Up-regulation of MicroRNAs-21 and -223 in a Sprague-Dawley Rat Model of Traumatic Spinal Cord Injury
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Design and Setting
2.2. Experimental Procedures
2.2.1. Establishment of an SD Model of TSCI
- (1)
- The sham group (n = 25): The SD rats undergoing insertion of an uninflated balloon catheter
- (2)
- The mild group (n = 25): The SD rats undergoing insertion of a 20-μL balloon catheter
- (3)
- The severe group (n = 25): The SD rats undergoing insertion of a 50-μL balloon catheter inflated at a volume of 50 μL.
2.2.2. Validation of an SD Model of TSCI
2.2.3. Isolation of miR Samples and the RT-PCR
2.3. Data Analysis
3. Results
3.1. Validation of an SD Model of TSCI
3.2. Alterations in the Level of miR-21 Expression according to the Time Course
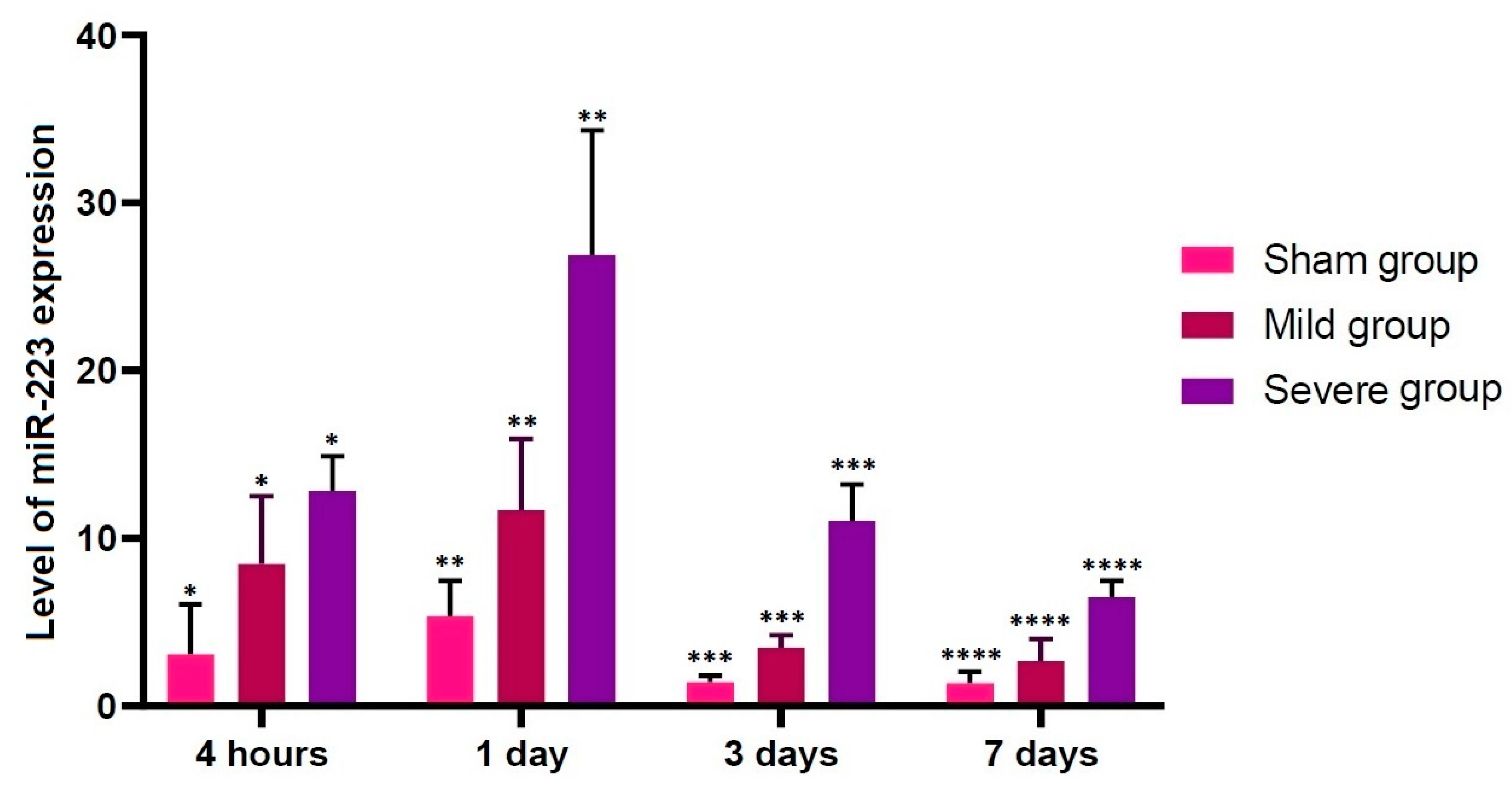
3.3. Alterations in the Level of miR-223 Expression according to the Time Course
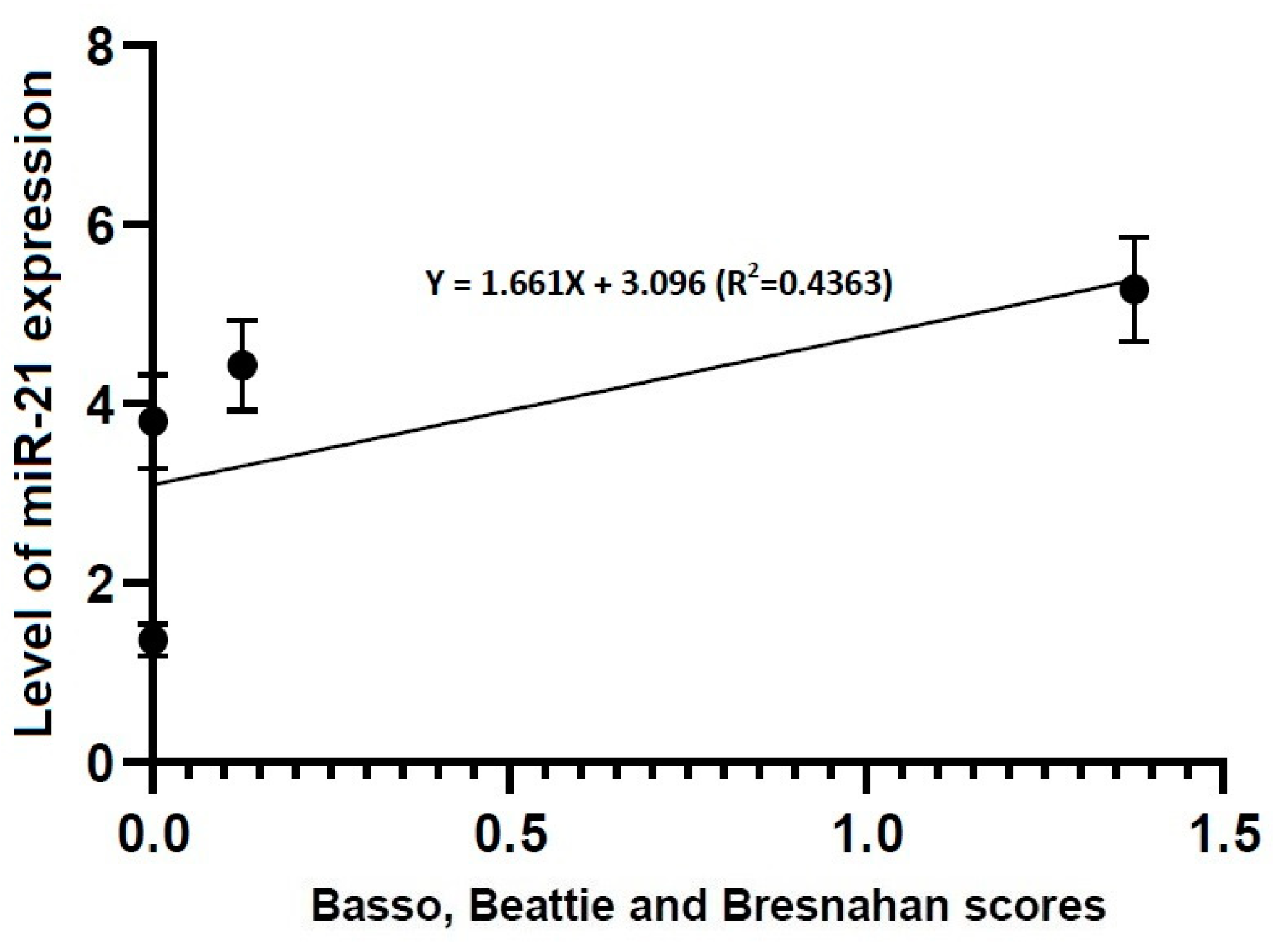
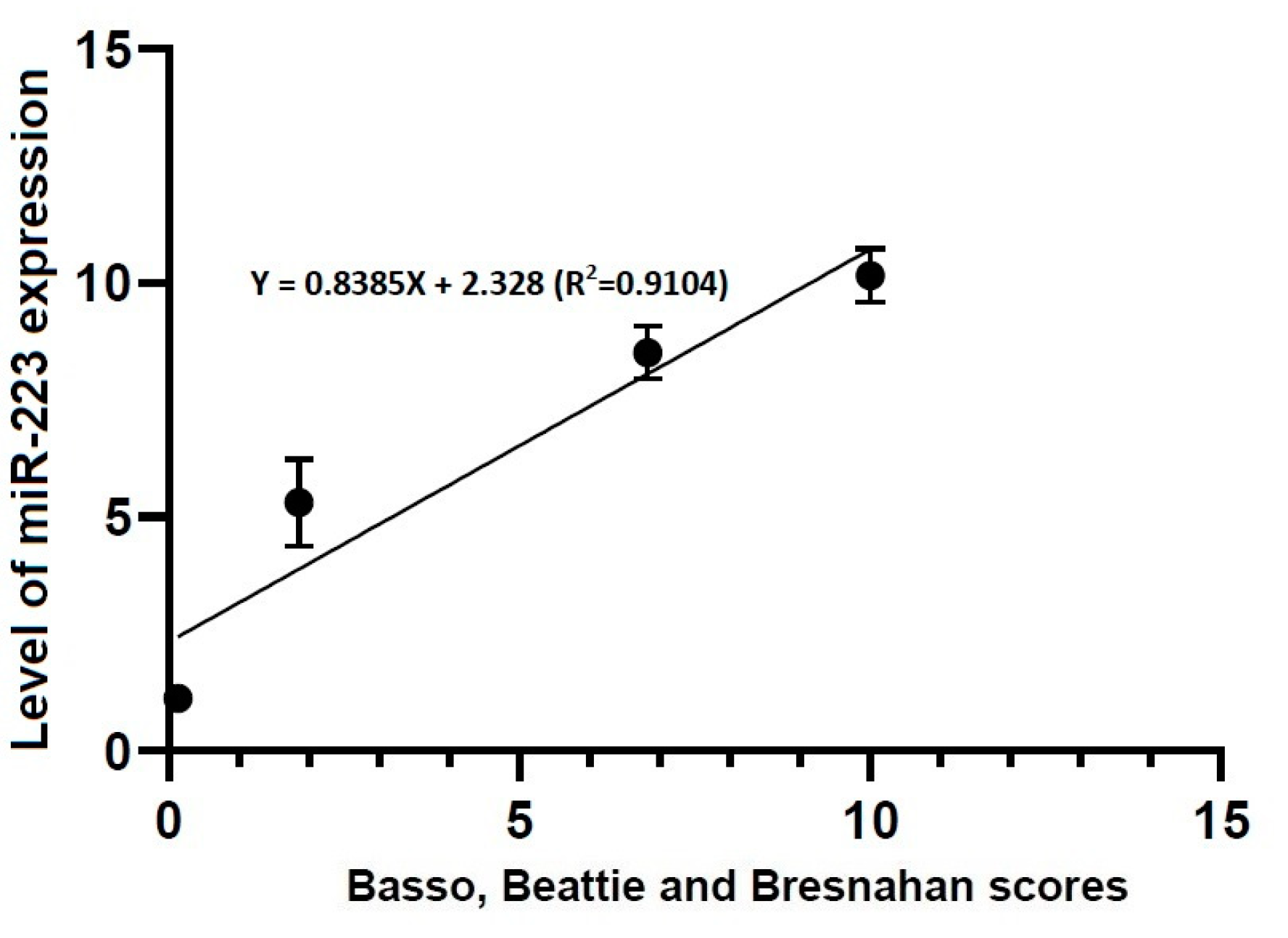
3.4. Correlations between Locomotor Rating Scale Scores and the Level of miR-21 or -223 Expression
4. Discussion
- (1)
- No differences in the level of miR-21 expression were found at the first time point studied (4 h post-lesion) between the three experimental groups, whereas such differences were significant at all the other time points (p < 0.05).
- (2)
- There were significant alterations in the level of miR-223 expression at all time points studied through all the experimental groups (p < 0.05).
- (3)
- Locomotor rating scale scores had a linear relationship with the level of miR-21 expression (R2 = 0.4363, Y = 1.661X + 3.096) and that of miR-223 one (R2 = 0.9104, Y = 0.8385X + 2.328).
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Cripps, R.A.; Lee, B.B.; Wing, P.; Weerts, E.; Mackay, J.; Brown, D. A global map for traumatic spinal cord injury epidemiology: Towards a living data repository for injury prevention. Spinal Cord 2011, 49, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Wyndaele, M.; Wyndaele, J.J. Incidence, prevalence and epidemiology of spinal cord injury: What learns a worldwide literature survey? Spinal Cord 2006, 44, 523–529. [Google Scholar] [CrossRef] [PubMed]
- García-Alté, A.; Pérez, K.; Novoa, A.; Suelves, J.M.; Bernabeu, M.; Vidal, J.; Arrufat, V.; Santamariña-Rubio, E.; Ferrando, J.; Cogollos, M.; et al. Spinal cord injury and traumatic brain injury: A cost-of-illness study. Neuroepidemiology 2012, 39, 103–108. [Google Scholar]
- Branco, F.; Cardenas, D.D.; Svircev, J.N. Spinal cord injury: A comprehensive review. Phys. Med. Rehabil. Clin. N. Am. 2007, 18, 651–679. [Google Scholar] [CrossRef]
- Singh, A.; Tetreault, L.; Kalsi-Ryan, S.; Nouri, A.; Fehlings, M.G. Global prevalence and incidence of traumatic spinal cord injury. Clin. Epidemiol. 2014, 6, 309–331. [Google Scholar]
- Center NSCIS. Spinal cord injury facts and figures at a glance. J. Spinal Cord Med. 2014, 37, 117–118. [Google Scholar] [CrossRef]
- Han, Z.A.; Lee, B.S.; Kim, W.; Lee, S.J.; Im, H.J.; Kim, C.; Song, K.; Ko, H.Y.; Bang, M.S.; Park, C.I. People with spinal cord injury in Korea. Am. J. Phys. Med. Rehabil. 2017, 96, S83–S85. [Google Scholar] [CrossRef]
- Bracken, M.B. Steroids for acute spinal cord injury. Cochrane Database Syst. Rev. 2012, 1, CD001046. [Google Scholar] [CrossRef] [PubMed]
- Budh, C.N.; Osteråker, A.L. Life satisfaction in individuals with a spinal cord injury and pain. Clin. Rehabil. 2007, 21, 89–96. [Google Scholar] [CrossRef]
- Ramer, L.M.; Ramer, M.S.; Steeves, J.D. Setting the stage for functional repair of spinal cord injuries: A cast of thousands. Spinal Cord 2005, 43, 134–161. [Google Scholar] [CrossRef]
- Park, E.; Velumian, A.A.; Fehlings, M.G. The role of excitotoxicity in secondary mechanisms of spinal cord injury: A review with an emphasis on the implications for white matter degeneration. J. Neurotrauma 2004, 21, 754–774. [Google Scholar] [CrossRef] [PubMed]
- Schwab, J.M.; Brechtel, K.; Mueller, C.A.; Failli, V.; Kaps, H.P.; Tuli, S.K.; Schluesener, H.J. Experimental strategies to promote spinal cord regeneration—An integrative perspective. Prog. Neurobiol. 2006, 78, 91–116. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.; Wang, Y.; Akyol, O.; Ho, W.M.; Ii, R.A.; Stier, G.; Martin, R.; Zhang, J.H. What’s new in traumatic brain injury: Update on tracking, monitoring and treatment. Int. J. Mol. Sci. 2015, 16, 11903–11965. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pearse, D.D. Therapeutic hypothermia in spinal cord injury: The status of its use and open questions. Int. J. Mol. Sci. 2015, 16, 16848–16879. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, L. Evaluation and management of neurogenic bladder: What is new in China? Int. J. Mol. Sci. 2015, 16, 18580–18600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nizamutdinov, D.; Shapiro, L.A. Overview of traumatic brain injury: An immunological context. Brain Sci. 2017, 7, 11. [Google Scholar] [CrossRef]
- Gutiérrez, Á.; Sepúlveda-Muñoz, D.; Gil-Agudo, Á.; de los Reyes Guzmán, A. Serious game platform with haptic feedback and EMG monitoring for upper limb rehabilitation and smoothness quantification on spinal cord injury patients. Appl. Sci. 2020, 10, 963. [Google Scholar] [CrossRef] [Green Version]
- Otzel, D.M.; Lee, J.; Ye, F.; Borst, S.E.; Yarrow, J.F. Activity-based physical rehabilitation with adjuvant testosterone to promote neuromuscular recovery after spinal cord injury. Int. J. Mol. Sci. 2018, 19, 1701. [Google Scholar] [CrossRef] [Green Version]
- Pereira, I.M.; Marote, A.; Salgado, A.J.; Silva, N.A. Filling the gap: Neural stem cells as a promising therapy for spinal cord injury. Pharmaceuticals 2019, 12, 65. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.Z.; Huang, J.H.; Zeng, L.; Wang, G.; Cao, M.; Lu, H.B. Anti-apoptotic effect of microRNA-21 after contusion spinal cord injury in rats. J. Neurotrauma 2013, 30, 1349–1360. [Google Scholar] [CrossRef]
- Izumi, B.; Nakasa, T.; Tanaka, N.; Nakanishi, K.; Kamei, N.; Yamamoto, R.; Nakamae, T.; Ohta, R.; Fujioka, Y.; Yamasaki, K.; et al. MicroRNA-223 expression in neutrophils in the early phase of secondary damage after spinal cord injury. Neurosci. Lett. 2011, 492, 114–118. [Google Scholar] [CrossRef]
- Vanický, I.; Urdzíková, L.; Saganová, K.; Cízková, D.; Gálik, J. A simple and reproducible model of spinal cord injury induced by epidural balloon inflation in the rat. J. Neurotrauma 2001, 18, 1399–1407. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.H.; Lee, J.H.; Chung, D.J.; Yang, W.J.; Lee, A.J.; Choi, C.B.; Chang, H.S.; Kim, D.H.; Chung, H.J.; Suh, H.J.; et al. Improved rat spinal cord injury model using spinal cord compression by percutaneous method. J. Vet. Sci. 2013, 14, 329–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.H.; Choi, C.B.; Chung, D.J.; Kang, E.H.; Chang, H.S.; Hwang, S.H.; Han, H.; Choe, B.Y.; Sur, J.H.; Lee, S.Y.; et al. Development of an improved canine model of percutaneous spinal cord compression injury by balloon catheter. J. Neurosci. Methods 2008, 167, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Chung, W.H.; Kang, E.H.; Chung, D.J.; Choi, C.B.; Chang, H.S.; Lee, J.H.; Hwang, S.H.; Han, H.; Choe, B.Y.; et al. Schwann cell-like remyelination following transplantation of human umbilical cord blood (hUCB)-derived mesenchymal stem cells in dogs with acute spinal cord injury. J. Neurol. Sci. 2011, 300, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.M.; Beattie, M.S.; Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Ridzon, D.A.; Broomer, A.J.; Zhou, Z.; Lee, D.H.; Nguyen, J.T.; Barbisin, M.; Xu, N.L.; Mahuvakar, V.R.; Andersen, M.R.; et al. Real-time quantification of microRNAs by stem-loop RT-PCR. Nucleic Acids Res. 2005, 33, e179. [Google Scholar] [CrossRef]
- Chambers, C.; Shuai, B. Profiling microRNA expression in Arabidopsis pollen using microRNA array and real-time PCR. BMC Plant Biol. 2009, 9, 87. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, K.; Nakasa, T.; Tanaka, N.; Ishikawa, M.; Yamada, K.; Yamasaki, K.; Kamei, N.; Izumi, B.; Adachi, N.; Miyaki, S.; et al. Responses of microRNAs 124a and 223 following spinal cord injury in mice. Spinal Cord 2010, 48, 192–196. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Liang, H.; Liao, Z.; Wang, Y.; Hu, X.; Chen, X.; Xu, L.; Hu, Z. MiR-203 enhances let-7 biogenesis by targeting LIN28B to suppress tumor growth in lung cancer. Sci. Rep. 2017, 7, 42680. [Google Scholar] [CrossRef] [Green Version]
- Wu, L.; Li, Y.; Fan, J.M.; Zhang, Z.M.; Ouyang, J.L.; Ni, T.T.; Wu, H.X.; Li, H. MicroRNA-204 targets signal transducer and activator of transcription 5 expression and inhibits proliferation of B-cell lymphoma cells. Mol. Med. Rep. 2015, 11, 4567–4572. [Google Scholar] [CrossRef] [PubMed]
- Navarro, I.C.; Ferreira, F.M.; Nakaya, H.I.; Baron, M.A.; Vilar-Pereira, G.; Pereira, I.R.; Silva, A.M.; Real, J.M.; De, B.T.; Chevillard, C.; et al. MicroRNA transcriptome profiling in heart of trypanosoma cruzi-infected mice: Parasitological and cardiological outcomes. PLoS Negl. Trop. Dis. 2015, 9, e0003828. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, H.; McDaniel, K.; Han, Y.; Liu, X.; Kennedy, L.; Yang, F.; McCarra, J.; Zhou, T.; Glaser, S.; Venter, J.; et al. Regulation of the extrinsic apoptotic pathway by microRNA-21 in alcoholic liver injury. J. Biol. Chem. 2014, 289, 27526–27539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2ˆ(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013, 3, 71–85. [Google Scholar]
- Wong, M.L.; Medrano, J.F. Real-time PCR for mRNA quantitation. Biotechniques 2005, 39, 75–85. [Google Scholar] [CrossRef]
- VanGuilder, H.D.; Vrana, K.E.; Freeman, W.M. Twenty-five years of quantitative PCR for gene expression analysis. Biotechniques 2008, 44, 619–626. [Google Scholar] [CrossRef] [Green Version]
- Yuan, J.S.; Reed, A.; Chen, F.; Stewart, C.N.J. Statistical analysis of real-time PCR data. BMC Bioinform. 2006, 7, 85. [Google Scholar] [CrossRef] [Green Version]
- Nieto-Diaz, M.; Esteban, F.J.; Reigada, D.; Muñoz-Galdeano, T.; Yunta, M.; Caballero-López, M.; Navarro-Ruiz, R.; Del Águila, A.; Maza, R.M. MicroRNA dysregulation in spinal cord injury: Causes, consequences and therapeutics. Front. Cell. Neurosci. 2014, 8, 53. [Google Scholar] [CrossRef]
- Dong, J.; Lu, M.; He, X.; Xu, J.; Qin, J.; Cheng, Z.; Liang, B.; Wang, D.; Li, H. Identifying the role of microRNAs in spinal cord injury. Neurol. Sci. 2014, 35, 1663–1671. [Google Scholar] [CrossRef]
- Fineberg, S.K.; Kosik, K.S.; Davidson, B.L. MicroRNAs potentiate neural development. Neuron 2009, 64, 303–309. [Google Scholar] [CrossRef] [Green Version]
- Genovese, T.; Esposito, E.; Mazzon, E.; Di, P.R.; Caminiti, R.; Bramanti, P.; Cappelani, A.; Cuzzocrea, S. Absence of endogenous interleukin-10 enhances secondary inflammatory process after spinal cord compression injury in mice. J. Neurochem. 2009, 108, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Dumont, R.J.; Okonkwo, D.O.; Verma, S.; Hurlbert, R.J.; Boulos, P.T.; Ellegala, D.B.; Dumont, A.S. Acute spinal cord injury, part I: Pathophysiologic mechanisms. Clin. Neuropharmacol. 2001, 24, 254–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakovlev, A.G.; Faden, A.I. Mechanisms of neural cell death: Implications for development of neuroprotective treatment strategies. NeuroRx 2004, 1, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, K.R.; Stoica, B.A.; Fricke, S.; Di, G.S.; Faden, A.I. Cell cycle activation contributes to post-mitotic cell death and secondary damage after spinal cord injury. Brain 2007, 130, 2977–2992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero, A.R.; Uchida, K.; Nakajima, H.; Watanabe, S.; Nakamura, M.; Johnson, W.E.; Baba, H. Blockade of interleukin-6 signaling inhibits the classic pathway and promotes an alternative pathway of macrophage activation after spinal cord injury in mice. J. Neuroinflamm. 2012, 9, 40. [Google Scholar] [CrossRef] [Green Version]
- Lüningschrör, P.; Hauser, S.; Kaltschmidt, B.; Kaltschmidt, C. MicroRNAs in pluripotency, reprogramming and cell fate induction. Biochim. Biophys. Acta 2013, 1833, 1894–1903. [Google Scholar] [CrossRef] [Green Version]
- Chan, J.A.; Krichevsky, A.M.; Kosik, K.S. MicroRNA-21 is an antiapoptotic factor in human glioblastoma cells. Cancer Res. 2005, 65, 6029–6033. [Google Scholar] [CrossRef] [Green Version]
- Sayed, D.; He, M.; Hong, C.; Gao, S.; Rane, S.; Yang, Z.; Abdellatif, M. MicroRNA-21 is a downstream effector of AKT that mediates its antiapoptotic effects via suppression of Fas ligand. J. Biol. Chem. 2010, 285, 20281–20290. [Google Scholar] [CrossRef] [Green Version]
- Sahni, V.; Mukhopadhyay, A.; Tysseling, V.; Hebert, A.; Birch, D.; Mcguire, T.L.; Stupp, S.I.; Kessler, J.A. BMPR1a and BMPR1b signaling exert opposing effects on gliosis after spinal cord injury. J. Neurosci. 2010, 30, 1839–1855. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Ding, F.; Gu, X. Non-coding RNAs as emerging regulators of neural injury responses and regeneration. Neurosci. Bull. 2016, 32, 253–264. [Google Scholar] [CrossRef] [Green Version]
- Liu, J. Control of protein synthesis and mRNA degradation by microRNAs. Curr. Opin. Cell Biol. 2008, 20, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Chao, K.; Ng, S.C.; Bai, A.H.; Yu, Q.; Yu, J.; Li, M.; Cui, Y.; Chen, M.; Hu, J.F.; et al. Pro-inflammatory miR-223 mediates the cross-talk between the IL23 pathway and the intestinal barrier in inflammatory bowel disease. Genome Biol. 2016, 17, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Z.; Zhou, H.; Lu, L.; Li, X.; Fu, Z.; Liu, J.; Kang, Y.; Wei, Z.; Pan, B.; Liu, L.; et al. The roles of microRNAs in spinal cord injury. Int. J. Neurosci. 2017, 127, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Byrnes, K.R.; Washington, P.M.; Knoblach, S.M.; Hoffman, E.; Faden, A.I. Delayed inflammatory mRNA and protein expression after spinal cord injury. J. Neuroinflamm. 2011, 8, 130. [Google Scholar] [CrossRef] [Green Version]
- Strickland, E.R.; Hook, M.A.; Balaraman, S.; Huie, J.R.; Grau, J.W.; Miranda, R.C. MicroRNA dysregulation following spinal cord contusion: Implications for neural plasticity and repair. Neuroscience 2011, 186, 146–160. [Google Scholar] [CrossRef] [Green Version]
- Urbanek, M.O.; Nawrocka, A.U.; Krzyzosiak, W.J. Small RNA detection by in situ hybridization methods. Int. J. Mol. Sci. 2015, 16, 13259–13286. [Google Scholar] [CrossRef] [Green Version]
- Baril, P.; Ezzine, S.; Pichon, C. Monitoring the spatiotemporal activities of miRNAs in small animal models using molecular imaging modalities. Int. J. Mol. Sci. 2015, 16, 4947–4972. [Google Scholar] [CrossRef] [Green Version]
- Miya Shaik, M.; Tamargo, I.A.; Abubakar, M.B.; Kamal, M.A.; Greig, N.H.; Gan, S.H. The role of microRNAs in Alzheimer’s disease and their therapeutic potentials. Genes 2018, 9, 174. [Google Scholar] [CrossRef] [Green Version]
- Marí-Alexandre, J.; Sánchez-Izquierdo, D.; Gilabert-Estellés, J.; Barceló-Molina, M.; Braza-Boïls, A.; Sandoval, J. MiRNAs regulation and its role as biomarkers in endometriosis. Int. J. Mol. Sci. 2016, 17, 93. [Google Scholar] [CrossRef] [Green Version]
- Kloosterman, W.P.; Wienholds, E.; de Bruijn, E.; Kauppinen, S.; Plasterk, R.H. In situ detection of miRNAs in animal embryos using LNA-modified oligonucleotide probes. Nat. Methods 2006, 3, 27–29. [Google Scholar] [CrossRef]
- Barnabé-Heider, F.; Frisén, J. Stem cells for spinal cord repair. Cell Stem Cell 2008, 3, 16–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hachem, L.D.; Ahuja, C.S.; Fehlings, M.G. Assessment and management of acute spinal cord injury: From point of injury to rehabilitation. J. Spinal Cord Med. 2017, 40, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Reis, C.; Gospodarev, V.; Reis, H.; Wilkinson, M.; Gaio, J.; Araujo, C.; Chen, S.; Zhang, J.H. Traumatic brain injury and stem cell: Pathophysiology and update on recent treatment modalities. Stem Cells Int. 2017, 2017, 6392592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozano, C.; Duroux-Richard, I.; Firat, H.; Schordan, E.; Apparailly, F. MicroRNAs: Key regulators to understand osteoclast differentiation? Front. Immunol. 2019, 10, 375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, D.C.P.D.; Carneiro, F.D.; Almeida, K.C.; Fernandes-Santos, C. Role of miRNAs on the pathophysiology of cardiovascular diseases. Arq. Bras. Cardiol. 2018, 111, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Podbielska, M.; Banik, N.L.; Kurowska, E.; Hogan, E.L. Myelin recovery in multiple sclerosis: The challenge of remyelination. Brain Sci. 2013, 3, 1282–1324. [Google Scholar] [CrossRef] [Green Version]
- Stallings, R.L. MicroRNA involvement in the pathogenesis of neuroblastoma: Potential for microRNA mediated therapeutics. Curr. Pharm. Des. 2009, 15, 456–462. [Google Scholar] [CrossRef] [Green Version]
- Pal, M.K.; Jaiswar, S.P.; Dwivedi, V.N.; Tripathi, A.K.; Dwivedi, A.; Sankhwar, P. MicroRNA: A new and promising potential biomarker for diagnosis and prognosis of ovarian cancer. Cancer Biol. Med. 2015, 12, 328–341. [Google Scholar]
- Jurkovicova, D.; Smolkova, B.; Magyerkova, M.; Sestakova, Z.; Kajabova, V.H.; Kulcsar, L.; Zmetakova, I.; Kalinkova, L.; Krivulcik, T.; Karaba, M.; et al. Down-regulation of traditional oncomiRs in plasma of breast cancer patients. Oncotarget 2017, 8, 77369–77384. [Google Scholar] [CrossRef] [Green Version]
- Martirosyan, N.L.; Carotenuto, A.; Patel, A.A.; Kalani, M.Y.; Yagmurlu, K.; Lemole, G.M.J.; Preul, M.C.; Theodore, N. The role of microRNA markers in the diagnosis, treatment, and outcome prediction of spinal cord injury. Front. Surg. 2016, 3, 56. [Google Scholar] [CrossRef] [Green Version]
- Kreth, S.; Hübner, M.; Hinske, L.C. MicroRNAs as clinical biomarkers and therapeutic tools in perioperative medicine. Anesth. Analg. 2018, 126, 670–681. [Google Scholar] [CrossRef] [PubMed]
- Gemmati, D.; Varani, K.; Bramanti, B.; Piva, R.; Bonaccorsi, G.; Trentini, A.; Manfrinato, M.C.; Tisato, V.; Carè, A.; Bellini, T. “Bridging the gap” everything that could have been avoided if we had applied gender medicine, pharmacogenetics and personalized medicine in the gender-omics and sex-omics era. Int. J. Mol. Sci. 2019, 21, 296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christopher, A.F.; Kaur, R.P.; Kaur, G.; Kaur, A.; Gupta, V.; Bansal, P. MicroRNA therapeutics: Discovering novel targets and developing specific therapy. Perspect. Clin. Res. 2016, 7, 68–74. [Google Scholar] [PubMed]
- Taneja, A.; Della, P.O.; Danhof, M. Challenges in translational drug research in neuropathic and inflammatory pain: The prerequisites for a new paradigm. Eur. J. Clin. Pharmacol. 2017, 73, 1219–1236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yunta, M.; Nieto-Díaz, M.; Esteban, F.J.; Caballero-López, M.; Navarro-Ruíz, R.; Reigada, D.; Pita-Thomas, D.W.; del, Á.A.; Muñoz-Galdeano, T.; Maza, R.M. MicroRNA dysregulation in the spinal cord following traumatic injury. PLoS ONE 2012, 7, e34534. [Google Scholar] [CrossRef] [PubMed]
- Bhalala, O.G.; Pan, L.; Sahni, V.; McGuire, T.L.; Gruner, K.; Tourtellotte, W.G.; Kessler, J.A. MicroRNA-21 regulates astrocytic response following spinal cord injury. J. Neurosci. 2012, 32, 17935–17947. [Google Scholar] [CrossRef] [PubMed]




| Group | Time Points | |||
|---|---|---|---|---|
| 4 H | 1 Day | 3 Days | 7 Days | |
| Sham | 21.00000 | 21.00000 | 21.00000 | 21.00000 |
| Mild | 0.12500 * | 1.85714 * | 6.83333 * | 10.00000 * |
| Severe | 0.00000 | 0.00000 ** | 0.12500 ** | 1.37500 ** |
| Group | Time Points | |||
|---|---|---|---|---|
| 4 H | 1 Day | 3 Days | 7 Days | |
| Sham | 1.27396 ± 0.09624 | 1.88336 ± 0.10513 * | 1.51919 ± 0.05863 ** | 2.62678 ± 0.58438 *** |
| Mild | 1.36184 ± 0.17934 | 3.80291 ± 0.52056 * | 4.43207 ± 0.50885 ** | 5.27714 ± 0.66994 *** |
| Severe | 1.11534 ± 0.10416 | 5.30909 ± 0.92265 * | 8.50091 ± 0.56952 ** | 10.1606 ± 0.42516 *** |
| Group | Time Points | |||
|---|---|---|---|---|
| 4 h | 1 Day | 3 Days | 7 Days | |
| Sham | 3.068116 ± 1.348989 * | 5.35168 ± 0.95415 ** | 1.40717 ± 0.17022 *** | 1.37411 ± 0.30366 **** |
| Mild | 8.465987 ± 1.812612 * | 11.6884 ± 1.89249 ** | 3.47943 ± 0.33655 *** | 2.67426 ± 0.59617 **** |
| Severe | 12.8211 ± 0.921337 * | 26.8762 ± 3.33326 ** | 11.0146 ± 0.9859 *** | 6.48124 ± 0.44328 **** |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, H.-J.; Chung, W.-H.; Do, S.-H.; Lee, J.-H.; Kim, H.-y. Up-regulation of MicroRNAs-21 and -223 in a Sprague-Dawley Rat Model of Traumatic Spinal Cord Injury. Brain Sci. 2020, 10, 141. https://doi.org/10.3390/brainsci10030141
Chung H-J, Chung W-H, Do S-H, Lee J-H, Kim H-y. Up-regulation of MicroRNAs-21 and -223 in a Sprague-Dawley Rat Model of Traumatic Spinal Cord Injury. Brain Sciences. 2020; 10(3):141. https://doi.org/10.3390/brainsci10030141
Chicago/Turabian StyleChung, Hyo-Jin, Wook-Hun Chung, Sun-Hee Do, Jae-Hoon Lee, and Hwi-yool Kim. 2020. "Up-regulation of MicroRNAs-21 and -223 in a Sprague-Dawley Rat Model of Traumatic Spinal Cord Injury" Brain Sciences 10, no. 3: 141. https://doi.org/10.3390/brainsci10030141





