Reporting Data on Auditory Brainstem Responses (ABR) in Rats: Recommendations Based on Review of Experimental Protocols and Literature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Search Strategy
- Auditory evoked potential” AND “rat”;
- Auditory brainstem response” AND “rat”.
2.2. Study Selection
- Article published in the last five years
- Original research
- Use of rats
- Use of acoustically evoked auditory brainstem responses
- Full text not available
- Literature review
- Lack of information about the experimental group
- Use of electrically evoked auditory brainstem responses
2.3. Survey
- Animals used (strain, gender, age)
- Animal housing
- Transfer of rats to the ABR experimental room
- The system used to measure ABR
- Time of ABR measurement
- Anesthesia
- Tympanic membrane evaluation
- Experimental area
- experimental room temperature (RT)
- Animal’s body temperature
- monitoring of the body temperature
- Experimental design
- Parameters measured using ABR
- ABR acquisition characteristics
- Stimulus characteristics
3. Results
3.1. Animals Used
3.2. Animal Housing
3.2.1. Animal Facility
3.2.2. Day/Night Cycle
3.2.3. The Temperature in the Facility
3.2.4. Humidity in the Facility
3.2.5. The Cage Type and Number of Rats per Cage
3.2.6. Acclimatization Time
3.3. Transfer of Rats to the ABR Experimental Room
3.4. The System Used to Measure ABR
3.5. Time of ABR Measurement
3.6. Anesthesia
3.7. Tympanic Membrane Evaluation
3.8. Experimental Area
Experimental Room Temperature
3.9. Animal’s Body Temperature
Methods for Monitoring of the Body Temperature
3.10. Experimental Design
3.11. Parameters Measured Using ABR
3.12. ABR Acquisition Characteristics
3.12.1. Signal Delivery/Speaker Placement
3.12.2. Electrodes
3.13. Stimulus Characteristics
3.13.1. Tested Frequencies and Intensity Range
3.13.2. Repetition Rate/Stimulus Rate
3.13.3. Polarity
3.13.4. Number of Averages and Analysis Time
3.13.5. Filters
3.13.6. Click ABR
4. Discussion
4.1. Animals Used
- Reporting recommendation: Include gender and age of animals in the study report.
4.2. Animal Housing
- Reporting recommendation: The level of noise and the temperature and humidity in the animal facility should be reported.
4.2.1. The Cage Type and Number of Rats per Cage
- Reporting recommendation: Report the housing conditions in the Supplementary Materials.
4.2.2. Acclimatization Time
- Reporting recommendation: Indicate an acclimatization period in the protocol.
4.3. Transfer to the ABR Experimental Room
- Reporting recommendation: Report the details concerning animal transfer from the animal facility to the experimental area. Indicate the time of the last cage cleaning.
4.4. The System Used to Measure ABR
- Reporting recommendation: Report the type of system, conditions, and consumables used to measure ABR.
4.5. Time of ABR Measurement
- Reporting recommendation: Indicate using light/night cycle, length of each phase, and time of the phase switch.
4.6. Anesthesia
- Reporting recommendation: Report the dosage of anesthetics and the route of administration.
4.7. Tympanic Membrane Evaluation
- Reporting recommendation: Report assessing (or not) the ear canal and tympanic membrane.
4.8. Experimental Area
- Reporting recommendation: Report the use (or lack of) of the Faraday cage and the actual temperature in the experimental room.
4.9. Animal’s Body Temperature
- Reporting recommendation: Report if and how the body temperature of animals was maintained and measured during the ABR recording.
4.10. Experimental Design
- Reporting recommendation: Report the number of ABR recordings per animal and the recovery time between consecutive recordings.
4.11. Parameters Measured Using ABR
- Reporting recommendation: Indicate the number of the ABR traces at near-threshold intensity.
4.12. ABR Acquisition Characteristics
4.12.1. Signal Delivery/Speaker Placement
- Reporting recommendation: When signal stimulation is not delivered directly to the ear, please report the distance between the speaker and the tested ear.
4.12.2. Electrodes
- Reporting recommendation: Report testing the electrode impedance before ABR; report the result of impedance test.
4.13. Stimulus Characteristics
4.13.1. Tested Frequencies and Intensity Range
- Reporting recommendation: Report all frequencies measured.
4.13.2. Repetition Rate/Stimulus Rate
- Reporting recommendation: Report the repetition rate and stimulus sequence.
4.13.3. Polarity
- Reporting recommendation: Report the type of polarity used.
4.13.4. Number of Averages and Analysis Time
- Reporting recommendation: Indicate the number of averages and the analysis time.
4.13.5. Filters
- Reporting recommendation: Report filters used.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Neitzel, R.L.; Fligor, B.J. Risk of noise-induced hearing loss due to recreational sound: Review and recommendations. J. Acoust. Soc. Am. 2019, 146, 3911–3921. [Google Scholar] [CrossRef] [PubMed]
- Arslan, E.; Orzan, E.; Santarelli, R. Global problem of drug-induced hearing loss. Ann. N. Y. Acad. Sci. 1999, 884, 1–14. [Google Scholar] [CrossRef]
- Mazurek, B.; Olze, H.; Haupt, H.; Szczepek, A.J. The more the worse: The grade of noise-induced hearing loss associates with the severity of tinnitus. Int. J. Environ. Res. Public Health 2010, 7, 3071–3079. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stevenson, J.S.; Clifton, L.; Kuzma, E.; Littlejohns, T.J. Speech-in-noise hearing impairment is associated with an increased risk of incident dementia in 82,039 UK Biobank participants. Alzheimer’s Dement. 2021. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J.; et al. Dementia prevention, intervention, and care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef] [Green Version]
- Kujawa, S.G.; Liberman, M.C. Translating animal models to human therapeutics in noise-induced and age-related hearing loss. Hear. Res. 2019, 377, 44–52. [Google Scholar] [CrossRef]
- Young, A.; Cornejo, J.; Spinner, A. Auditory Brainstem Response. In StatPearls; StatPearls Publishing LLC.: Treasure Island, FL, USA, 2021. [Google Scholar]
- Escabi, C.D.; Frye, M.D.; Trevino, M.; Lobarinas, E. The rat animal model for noise-induced hearing loss. J. Acoust Soc. Am. 2019, 146, 3692–3709. [Google Scholar] [CrossRef] [Green Version]
- Heffner, H.E.; Heffner, R.S. Hearing ranges of laboratory animals. J. Am. Assoc. Lab. Anim. Sci. 2007, 46, 20–22. [Google Scholar]
- Alvarado, J.C.; Fuentes-Santamaría, V.; Gabaldón-Ull, M.C.; Blanco, J.L.; Juiz, J.M. Wistar rats: A forgotten model of age-related hearing loss. Front. Aging Neurosci. 2014, 6, 29. [Google Scholar] [CrossRef] [Green Version]
- Rüttiger, L.; Singer, W.; Panford-Walsh, R.; Matsumoto, M.; Lee, S.C.; Zuccotti, A.; Zimmermann, U.; Jaumann, M.; Rohbock, K.; Xiong, H.; et al. The reduced cochlear output and the failure to adapt the central auditory response causes tinnitus in noise exposed rats. PLoS ONE 2013, 8, e57247. [Google Scholar] [CrossRef] [Green Version]
- Henry, K.R. Differential changes of auditory nerve and brain stem short latency evoked potentials in the laboratory mouse. Electroencephalogr. Clin. Neurophysiol. 1979, 46, 452–459. [Google Scholar] [CrossRef]
- Bracken, M.B. Why animal studies are often poor predictors of human reactions to exposure. J. R. Soc. Med. 2009, 102, 120–122. [Google Scholar] [CrossRef] [Green Version]
- Domarecka, E.; Olze, H.; Szczepek, A.J. Auditory Brainstem Responses (ABR) of Rats during Experimentally Induced Tinnitus: Literature Review. Brain Sci. 2020, 10, 901. [Google Scholar] [CrossRef] [PubMed]
- Sotocinal, S.G.; Sorge, R.E.; Zaloum, A.; Tuttle, A.H.; Martin, L.J.; Wieskopf, J.S.; Mapplebeck, J.C.; Wei, P.; Zhan, S.; Zhang, S.; et al. The Rat Grimace Scale: A partially automated method for quantifying pain in the laboratory rat via facial expressions. Mol. Pain 2011, 7, 55. [Google Scholar] [CrossRef] [Green Version]
- Ritschl, L.M.; Fichter, A.M.; Häberle, S.; von Bomhard, A.; Mitchell, D.A.; Wolff, K.D.; Mücke, T. Ketamine-Xylazine Anesthesia in Rats: Intraperitoneal versus Intravenous Administration Using a Microsurgical Femoral Vein Access. J. Reconstr. Microsurg. 2015, 31, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Overbeck, G.W.; Church, M.W. Effects of tone burst frequency and intensity on the auditory brainstem response (ABR) from albino and pigmented rats. Hear. Res. 1992, 59, 129–137. [Google Scholar] [CrossRef]
- Turner, J.G.; Parrish, J.L.; Hughes, L.F.; Toth, L.A.; Caspary, D.M. Hearing in laboratory animals: Strain differences and nonauditory effects of noise. Comp. Med. 2005, 55, 12–23. [Google Scholar] [PubMed]
- Charlton, P.E.; Schatz, K.C.; Burke, K.; Paul, M.J.; Dent, M.L. Sex differences in auditory brainstem response audiograms from vasopressin-deficient Brattleboro and wild-type Long-Evans rats. PLoS ONE 2019, 14, e0222096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Church, M.W.; Williams, H.L.; Holloway, J.A. Postnatal development of the brainstem auditory evoked potential and far-field cochlear microphonic in non-sedated rat pups. Brain Res. 1984, 316, 23–31. [Google Scholar] [CrossRef]
- Church, M.W.; Williams, H.L.; Holloway, J.A. Brain-stem auditory evoked potentials in the rat: Effects of gender, stimulus characteristics and ethanol sedation. Electroencephalogr. Clin. Neurophysiol. 1984, 59, 328–339. [Google Scholar] [CrossRef]
- Balogová, Z.; Popelář, J.; Chiumenti, F.; Chumak, T.; Burianová, J.S.; Rybalko, N.; Syka, J. Age-Related Differences in Hearing Function and Cochlear Morphology between Male and Female Fischer 344 Rats. Front. Aging Neurosci. 2017, 9, 428. [Google Scholar] [CrossRef] [Green Version]
- Beltrame, A.K.; Dahms, N.M.; Runge, C.L. Auditory brainstem responses in aging dark agouti rats. Biosci. Rep. 2021, 41, BSR20202724. [Google Scholar] [CrossRef]
- Yadav, A.; Tandon, O.P.; Vaney, N. Auditory evoked responses during different phases of menstrual cycle. Indian J. Physiol. Pharmacol. 2002, 46, 449–456. [Google Scholar] [PubMed]
- Mann, N.; Sidhu, R.S.; Babbar, R. Brainstem auditory evoked responses in different phases of menstrual cycle. J. Clin. Diagn Res. 2012, 6, 1640–1643. [Google Scholar] [CrossRef] [PubMed]
- Delhez, A.; Lefebvre, P.; Péqueux, C.; Malgrange, B.; Delacroix, L. Auditory function and dysfunction: Estrogen makes a difference. Cell Mol. Life Sci. 2020, 77, 619–635. [Google Scholar] [CrossRef]
- Marcondes, F.K.; Bianchi, F.J.; Tanno, A.P. Determination of the estrous cycle phases of rats: Some helpful considerations. Braz. J. Biol. 2002, 62, 609–614. [Google Scholar] [CrossRef] [Green Version]
- Evans, A.M. Age at puberty and first litter size in early and late paired rats. Biol. Reprod. 1986, 34, 322–326. [Google Scholar] [CrossRef]
- Bonthuis, P.J.; Cox, K.H.; Searcy, B.T.; Kumar, P.; Tobet, S.; Rissman, E.F. Of mice and rats: Key species variations in the sexual differentiation of brain and behavior. Front. Neuroendocr. 2010, 31, 341–358. [Google Scholar] [CrossRef] [Green Version]
- Lewis, E.M.; Barnett, J.F., Jr.; Freshwater, L.; Hoberman, A.M.; Christian, M.S. Sexual maturation data for Crl Sprague-Dawley rats: Criteria and confounding factors. Drug Chem. Toxicol. 2002, 25, 437–458. [Google Scholar] [CrossRef] [PubMed]
- Lauer, A.M.; Larkin, G.; Jones, A.; May, B.J. Behavioral Animal Model of the Emotional Response to Tinnitus and Hearing Loss. J. Assoc. Res. Otolaryngol. 2018, 19, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Milligan, S.R.; Sales, G.D.; Khirnykh, K. Sound levels in rooms housing laboratory animals: An uncontrolled daily variable. Physiol. Behav. 1993, 53, 1067–1076. [Google Scholar] [CrossRef]
- Li, P.; Bing, D.; Wang, S.; Chen, J.; Du, Z.; Sun, Y.; Qi, F.; Zhang, Y.; Chu, H. Sleep Deprivation Modifies Noise-Induced Cochlear Injury Related to the Stress Hormone and Autophagy in Female Mice. Front. Neurosci. 2019, 13, 1297. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.H.; Kim, M.; Lee, S.J.; Lee, E.; Lee, S.A.; Lee, J.D.; Choi, J.H.; Kim, B.G. Effect of sleep deprivation on hearing levels in rats. Int. J. Pediatr. Otorhinolaryngol. 2018, 112, 169–175. [Google Scholar] [CrossRef]
- Abou-Ismail, U.A.; Burman, O.H.; Nicol, C.J.; Mendl, M. The effects of enhancing cage complexity on the behaviour and welfare of laboratory rats. Behav. Process. 2010, 85, 172–180. [Google Scholar] [CrossRef]
- Reeb, C.; Jones, R.; Bearg, D.; Bedigan, H.; Myers, D.; Paigen, B. Microenvironment in Ventilated Animal Cages with Differing Ventilation Rates, Mice Populations, and Frequency of Bedding Changes. Contemp. Top. Lab. Anim. Sci. 1998, 37, 43–49. [Google Scholar] [PubMed]
- Lovejoy, H.M.; McGuirt, W.F.; Ayres, P.H.; Hayes, A.W.; Coggins, C.R.; Sagartz, J. Effects of low humidity on the rat middle ear. Laryngoscope 1994, 104, 1055–1058. [Google Scholar] [CrossRef]
- Brown, K.J.; Grunberg, N.E. Effects of housing on male and female rats: Crowding stresses male but calm females. Physiol. Behav. 1995, 58, 1085–1089. [Google Scholar] [CrossRef]
- Gaskill, B.N.; Pritchett-Corning, K.R. Effect of Cage Space on Behavior and Reproduction in Crl:CD(SD) and BN/Crl Laboratory Rats. J. Am. Assoc. Lab. Anim. Sci. 2015, 54, 497–506. [Google Scholar] [PubMed]
- Barker, T.H.; George, R.P.; Howarth, G.S.; Whittaker, A.L. Assessment of housing density, space allocation and social hierarchy of laboratory rats on behavioural measures of welfare. PLoS ONE 2017, 12, e0185135. [Google Scholar] [CrossRef] [Green Version]
- Mazurek, B.; Haupt, H.; Joachim, R.; Klapp, B.F.; Stöver, T.; Szczepek, A.J. Stress induces transient auditory hypersensitivity in rats. Hear. Res. 2010, 259, 55–63. [Google Scholar] [CrossRef]
- Arts, J.W.; Kramer, K.; Arndt, S.S.; Ohl, F. The impact of transportation on physiological and behavioral parameters in Wistar rats: Implications for acclimatization periods. ILAR J. 2012, 53, E82–E98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capdevila, S.; Giral, M.; Ruiz de la Torre, J.L.; Russell, R.J.; Kramer, K. Acclimatization of rats after ground transportation to a new animal facility. Lab. Anim. 2007, 41, 255–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swallow, J.; Anderson, D.; Buckwell, A.C.; Harris, T.; Hawkins, P.; Kirkwood, J.; Lomas, M.; Meacham, S.; Peters, A.; Prescott, M.; et al. Guidance on the transport of laboratory animals. Lab. Anim. 2005, 39, 1–39. [Google Scholar] [CrossRef] [PubMed]
- Barahona, M.J.; Rojas, J.; Uribe, E.A.; García-Robles, M.A. Tympanic Membrane Rupture During Stereotaxic Surgery Disturbs the Normal Feeding Behavior in Rats. Front. Behav. Neurosci. 2020, 14, 591204. [Google Scholar] [CrossRef]
- Yelvington, D.B.; Weiss, G.K.; Ratner, A. Habituation of the prolactin response in rats to psychological stress. Psychoneuroendocrinology 1985, 10, 95–102. [Google Scholar] [CrossRef]
- Balcombe, J.P.; Barnard, N.D.; Sandusky, C. Laboratory routines cause animal stress. Contemp. Top. Lab. Anim. Sci. 2004, 43, 42–51. [Google Scholar]
- Castelhano-Carlos, M.J.; Baumans, V. The impact of light, noise, cage cleaning and in-house transport on welfare and stress of laboratory rats. Lab. Anim. 2009, 43, 311–327. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, S.; Miller, M.M.; Filipski, S.B.; Tolwani, R.J. Cage change influences serum corticosterone and anxiety-like behaviors in the mouse. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 479–483. [Google Scholar]
- Harkin, A.; O’Donnell, J.M.; Kelly, J.P. A study of VitalView for behavioural and physiological monitoring in laboratory rats. Physiol. Behav. 2002, 77, 65–77. [Google Scholar] [CrossRef]
- Gerdin, A.K.; Igosheva, N.; Roberson, L.A.; Ismail, O.; Karp, N.; Sanderson, M.; Cambridge, E.; Shannon, C.; Sunter, D.; Ramirez-Solis, R.; et al. Experimental and husbandry procedures as potential modifiers of the results of phenotyping tests. Physiol. Behav. 2012, 106, 602–611. [Google Scholar] [CrossRef] [Green Version]
- Van Driel, K.S.; Talling, J.C. Familiarity increases consistency in animal tests. Behav. Brain Res. 2005, 159, 243–245. [Google Scholar] [CrossRef] [PubMed]
- Obernier, J.A.; Baldwin, R.L. Establishing an appropriate period of acclimatization following transportation of laboratory animals. ILAR J. 2006, 47, 364–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- National Research Council Committee on, R.; Alleviation of Pain in Laboratory, A. The National Academies Collection: Reports funded by National Institutes of Health. In Recognition and Alleviation of Pain in Laboratory Animals; National Academies Press: Washington, DC, USA, 2009. [Google Scholar]
- Lundt, A.; Soos, J.; Henseler, C.; Arshaad, M.I.; Müller, R.; Ehninger, D.; Hescheler, J.; Sachinidis, A.; Broich, K.; Wormuth, C.; et al. Data Acquisition and Analysis In Brainstem Evoked Response Audiometry In Mice. J. Vis. Exp. 2019, 147. [Google Scholar] [CrossRef]
- Frankland, P.W.; Ralph, M.R. Circadian modulation in the rat acoustic startle circuit. Behav. Neurosci. 1995, 109, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Meltser, I.; Cederroth, C.R.; Basinou, V.; Savelyev, S.; Lundkvist, G.S.; Canlon, B. TrkB-mediated protection against circadian sensitivity to noise trauma in the murine cochlea. Curr. Biol. 2014, 24, 658–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von der Behrens, W. Animal models of subjective tinnitus. Neural. Plast. 2014, 2014, 741452. [Google Scholar] [CrossRef] [Green Version]
- Church, M.W.; Gritzke, R. Effects of ketamine anesthesia on the rat brain-stem auditory evoked potential as a function of dose and stimulus intensity. Electroencephalogr. Clin. Neurophysiol. 1987, 67, 570–583. [Google Scholar] [CrossRef]
- Avsaroglu, H.; van der Sar, A.S.; van Lith, H.A.; van Zutphen, L.F.; Hellebrekers, L.J. Differences in response to anaesthetics and analgesics between inbred rat strains. Lab. Anim. 2007, 41, 337–344. [Google Scholar] [CrossRef] [Green Version]
- Ruebhausen, M.R.; Brozoski, T.J.; Bauer, C.A. A comparison of the effects of isoflurane and ketamine anesthesia on auditory brainstem response (ABR) thresholds in rats. Hear. Res. 2012, 287, 25–29. [Google Scholar] [CrossRef]
- Turner, P.V.; Albassam, M.A. Susceptibility of rats to corneal lesions after injectable anesthesia. Comp. Med. 2005, 55, 175–182. [Google Scholar]
- Stein, A.B.; Tiwari, S.; Thomas, P.; Hunt, G.; Levent, C.; Stoddard, M.F.; Tang, X.L.; Bolli, R.; Dawn, B. Effects of anesthesia on echocardiographic assessment of left ventricular structure and function in rats. Basic Res. Cardiol. 2007, 102, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Ming, Z.; Dart, A.M.; Du, X.J. Optimizing dosage of ketamine and xylazine in murine echocardiography. Clin. Exp. Pharmacol. Physiol. 2007, 34, 499–507. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, K.; Flecknell, P. Retrospective review of anesthetic and analgesic regimens used in animal research proposals. ALTEX 2019, 36, 65–80. [Google Scholar] [CrossRef]
- Machholz, E.; Mulder, G.; Ruiz, C.; Corning, B.F.; Pritchett-Corning, K.R. Manual restraint and common compound administration routes in mice and rats. J. Vis. Exp. 2012. [Google Scholar] [CrossRef] [Green Version]
- Teixeira da Silva, J.A. Room temperature in scientific protocols and experiments should be defined: A reproducibility issue. Biotechniques 2021, 70, 306–308. [Google Scholar] [CrossRef]
- Rossi, G.T.; Britt, R.H. Effects of hypothermia on the cat brain-stem auditory evoked response. Electroencephalogr. Clin. Neurophysiol. 1984, 57, 143–155. [Google Scholar] [CrossRef]
- Church, M.W.; Shucard, D.W. Age-related hearing loss in BDF1 mice as evidenced by the brainstem auditory evoked potential. Audiology 1986, 25, 363–372. [Google Scholar] [CrossRef]
- Lomax, P. Measurement of ‘core’ temperature in the rat. Nature 1966, 210, 854–855. [Google Scholar] [CrossRef] [PubMed]
- Taylor, D.K. Study of two devices used to maintain normothermia in rats and mice during general anesthesia. J. Am. Assoc. Lab. Anim. Sci. 2007, 46, 37–41. [Google Scholar]
- Rufiange, M.; Leung, V.S.Y.; Simpson, K.; Pang, D.S.J. Pre-warming following premedication limits hypothermia before and during anesthesia in Sprague-Dawley rats (Rattus norvegicus). Can. J. Vet. Res. 2021, 85, 106–111. [Google Scholar]
- Schuster, C.J.; Pang, D.S.J. Forced-air pre-warming prevents peri-anaesthetic hypothermia and shortens recovery in adult rats. Lab. Anim. 2018, 52, 142–151. [Google Scholar] [CrossRef]
- Colmenárez-Raga, A.C.; Díaz, I.; Pernia, M.; Pérez-González, D.; Delgado-García, J.M.; Carro, J.; Plaza, I.; Merchán, M.A. Reversible Functional Changes Evoked by Anodal Epidural Direct Current Electrical Stimulation of the Rat Auditory Cortex. Front. Neurosci. 2019, 13, 356. [Google Scholar] [CrossRef] [PubMed]
- Backoff, P.M.; Caspary, D.M. Age-related changes in auditory brainstem responses in Fischer 344 rats: Effects of rate and intensity. Hear. Res. 1994, 73, 163–172. [Google Scholar] [CrossRef]
- Flydal, K.; Hermansen, A.; Enger, P.S.; Reimers, E. Hearing in reindeer (Rangifer tarandus). J. Comp. Physiol. A 2001, 187, 265–269. [Google Scholar] [CrossRef]
- Akil, O.; Oursler, A.E.; Fan, K.; Lustig, L.R. Mouse Auditory Brainstem Response Testing. Bio-Protocol 2016, 6, e1768. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrofsky, J. The effect of the subcutaneous fat on the transfer of current through skin and into muscle. Med. Eng. Phys. 2008, 30, 1168–1176. [Google Scholar] [CrossRef]
- Jung, S.Y.; Kim, S.H.; Yeo, S.G. Association of Nutritional Factors with Hearing Loss. Nutrients 2019, 11, 307. [Google Scholar] [CrossRef] [Green Version]
- Petrofsky, J.; Schwab, E. A re-evaluation of modelling of the current flow between electrodes: Consideration of blood flow and wounds. J. Med. Eng. Technol. 2007, 31, 62–74. [Google Scholar] [CrossRef] [PubMed]
- Virgen-Ortiz, A.; Apolinar-Iribe, A.; Muniz, J. Gender-effect on the contractile properties of skeletal muscle in streptozotocin-induced diabetic rats. J. Musculoskelet. Neuronal. Interact. 2018, 18, 255–261. [Google Scholar]
- Quiros Cognuck, S.; Reis, W.L.; Silva, M.; Debarba, L.K.; Mecawi, A.S.; de Paula, F.J.A.; Rodrigues Franci, C.; Elias, L.L.K.; Antunes-Rodrigues, J. Sex differences in body composition, metabolism-related hormones, and energy homeostasis during aging in Wistar rats. Physiol. Rep. 2020, 8, e14597. [Google Scholar] [CrossRef]
- Brudzynski, S.M. Communication of adult rats by ultrasonic vocalization: Biological, sociobiological, and neuroscience approaches. ILAR J. 2009, 50, 43–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brudzynski, S.M. Biological Functions of Rat Ultrasonic Vocalizations, Arousal Mechanisms, and Call Initiation. Brain Sci. 2021, 11, 605. [Google Scholar] [CrossRef]
- Borszcz, G.S. Contribution of the ventromedial hypothalamus to generation of the affective dimension of pain. Pain 2006, 123, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Sanz-Fernández, R.; Sánchez-Rodriguez, C.; Granizo, J.J.; Durio-Calero, E.; Martín-Sanz, E. Utility of auditory steady-state and brainstem responses in age-related hearing loss in rats. Acta Otolaryngol. 2015, 135, 35–41. [Google Scholar] [CrossRef] [PubMed]
- ABR User Guide. 2021. Available online: https://www.tdt.com/files/manuals/ABRGuide.pdf (accessed on 20 June 2021).
- Buran, B.N.; Elkins, S.; Kempton, J.B.; Porsov, E.V.; Brigande, J.V.; David, S.V. Optimizing Auditory Brainstem Response Acquisition Using Interleaved Frequencies. J. Assoc. Res. Otolaryngol. 2020, 21, 225–242. [Google Scholar] [CrossRef]
- Bogaerts, S.; Clements, J.D.; Sullivan, J.M.; Oleskevich, S. Automated threshold detection for auditory brainstem responses: Comparison with visual estimation in a stem cell transplantation study. BMC Neurosci. 2009, 10, 104. [Google Scholar] [CrossRef] [Green Version]




| Strain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Wistar (used in 8 studies) | Sprague-Dawley (used in 6 studies) | Long-Evans (used in 2 studies) | Fischer 344, Fischer344/NHsd (used in 2 studies) | Lewis (used in 1 study) | |||||
| outbred, albino | outbred, albino | outbred, pigmented | inbred, albino | inbred, albino | |||||
| Gender | |||||||||
| male | female | male | female | male | female | male | female | male | female |
| Age (Range in Months) | |||||||||
| 1–6 7–18 | 1–3 | 1–3 | 3–24 | 1–1.5 and 3–24 | Not used | 1–1.5 | |||
| Usage of Reflexes | Number of Reporting Protocols |
|---|---|
| eyelid reflex | 7 |
| toe reflex | 13 |
| tail-flick reflex | 4 |
| nose and vibrissae | 5 |
| Anesthesia Monitoring | Number of Reporting Protocols |
|---|---|
| heart rate | 2 |
| body temperature | 9 |
| mucosal membrane color | 3 |
| breathing frequency | 9 |
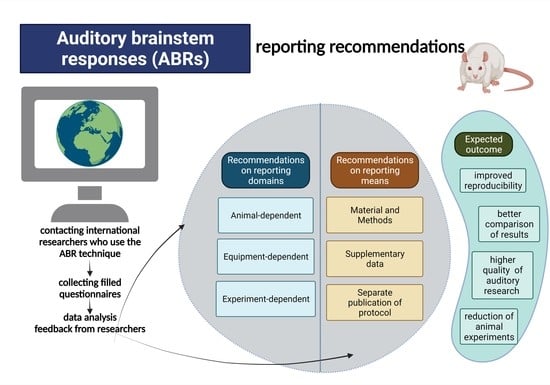
| Domains | Factors | Recommended to Be Reported in the Central Part of the Publication (Materials and Methods) | Recommended to Be Reported as Supplementary Information |
|---|---|---|---|
| Animal-dependent domain | Animals used | strain, age, number of animals *, gender, vendor, feeding * | Exclusion criteria: observations regarding a general state of health of animals (abnormal appearance, tumors, inner ear infections, tympanic membrane perforations) * |
| Animal housing | Reversed day/night cycle, number of rats per cage, acclimatization time | Conditions in the animal facility (temperature, humidity), the beginning of the light and dark phase, type and size of the cage | |
| Transfer of rats to the experimental area | - | Acclimatization time to the experimental area, number of rats transferred to the experimental room, a method for stress assessment | |
| Tympanic membrane evaluation | Performing otoscopy | - | |
| Equipment-dependent domain | The system used to measure ABR | Company name, year of purchase | - |
| Experimental area | Usage of Faraday cage | Size of the room or chamber where ABR is performed | |
| Experimental room temperature | The temperature in the experimental room | ||
| Animal’s body temperature | Body temperature during experiments; describe the monitoring system | ||
| Monitoring of the body temperature | The monitoring system for rat’s body temperature | ||
| Experiment-dependent domain | Time of ABR measurement | Time and length of ABR measurement | - |
| Anesthesia | Anesthetics (dosage), route of administration | Anesthesia monitoring, covering eyes | |
| Experimental design | Number of ABR measurement sessions per rat | The recovery process after anesthesia | |
| ABR analyses parameters | threshold, latency, amplitude; the number of ABR traces at near-threshold intensity | ||
| ABR acquisition characteristics | Signal delivery, electrodes: type, impedance, number, placement, the methods of placement (e.g., surgically fixed, subdermal) | Usage time of electrodes, disinfecting agent for electrodes | |
| Stimulus characteristics | Sound stimuli (type, duration, number of cycles), tested frequencies, intensity range, repetition rate, number of averageness, filters, usage of notch filters, sampling rate |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domarecka, E.; Kalcioglu, M.T.; Mutlu, A.; Özgür, A.; Smit, J.; Olze, H.; Szczepek, A.J. Reporting Data on Auditory Brainstem Responses (ABR) in Rats: Recommendations Based on Review of Experimental Protocols and Literature. Brain Sci. 2021, 11, 1596. https://doi.org/10.3390/brainsci11121596
Domarecka E, Kalcioglu MT, Mutlu A, Özgür A, Smit J, Olze H, Szczepek AJ. Reporting Data on Auditory Brainstem Responses (ABR) in Rats: Recommendations Based on Review of Experimental Protocols and Literature. Brain Sciences. 2021; 11(12):1596. https://doi.org/10.3390/brainsci11121596
Chicago/Turabian StyleDomarecka, Ewa, Mahmut Tayyar Kalcioglu, Ahmet Mutlu, Abdulkadir Özgür, Jasper Smit, Heidi Olze, and Agnieszka J. Szczepek. 2021. "Reporting Data on Auditory Brainstem Responses (ABR) in Rats: Recommendations Based on Review of Experimental Protocols and Literature" Brain Sciences 11, no. 12: 1596. https://doi.org/10.3390/brainsci11121596