; Mukohata, Y. Two Possible Roles of Bacteriorhodopsin; a Comparative Study of Strains of Halobacz’eril’m Halobium Differing in Pigmentation. Biochem. Biophys. Res. Commun. 1977, 78, 237–243. [Google Scholar] [CrossRef]
- Inoue, K.; Ono, H.; Abe-Yoshizumi, R.; Yoshizawa, S.; Ito, H.; Kogure, K.; Kandori, K. A light-driven sodium ion pump in marine bacteria. Nat. Commun. 2013, 4, 1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inoue, K.; Ito, S.; Kato, Y.; Nomura, Y.; Shibata, M.; Uchihashi, T.; Tsunoda, S.P.; Kandori, H. A natural light-driven inward proton pump. Nat. Commun. 2016, 7, 13415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polovinkin, V.; Rodriguez-Valera, F.; Bueldt, G.; Kovalev, K.; Alekseev, A.; Gordeliy, V.; Chizhov, I.; Juettner, J.; Bamann, C.; Manstein, D.J.; et al. Inward H + pump xenorhodopsin: Mechanism and alternative optogenetic approach. Sci. Adv. 2017, 3, e1603187. [Google Scholar]
- Nagel, G.; Ollig, D.; Fuhrmann, M.; Kateriya, S.; Musti, A.M.; Bamberg, E.; Hegemann, P. Channelrhodopsin-1: A Light-Gated Proton Channel in Green Algae. Science 2002, 296, 2395–2398. [Google Scholar] [CrossRef]
- Nagel, G.; Szellas, T.; Huhn, W.; Kateriya, S.; Adeishvili, N.; Berthold, P.; Ollig, D.; Hegemann, P.; Bamberg, E. Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc. Natl. Acad. Sci. USA 2003, 100, 13940–13945. [Google Scholar] [CrossRef] [Green Version]
- Kato, H.E.; Zhang, F.; Yizhar, O.; Ramakrishnan, C.; Nishizawa, T.; Hirata, K.; Ito, J.; Aita, Y.; Tsukazaki, T.; Hayashi, S.; et al. Crystal structure of the channelrhodopsin light-gated cation channel. Nature 2012, 482, 369–374. [Google Scholar] [CrossRef] [Green Version]
- Volkov, O.; Kovalev, K.; Polovinkin, V.; Borshchevskiy, V.; Bamann, C.; Astashkin, R.; Marin, E.; Popov, A.; Balandin, T.; Willbold, D.; et al. Structural insights into ion conduction by channelrhodopsin 2. Science 2017, 358, pii: eaan8862. [Google Scholar] [CrossRef]
- Boyden, E.S.; Zhang, F.; Bamberg, E.; Nagel, G.; Deisseroth, K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat. Neurosci. 2005, 8, 1263–1268. [Google Scholar] [CrossRef]
- Ishizuka, T.; Kakuda, M.; Araki, R.; Yawo, H. Kinetic evaluation of photosensitivity in genetically engineered neurons expressing green algae light-gated channels. Neurosci. Res. 2006, 54, 85–94. [Google Scholar] [CrossRef]
- Schneider, F.; Grimm, C.; Hegemann, P. Biophysics of Channelrhodopsin. Annu. Rev. Biophys. 2015, 44, 167–186. [Google Scholar] [CrossRef] [Green Version]
- Prigge, M.; Schneider, F.; Tsunoda, S.P.; Shilyansky, C.; Wietek, J.; Deisseroth, K.; Hegemann, P. Color-tuned channelrhodopsins for multiwavelength optogenetics. J. Biol. Chem. 2012, 287, 31804–31812. [Google Scholar] [CrossRef]
- Wang, H.; Sugiyama, Y.; Hikima, T.; Sugano, E.; Tomita, H.; Takahashi, T.; Ishizuka, T.; Yawo, H. Molecular determinants differentiating photocurrent properties of two channelrhodopsins from Chlamydomonas. J. Biol. Chem. 2009, 284, 5685–5696. [Google Scholar] [CrossRef]
- Tsunoda, S.P.; Hegemann, P. Glu 87 of channelrhodopsin-1 causes pH-dependent color tuning and fast photocurrent inactivation. Photochem. Photobiol. 2009, 85, 564–569. [Google Scholar] [CrossRef]
- Klapoetke, N.C.; Murata, Y.; Kim, S.S.; Pulver, S.R.; Birdsey-Benson, A.; Cho, Y.K.; Morimoto, T.K.; Chuong, A.S.; Carpenter, E.J.; Tian, Z.; et al. Independent optical excitation of distinct neural populations. Nat. Methods 2014, 11, 338–346. [Google Scholar] [CrossRef] [Green Version]
- Govorunova, E.G.; Sineshchekov, O.A.; Li, H.; Janz, R.; Spudich, J.L. Characterization of a highly efficient blue-shifted channelrhodopsin from the marine alga platymonas subcordiformis. J. Biol. Chem. 2013, 288, 29911–29922. [Google Scholar] [CrossRef]
- Berndt, A.; Yizhar, O.; Gunaydin, L.A.; Hegemann, P.; Deisseroth, K. Bi-stable neural state switches. Nat. Neurosci. 2009, 12, 229–234. [Google Scholar] [CrossRef]
- Yizhar, O.; Fenno, L.E.; Prigge, M.; Schneider, F.; Davidson, T.J.; Ogshea, D.J.; Sohal, V.S.; Goshen, I.; Finkelstein, J.; Paz, J.T.; et al. Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 2011, 477, 171–178. [Google Scholar] [CrossRef]
- Kleinlogel, S.; Feldbauer, K.; Dempski, R.E.; Fotis, H.; Wood, P.G.; Bamann, C.; Bamberg, E. Ultra light-sensitive and fast neuronal activation with the Ca2+-permeable channelrhodopsin CatCh. Nat. Neurosci. 2011, 14, 513–518. [Google Scholar] [CrossRef]
- Vierock, J.; Grimm, C.; Nitzan, N.; Hegemann, P. Molecular determinants of proton selectivity and gating in the red-light activated channelrhodopsin Chrimson. Sci. Rep. 2017, 7, 1–15. [Google Scholar] [CrossRef]
- Wietek, J.; Wiegert, J.S.; Adeishvili, N.; Schneider, F.; Watanabe, H.; Tsunoda, S.P.; Vogt, A.; Elstner, M.; Oertner, T.G.; Hegemann, P. Conversion of channelrhodopsin into a light-gated chloride channel. Science 2014, 344, 409–412. [Google Scholar] [CrossRef]
- Berndt, A.; Lee, S.Y.; Ramakrishnan, C.; Deisseroth, K. Structure-guided transformation of channelrhodopsin into a light-activated chloride channel. Science 2014, 344, 420–424. [Google Scholar] [CrossRef]
- Govorunova, E.G.; Sineshchekov, O.A.; Janz, R.; Liu, X.; Spudich, J.L. NEUROSCIENCE. Natural light-gated anion channels: A family of microbial rhodopsins for advanced optogenetics. Science 2015, 349, 647–650. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kato, H.E.; Yamashita, K.; Ito, S.; Inoue, K.; Ramakrishnan, C.; Fenno, L.E.; Evans, K.E.; Paggi, J.M.; Dror, R.O.; et al. Crystal structure of the natural anion-conducting channelrhodopsin GtACR1. Nature 2018, 561, 343–348. [Google Scholar] [CrossRef]
- Kato, H.E.; Kim, Y.S.; Paggi, J.M.; Evans, K.E.; Allen, W.E.; Richardson, C.; Inoue, K.; Ito, S.; Ramakrishnan, C.; Fenno, L.E.; et al. Structural mechanisms of selectivity and gating in anion channelrhodopsins. Nature 2018, 561, 349–371. [Google Scholar] [CrossRef]
- Spudich, J.L.; Li, H.; Huang, C.-Y.; Wang, M.; Schafer, C.T.; Sineshchekov, O.A.; Zheng, L.; Govorunova, E.G. Crystal structure of a natural light-gated anion channelrhodopsin. Elife 2019, 8, 1–21. [Google Scholar]
- Govorunova, E.G.; Sineshchekov, O.A.; Spudich, J.L. Structurally Distinct Cation Channelrhodopsins from Cryptophyte Algae. Biophys. J. 2016, 110, 2302–2304. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, Y.; Konno, M.; Ito, S.; Tsunoda, S.P.; Inoue, K.; Kandori, H. Molecular properties of a DTD channelrhodopsin from Guillardia theta. Biophys. Physicobiol. 2017, 14, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Sugiyama, Y.; Wang, H.; Hikima, T.; Sato, M.; Kuroda, J.; Takahashi, T.; Ishizuka, T.; Yawo, H. Photocurrent attenuation by a single polar-to-nonpolar point mutation of channelrhodopsin-2. Photochem. Photobiol. Sci. 2009, 8, 328–336. [Google Scholar] [CrossRef]
- Dawydow, A.; Gueta, R.; Ljaschenko, D.; Ullrich, S.; Hermann, M.; Ehmann, N.; Gao, S.; Fiala, A.; Langenhan, T.; Nagel, G.; et al. Channelrhodopsin-2-XXL, a powerful optogenetic tool for low-light applications. Proc. Natl. Acad. Sci. USA 2014, 111, 13972–13977. [Google Scholar] [CrossRef]
- Nack, M.; Radu, I.; Gossing, M.; Bamann, C.; Bamberg, E.; von Mollard, G.F.; Heberle, J.; Hofkens, J.; Pozzo, J.L.; Tosic, O.; et al. The DC gate in Channelrhodopsin-2: Crucial hydrogen bonding interaction between C128 and D156. Photochem. Photobiol. Sci.USA 2010, 9, 194–198. [Google Scholar] [CrossRef]
- Sineshchekov, O.A.; Govorunova, E.G.; Li, H.; Spudich, J.L. Bacteriorhodopsin-like channelrhodopsins: Alternative mechanism for control of cation conductance. Proc. Natl. Acad. Sci. 2017, 114, E9512–E9519. [Google Scholar] [CrossRef] [Green Version]
- Lorenz-Fonfria, V.A.; Resler, T.; Krause, N.; Nack, M.; Gossing, M.; Fischer von Mollard, G.; Bamann, C.; Bamberg, E.; Schlesinger, R.; Heberle, J. Transient protonation changes in channelrhodopsin-2 and their relevance to channel gating. Proc. Natl. Acad. Sci. 2013, 110, E1273–E1281. [Google Scholar] [CrossRef] [Green Version]
- Wood, J.N.; Bevan, S.J.; Coote, P.R.; Dunn, P.M.; Harmar, A.; Hogan, P.; Latchman, D.S.; Morrison, C.; Rougon, G.; Theveniau, M.; et al. Novel Cell Lines Display Properties of Nociceptive Sensory Neurons. Proc. R. Soc. B Biol. Sci. 1990, 241, 187–194. [Google Scholar]
- Owen, S.F.; Liu, M.H.; Kreitzer, A.C. Thermal constraints on in vivo optogenetic manipulations. Nat. Neurosci. 2019, 22, 1061–1065. [Google Scholar] [CrossRef]
- Duan, X.; Nagel, G.; Gao, S. Mutated Channelrhodopsins with Increased Sodium and Calcium Permeability. Appl. Sci. 2019, 9, 664. [Google Scholar] [CrossRef]
- Kuhne, J.; Vierock, J.; Tennigkeit, S.A.; Dreier, M.-A.; Wietek, J.; Petersen, D.; Gavriljuk, K.; El-Mashtoly, S.F.; Hegemann, P.; Gerwert, K. Unifying photocycle model for light adaptation and temporal evolution of cation conductance in channelrhodopsin-2. Proc. Natl. Acad. Sci USA 2019, 116, 9380–9389. [Google Scholar] [CrossRef] [Green Version]
- Kawanabe, A.; Furutani, Y.; Jung, K.H.; Kandori, H. Photochromism of Anabaena sensory rhodopsin. J. Am. Chem. Soc. 2007, 129, 8644–8649. [Google Scholar] [CrossRef]
- Zamani, A.; Sakuragi, S.; Ishizuka, T.; Yawo, H. Kinetic characteristics of chimeric channelrhodopsins implicate the molecular identity involved in desensitization. Biophys. Physicobiol. 2017, 14, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.Y. A User’s Guide to Channelrhodopsin Variants: Features, Limitations and Future Developments. Exp. Physiol. 2011, 96, 19–25. [Google Scholar] [CrossRef]


© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shigemura, S.; Hososhima, S.; Kandori, H.; Tsunoda, S.P. Ion Channel Properties of a Cation Channelrhodopsin, Gt_CCR4. Appl. Sci. 2019, 9, 3440. https://doi.org/10.3390/app9173440
Shigemura S, Hososhima S, Kandori H, Tsunoda SP. Ion Channel Properties of a Cation Channelrhodopsin, Gt_CCR4. Applied Sciences. 2019; 9(17):3440. https://doi.org/10.3390/app9173440
Chicago/Turabian StyleShigemura, Shunta, Shoko Hososhima, Hideki Kandori, and Satoshi P. Tsunoda. 2019. "Ion Channel Properties of a Cation Channelrhodopsin, Gt_CCR4" Applied Sciences 9, no. 17: 3440. https://doi.org/10.3390/app9173440



