Improved Capacity Retention of SiO2-Coated LiNi0.6Mn0.2Co0.2O2 Cathode Material for Lithium-Ion Batteries
Abstract
:1. Introduction
2. Experiment
2.1. Material Preparation
2.2. Microstructure Characterization
2.3. Electrochemical Measurement
3. Results and Discussion
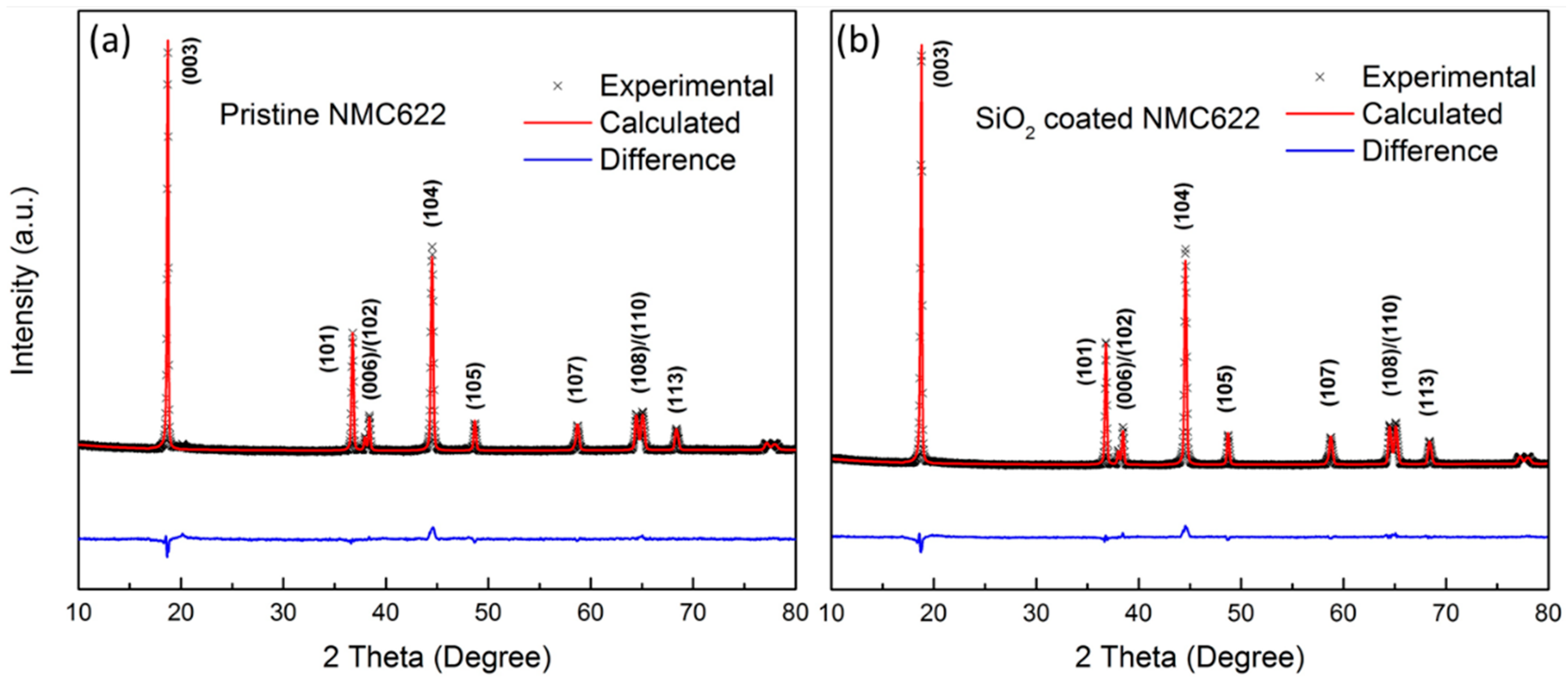
3.1. Material Characterization
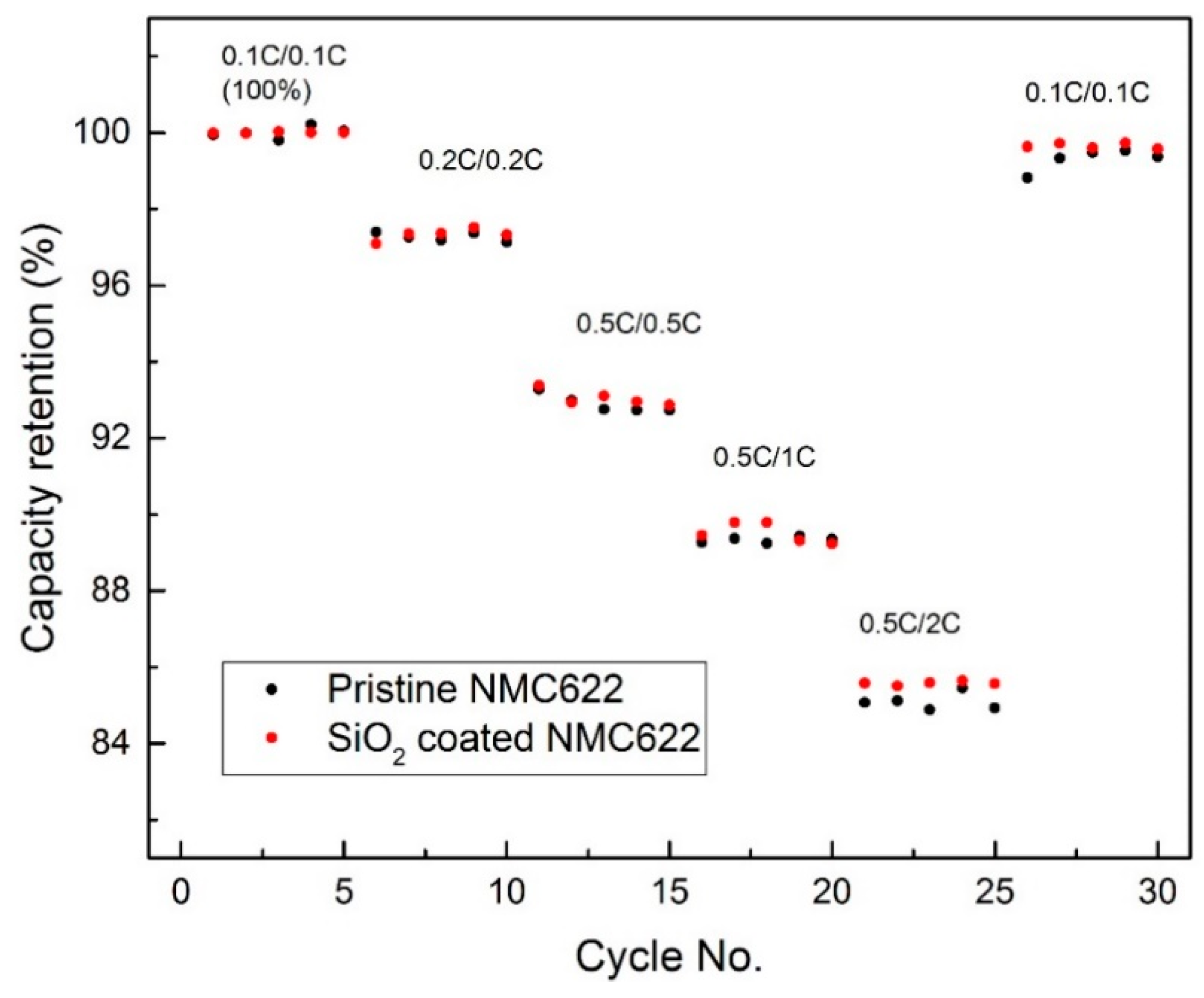
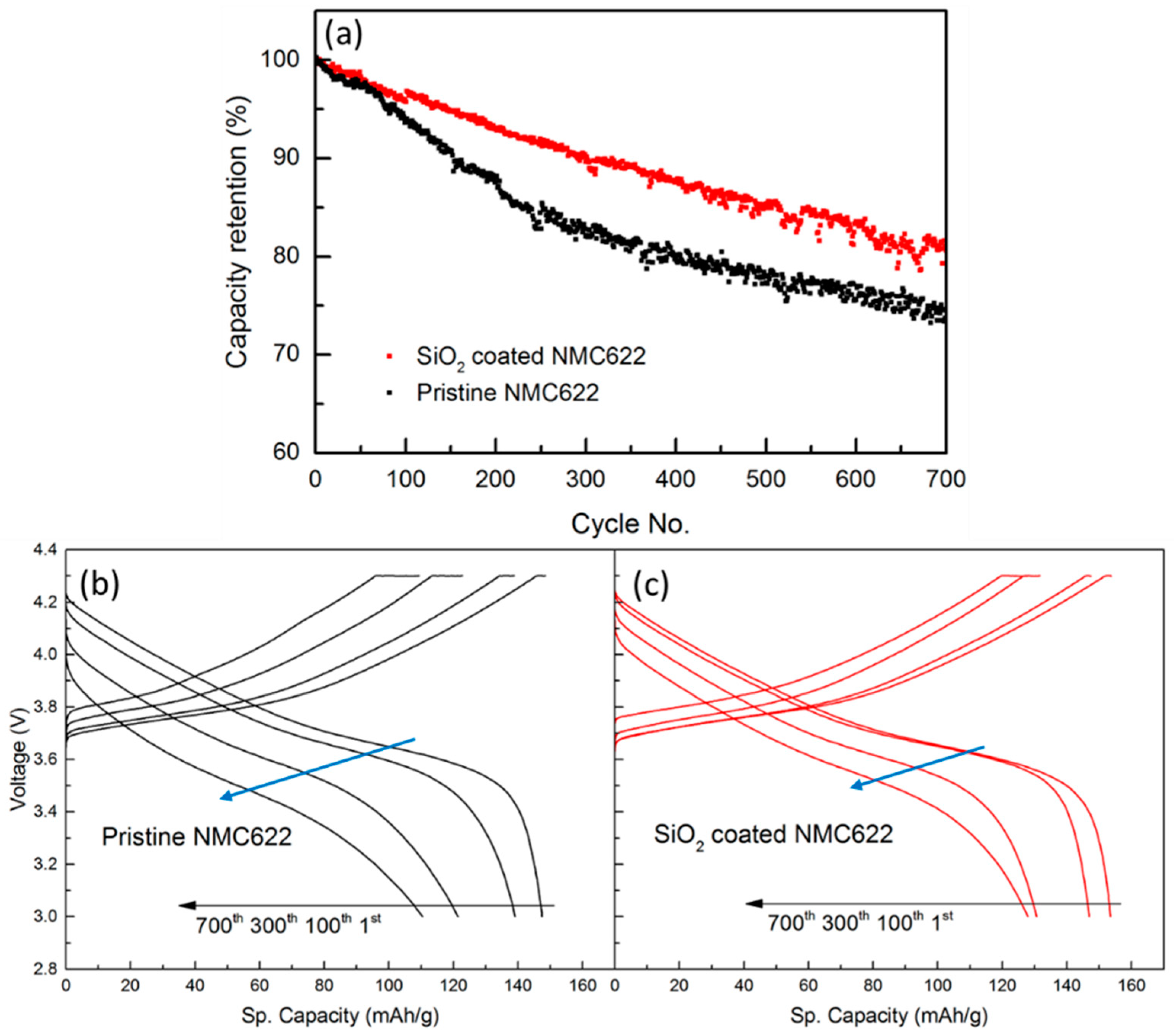
3.2. Electrochemical Measurement
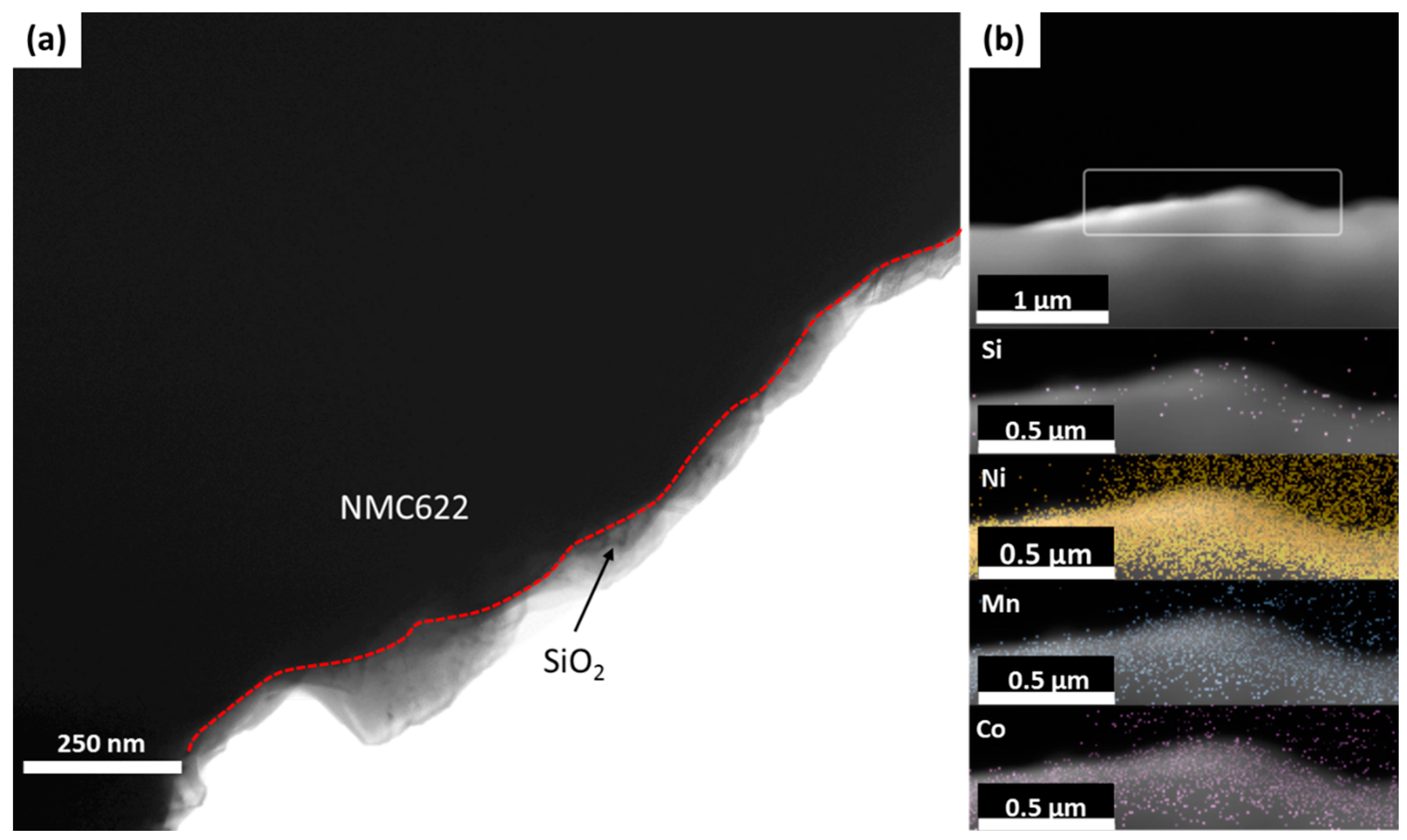
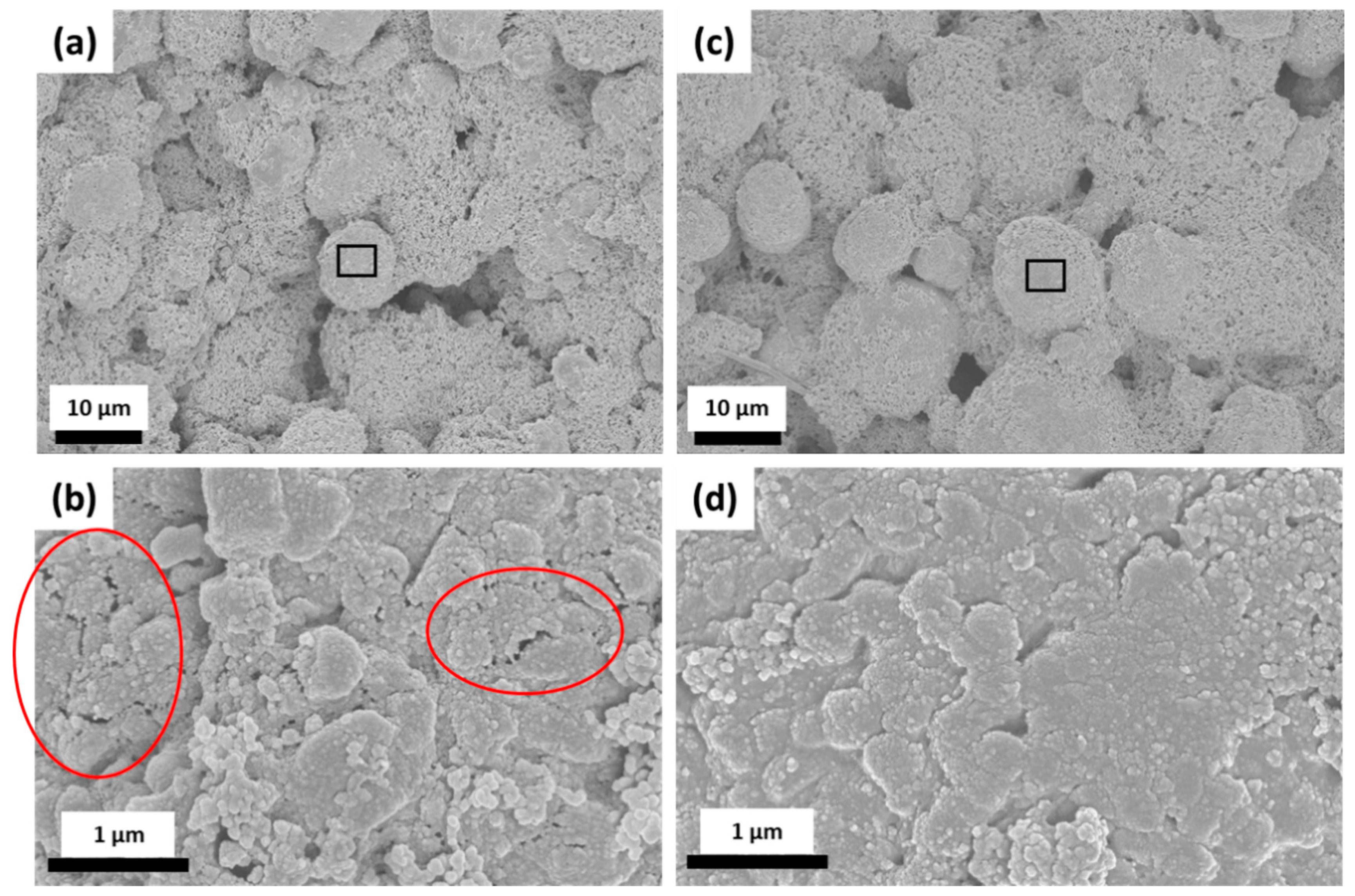
3.3. Post-Mortem Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wood, D.L., III; Li, J.; Daniel, C. Prospects for reducing the processing coast of lithium ion batteries. J. Power Sour. 2015, 275, 234–242. [Google Scholar] [CrossRef]
- Ding, Y.; Cano, Z.P.; Yu, A.; Lu, J.; Chen, Z. Automotive Li-ion batteries: Current status and future perspectives. Electrochem. Energy Rev. 2019, 2, 1–28. [Google Scholar] [CrossRef]
- Andre, D.; Kim, S.J.; Lamp, P.; Lux, S.F.; Maglia, F.; Paschos, O.; Stiaszny, B. Future generations of cathode materials: An automative industry perspective. J. Mater. Chem. A 2015, 3, 6709–6732. [Google Scholar] [CrossRef]
- Noh, H.J.; Youn, S.; Yoon, C.S.; Sun, Y.K. Comparison of the structural and electrochemical properties of layered Li[NixCoyMnz]O2 (x = 1/3, 0.5, 0.6, 0.7, 0.8, and 0.85) cathode material for lithium-ion batteries. J. Power Sour. 2013, 233, 121–130. [Google Scholar] [CrossRef]
- Han, J.G.; Kim, K.; Lee, Y.; Choi, N.S. Scavenging materials to stabilize LiPF6-containing carbonate-based electrolytes for Li-ion batteries. Adv. Mater. 2018, 31, 1804822. [Google Scholar] [CrossRef] [PubMed]
- Ryu, H.H.; Park, K.J.; Yoon, C.S.; Sun, Y.K. Capacity fading of ni-rich Li[NixCoyMn1-x-y]O2 (0.6 ≤ x ≤ 0.95) cathodes for high-energy-density lithium-ion batteries: Bulk or surface degradation? Chem. Mater. 2018, 30, 1155–1163. [Google Scholar] [CrossRef]
- Wise, A.M.; Ban, C.; Weker, J.N.; Misra, S.; Cavanagh, A.S.; Wu, Z.; Li, Z.; Whittingham, M.S.; Xu, K.; George, S.M.; et al. Effect of Al2O3 coating on stabilizing LiNi0.4Mn0.4Co0.2O2 cathodes. Chem. Mater. 2015, 27, 6146–6154. [Google Scholar] [CrossRef]
- Neudeck, S.; Strauss, F.; Garcia, G.; Wolf, H.; Janek, J.; Harmann, P.; Brezesinski, T. Room temperature, liquid-phase Al2O3 surface. Chem. Commun. 2019, 55, 2174–2177. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.; Kim, S.M.; Lee, K.W.; Song, J.H.; Jo, Y.N.; Kim, H.; Kim, J.S.; Kim, Y.J. Investigation of new manganese orthophosphate Mn3(PO4)2 coating for nickel-rich LiNi0.6Co0.2Mn0.2O2 cathode and improvement of its thermal properties. Electrochim. Acta 2016, 198, 77–83. [Google Scholar] [CrossRef]
- Cao, Y.; Qi, X.; Hu, K.; Wang, Y.; Gan, Z.; Li, Y.; Hu, G.; Peng, Z.; Du, K. Conductive polymers encapsulation to enhance electrochemical performance of Ni-rich cathode materials for Li-ion batteries. ACS Appl. Mater. Interfaces 2018, 10, 18270–18280. [Google Scholar] [CrossRef] [PubMed]
- Cho, W.; Kim, S.M.; Song, J.H.; Yim, T.; Woo, S.G.; Lee, K.W.; Kim, J.S.; Kim, Y.J. Improved electrochemical and thermal properties of nickel rich LiNi0.6Co0.2Mn0.2O2 cathode materials by SiO2 coating. J. Power Sour. 2015, 282, 45–50. [Google Scholar] [CrossRef]
- Li, Y.D.; Zhao, S.X.; Nan, C.W.; Li, B.H. Electrochemical performance of SiO2-coated LiFePO4 cathode materials for lithium ion battery. J. Alloy. Compd. 2011, 509, 957–960. [Google Scholar] [CrossRef]
- Zhang, L.L.; Liang, G.; Peng, G.; Zou, F.; Huang, Y.H.; Croft, M.C. Significantly improved electrochemical performance in Li3V2(PO4)3/C Promoted by SiO2 coating for lithium-ion batteries. J. Phys. Chem. C 2012, 116, 12401–12408. [Google Scholar] [CrossRef]
- Zheng, H.; Yang, R.; Liu, G.; Song, X.; Battaglia, V.S. Cooperation between active material, polymeric binder and conductive carbon additive in lithium ion battery cathode. J. Phys. Chem. C 2012, 116, 4875–4882. [Google Scholar] [CrossRef]
- Periasamy, P.; Kalaiselvi, N.; Kim, H. High voltage and high capacity characteristics of LiNi1/3Co1/3Mn1/3O2 cathode for lithium battery applications. Int. J. Electrochem. Sci. 2007, 2, 689–699. [Google Scholar]
- Zhang, X.; Jiang, W.; Mauger, A.; Qilu; Gendron, F.; Julien, C. Minimization of the cation mixing in Li1+x(NMC)1-xO2 as cathode material. J. Power Sour. 2010, 195, 1292–1301. [Google Scholar] [CrossRef]
- Liang, L.; Hu, G.; Jiang, F.; Cao, Y. Electrochemical behaviors of SiO2-coated LiNi0.8Co0.1Mn0.1O2. J. Alloy. Compd. 2016, 657, 570–581. [Google Scholar] [CrossRef]
- Ruan, Y.; Song, X.; Fu, Y.; Song, C.; Battaglia, V. Structural evolution and capacity degradation mechanism of LiNi0.6Mn0.2Co0.2O2 cathode materials. J. Power Sour. 2018, 400, 539–548. [Google Scholar] [CrossRef]



| Sample | Li | Ni | Mn | Co | O | Si | C |
|---|---|---|---|---|---|---|---|
| Pristine NMC622 | 7.17 ± 0.18 | 35.05 ± 0.60 | 10.83 ± 0.23 | 12.29 ± 0.26 | 34.6 ± 3.0 | <0.005 | 0.0876 ± 0.0069 |
| SiO2-coated NMC622 | 7.18 ± 0.18 | 34.85 ± 0.59 | 10.79 ± 0.23 | 12.27 ± 0.26 | 33.9 ± 2.9 | 0.263 ± 0.011 | 0.0605 ± 0.0048 |
| Sample | a (Å) | c (Å) | c/a | V (Å3) | I003/I104 | r1 | Rp (%) | Χ2 | Rwp (%) |
|---|---|---|---|---|---|---|---|---|---|
| Pristine NMC622 | 2.8666 (8) | 14.222 (8) | 4.961 (4) | 101.22 (1) | 1.690 | 0.497 | 0.647 | 5.76 | 0.996 |
| SiO2-coated NMC622 | 2.8671 (2) | 14.220 (1) | 4.959 (7) | 101.23 (4) | 1.731 | 0.491 | 0..668 | 4.79 | 1.045 |
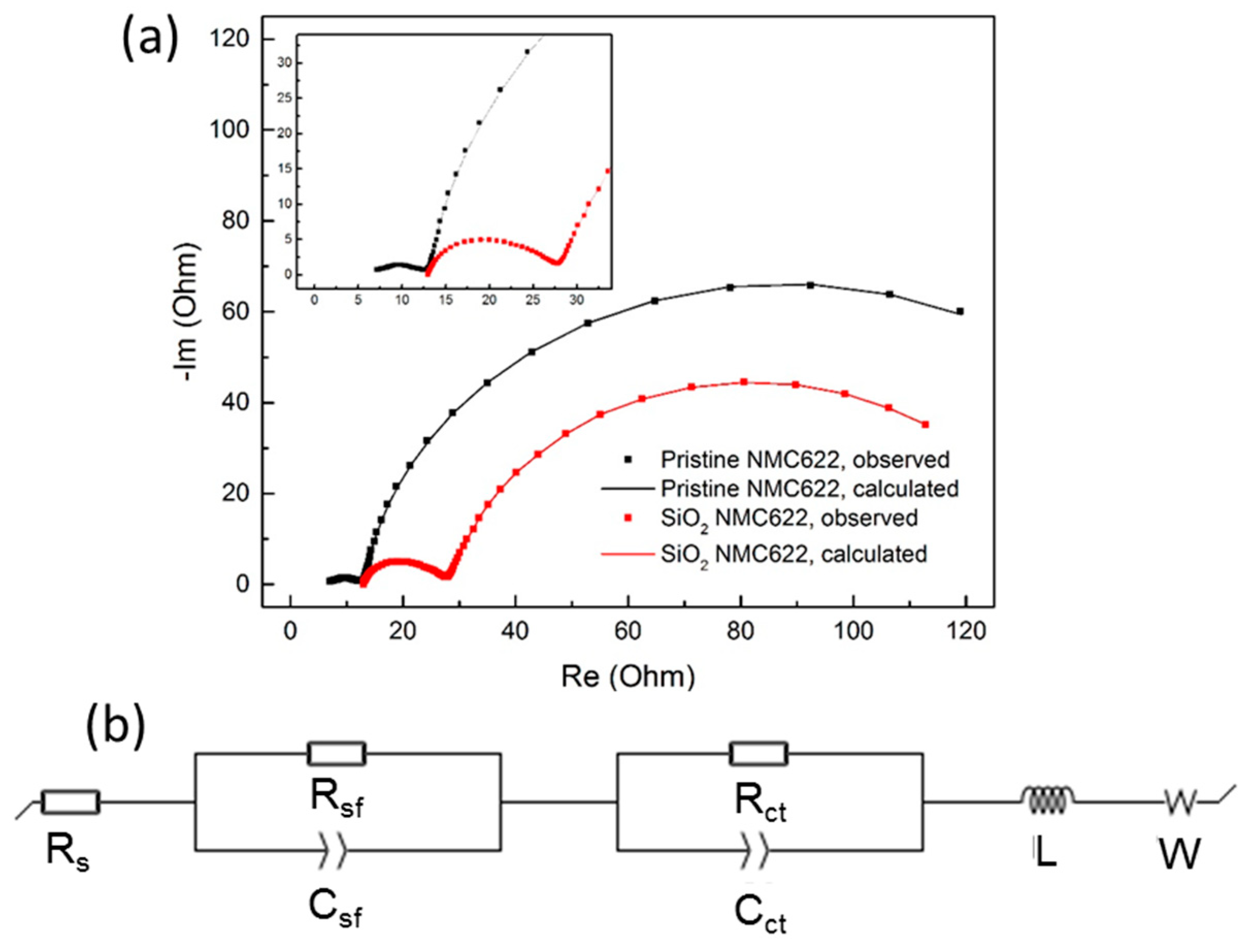
| Sample | Rs (Ω) | Rsf (Ω) | Rct (Ω) | Cf (F/cm2) | Cct (F/cm2) | L (H) | W (Ω·s−1/2) | Χ2 |
|---|---|---|---|---|---|---|---|---|
| Pristine NMC622 | 6.279 | 6.875 | 149.400 | 5.45 e-4 | 6.24 e-3 | 7.484 e-9 | 0.093 | 1.41 |
| SiO2-coated NMC622 | 12.500 | 15.720 | 107.200 | 5.30 e-5 | 6.42 e-3 | 1.160 e-7 | 6.387 | 5.43 |
| Sample | Uncycled Pristine NMC622 | Cycled Pristine NMC622 | Cycled SiO2-Coated NMC622 |
|---|---|---|---|
| Crystallite size (Å) | 470 ± 48 | 253 ± 72 | 452 ± 54 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, X.; Zhang, N.; Jahn, M.; Pfleging, W.; Seifert, H.J. Improved Capacity Retention of SiO2-Coated LiNi0.6Mn0.2Co0.2O2 Cathode Material for Lithium-Ion Batteries. Appl. Sci. 2019, 9, 3671. https://doi.org/10.3390/app9183671
Lu X, Zhang N, Jahn M, Pfleging W, Seifert HJ. Improved Capacity Retention of SiO2-Coated LiNi0.6Mn0.2Co0.2O2 Cathode Material for Lithium-Ion Batteries. Applied Sciences. 2019; 9(18):3671. https://doi.org/10.3390/app9183671
Chicago/Turabian StyleLu, Xiaoxue, Ningxin Zhang, Marcus Jahn, Wilhelm Pfleging, and Hans J. Seifert. 2019. "Improved Capacity Retention of SiO2-Coated LiNi0.6Mn0.2Co0.2O2 Cathode Material for Lithium-Ion Batteries" Applied Sciences 9, no. 18: 3671. https://doi.org/10.3390/app9183671






