Site Dependency of Anodal Transcranial Direct-Current Stimulation on Reaction Time and Transfer of Learning during a Sequential Visual Isometric Pinch Task
Abstract
:1. Introduction
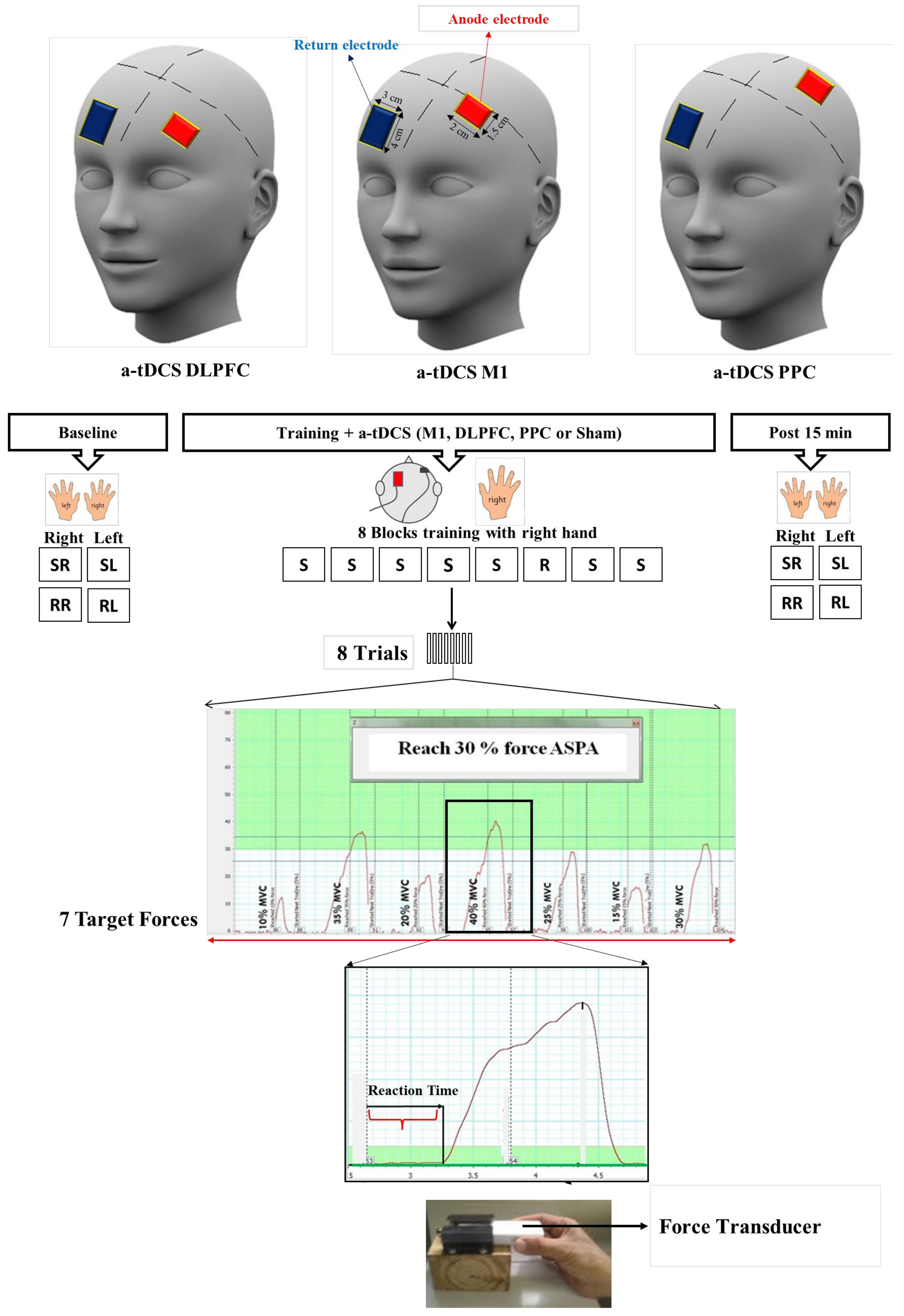
2. Methods and Materials
2.1. Participants and Study Design
2.2. Procedure
2.3. Transcranial Direct-Current Stimulation (tDCS)
2.4. Data Analysis
3. Results
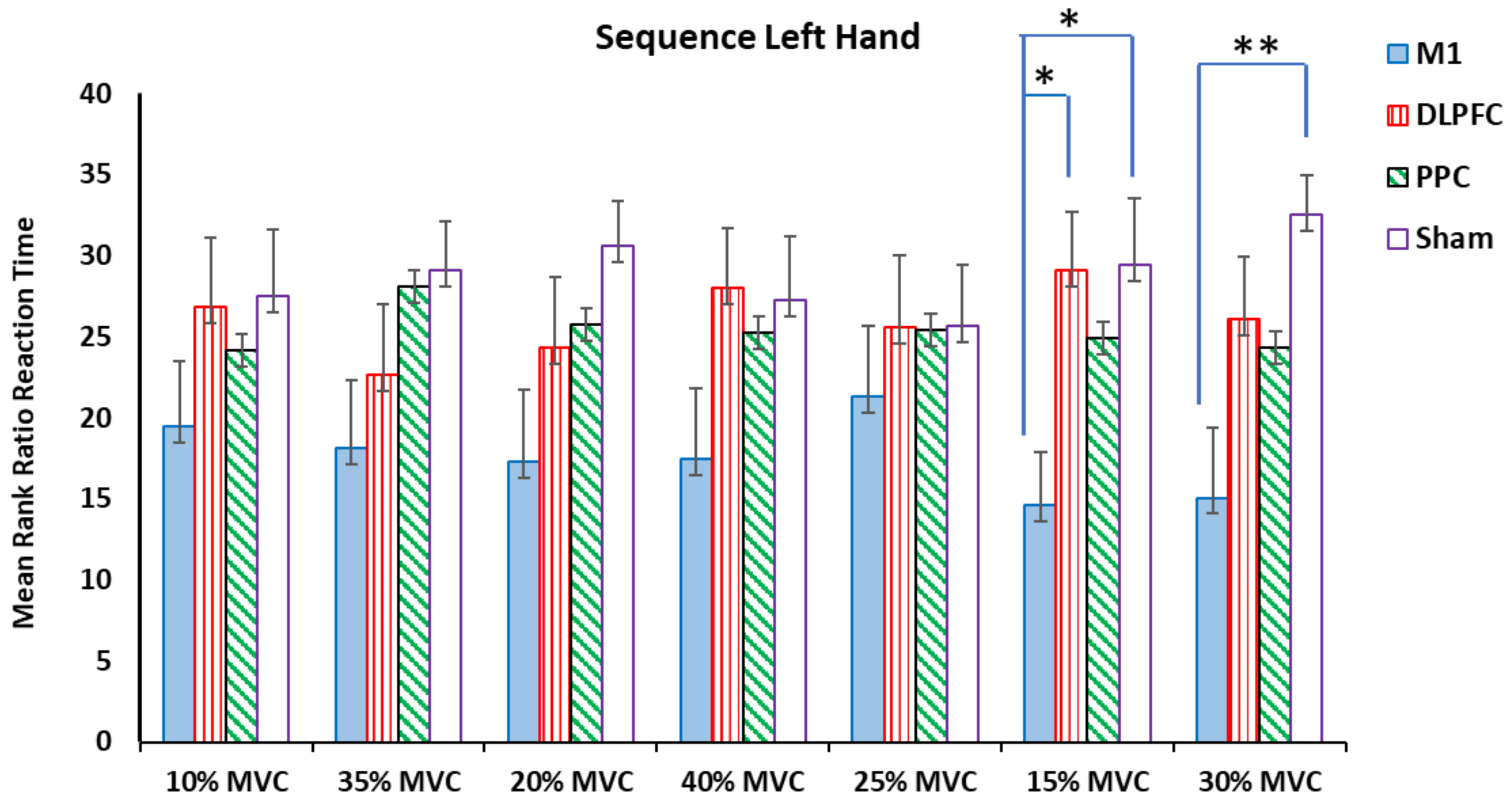
3.1. Ratio RT for Sequence Blocks in Both Right and Left Hands
3.2. Ratio RT for Random Blocks in Both Right and Left Hands
4. Discussion
4.1. The Effects of M1 Stimulation on the RTs
4.2. The Effects of DLPFC and PPC Stimulation on the RTs
4.3. The Effects of Stimulation on Transfer Learning
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cohen, A.; Ivry, R.I.; Keele, S.W. Attention and structure in sequence learning. J. Exp. Psychol. Learn. Mem. Cogn. 1990, 16, 17. [Google Scholar] [CrossRef]
- Cohen, N.J.; Squire, L.R. Preserved learning and retention of pattern-analyzing skill in amnesia: Dissociation of knowing how and knowing that. Science 1980, 210, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Parlow, S.E.; Kinsbourne, M. Asymmetrical transfer of training between hands: Implications for interhemispheric communication in normal brain. Brain Cogn. 1989, 11, 98–113. [Google Scholar] [CrossRef] [PubMed]
- Japikse, K.C.; Negash, S.; Howard, J.H., Jr.; Howard, D.V. Intermanual transfer of procedural learning after extended practice of probabilistic sequences. Exp. Brain Res. Exp. Hirnforschung. Exp. Cerebrale 2003, 148, 38–49. [Google Scholar] [CrossRef] [PubMed]
- Perez, M.; Tanaka, S.; Wise, S.; Sadato, N.; Tanabe, H.; Willingham, D.; Cohen, L. Neural substrates of intermanual transfer of a newly acquired motor skill. Curr. Biol. 2007, 17, 1896–1902. [Google Scholar] [CrossRef] [PubMed]
- Doyon, J.; Penhune, V.; Ungerleider, L.G. Distinct contribution of the cortico-striatal and cortico-cerebellar systems to motor skill learning. Neuropsychologia 2003, 41, 252–262. [Google Scholar] [CrossRef] [PubMed]
- Karni, A.; Meyer, G.; Jezzard, P.; Adams, M.M.; Turner, R.; Ungerleider, L.G. Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature 1995, 377, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Robertson, E.M.; Tormos, J.M.; Maeda, F.; Pascual-Leone, A. The Role of the Dorsolateral Prefrontal Cortex during Sequence Learning is Specific for Spatial Information. Cereb. Cortex 2001, 11, 628. [Google Scholar] [CrossRef]
- Press, D.Z.; Casement, M.D.; Pascual-Leone, A.; Robertson, E.M. The time course of off-line motor sequence learning. Brain Res. Cogn. Brain Res. 2005, 25, 375–378. [Google Scholar] [CrossRef]
- Calford, M.B.; Tweedale, R. Interhemispheric transfer of plasticity in the cerebral cortex. Science 1990, 249, 805–807. [Google Scholar] [CrossRef]
- Gordon, A.M.; Forssberg, H.; Iwasaki, N. Formation and lateralization of internal representations underlying motor commands during precision grip. Neuropsychologia 1994, 32, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Sainburg, R.L.; Wang, J. Interlimb transfer of visuomotor rotations: Independence of direction and final position information. Exp. Brain Res. 2002, 145, 437–447. [Google Scholar] [CrossRef] [PubMed]
- Liang, N.; Takahashi, M.; Ni, Z.; Yahagi, S.; Funase, K.; Kato, T.; Kasai, T. Effects of intermanual transfer induced by repetitive precision grip on input–output properties of untrained contralateral limb muscles. Exp. Brain Res. 2007, 182, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Criscimagna-Hemminger, S.E.; Donchin, O.; Gazzaniga, M.S.; Shadmehr, R. Learned dynamics of reaching movements generalize from dominant to nondominant arm. J. Neurophysiol. 2003, 89, 168–176. [Google Scholar] [CrossRef] [PubMed]
- Pecenka, N.; Engel, A.; Keller, P.E. Neural correlates of auditory temporal predictions during sensorimotor synchronization. Front. Hum. Neurosci. 2013, 7, 380. [Google Scholar] [CrossRef] [PubMed]
- Karabanov, A.; Blom, Ö.; Forsman, L.; Ullén, F. The dorsal auditory pathway is involved in performance of both visual and auditory rhythms. NeuroImage 2009, 44, 480–488. [Google Scholar] [CrossRef] [PubMed]
- Coull, J.; Nobre, A. Dissociating explicit timing from temporal expectation with fMRI. Curr. Opin. Neurobiol. 2008, 18, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Coull, J.T.; Cotti, J.; Vidal, F. Differential roles for parietal and frontal cortices in fixed versus evolving temporal expectations: Dissociating prior from posterior temporal probabilities with fMRI. NeuroImage 2016, 141, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Urbina, G.N.; Batsikadze, G.; Molero-Chamizo, A.; Paulus, W.; Kuo, M.F.; Nitsche, M.A. Parietal transcranial direct current stimulation modulates primary motor cortex excitability. Eur. J. Neurosci. 2015, 41, 845–855. [Google Scholar] [CrossRef]
- Brazovskaya, F.; Malikova, A.; Pavlygina, R. After-effects of anodal polarization in the cat cerebral cortex. Neurophysiology 1972, 4, 194–199. [Google Scholar] [CrossRef]
- Hodgson, R.; Ji, Z.; Standish, S.; Boyd-Hodgson, T.; Henderson, A.; Racine, R. Training-induced and electrically induced potentiation in the neocortex. Neurobiol. Learn. Mem. 2005, 83, 22–32. [Google Scholar] [CrossRef] [PubMed]
- Monfils, M.H.; Teskey, G.C. Skilled-learning-induced potentiation in rat sensorimotor cortex: A transient form of behavioural long-term potentiation. Neuroscience 2004, 125, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Rioult-Pedotti, M.S.; Friedman, D.; Donoghue, J.P. Learning-induced LTP in neocortex. Science 2000, 290, 533–536. [Google Scholar] [CrossRef] [PubMed]
- Muellbacher, W.; Ziemann, U.; Wissel, J.; Dang, N.; Kofler, M.; Facchini, S.; Boroojerdi, B.; Poewe, W.; Hallett, M. Early consolidation in human primary motor cortex. Nature 2002, 415, 640–644. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527 Pt 3, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Cohen, L.G.; Wassermann, E.M.; Priori, A.; Lang, N.; Antal, A.; Paulus, W.; Hummel, F.; Boggio, P.S.; Fregni, F.; et al. Transcranial direct current stimulation: State of the art 2008. Brain Stimul. 2008, 1, 206–223. [Google Scholar] [CrossRef] [PubMed]
- Priori, A.; Berardelli, A.; Rona, S.; Accornero, N.; Manfredi, M. Polarization of the human motor cortex through the scalp. Neuroreport 1998, 9, 2257–2260. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Paulus, W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 2001, 57, 1899–1901. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Liebetanz, D.; Antal, A.; Lang, N.; Tergau, F.; Paulus, W. Modulation of cortical excitability by weak direct current stimulation—Technical, safety and functional aspects. Suppl. Clin. Neurophysiol. 2003, 56, 255–276. [Google Scholar]
- Liebetanz, D.; Nitsche, M.A.; Tergau, F.; Paulus, W. Pharmacological approach to the mechanisms of transcranial DC-stimulation-induced after-effects of human motor cortex excitability. Brain 2002, 125, 2238–2247. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Schauenburg, A.; Lang, N.; Liebetanz, D.; Exner, C.; Paulus, W.; Tergau, F. Facilitation of implicit motor learning by weak transcranial direct current stimulation of the primary motor cortex in the human. J. Cogn. Neurosci. 2003, 15, 619. [Google Scholar] [CrossRef] [PubMed]
- Cuypers, K.; Leenus, D.J.; van den Berg, F.E.; Nitsche, M.A.; Thijs, H.; Wenderoth, N.; Meesen, R.L. Is Motor Learning Mediated by tDCS Intensity? PLoS ONE 2013, 8, e67344. [Google Scholar] [CrossRef] [PubMed]
- Stagg, C.J.; Jayaram, G.; Pastor, D.; Kincses, Z.T.; Matthews, P.M.; Johansen-Berg, H. Polarity and timing-dependent effects of transcranial direct current stimulation in explicit motor learning. Neuropsychologia 2011, 49, 800–804. [Google Scholar] [CrossRef] [PubMed]
- Kantak, S.S.; Mummidisetty, C.K.; Stinear, J.W. Primary motor and premotor cortex in implicit sequence learning—Evidence for competition between implicit and explicit human motor memory systems. Eur. J. Neurosci. 2012, 36, 2710–2715. [Google Scholar] [CrossRef] [PubMed]
- Schambra, H.M.; Abe, M.; Luckenbaugh, D.A.; Reis, J.; Krakauer, J.W.; Cohen, L.G. Probing for hemispheric specialization for motor skill learning: A transcranial direct current stimulation study. J. Neurophysiol. 2011, 106, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Saucedo Marquez, C.M.; Zhang, X.; Swinnen, S.P.; Meesen, R.; Wenderoth, N. Task-specific effect of transcranial direct current stimulation on motor learning. Front. Hum. Neurosci. 2013, 7, 333. [Google Scholar] [CrossRef] [PubMed]
- Reis, J.; Schambra, H.M.; Cohen, L.G.; Buch, E.R.; Fritsch, B.; Zarahn, E.; Celnik, P.A.; Krakauer, J.W. Noninvasive cortical stimulation enhances motor skill acquisition over multiple days through an effect on consolidation. Proc. Natl. Acad. Sci. USA 2009, 106, 1590–1595. [Google Scholar] [CrossRef] [PubMed]
- Thomas, C.; Ghodratitoostani, I.; Delbem, A.C.; Ali, A.; Datta, A. Influence of gender-related differences in transcranial direct current stimulation: A Computational Study. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 5196–5199. [Google Scholar]
- Hashemirad, F.; Fitzgerald, P.B.; Zoghi, M.; Hashemirad, M.; Jaberzadeh, S. The effects of inter-trial interval on implicit learning of sequential visual isometric pinch task. J. Bodyw. Mov. Ther. 2017, 21, 626–632. [Google Scholar] [CrossRef] [PubMed]
- Vaseghi, B.; Zoghi, M.; Jaberzadeh, S. The effects of anodal-tDCS on corticospinal excitability enhancement and its after-effects: Conventional vs. unihemispheric concurrent dual-site stimulation. Front. Hum. Neurosci. 2015, 9, 533. [Google Scholar] [CrossRef]
- Vaseghi, B.; Zoghi, M.; Jaberzadeh, S. How does anodal transcranial direct current stimulation of the pain neuromatrix affect brain excitability and pain perception? A randomised, double-blind, sham-control study. PLoS ONE 2015, 10, e0118340. [Google Scholar] [CrossRef]
- Koechlin, E.; Basso, G.; Pietrini, P.; Panzer, S.; Grafman, J. The role of the anterior prefrontal cortex in human cognition. Nature 1999, 399, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Poreisz, C.; Boros, K.; Antal, A.; Paulus, W. Safety aspects of transcranial direct current stimulation concerning healthy subjects and patients. Brain Res. Bull. 2007, 72, 208–214. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences; Psychology Press: New York, NY, USA, 1988. [Google Scholar]
- Waters-Metenier, S.; Husain, M.; Wiestler, T.; Diedrichsen, J. Bihemispheric transcranial direct current stimulation enhances effector-independent representations of motor synergy and sequence learning. J. Neurosci. 2014, 34, 1037–1050. [Google Scholar] [CrossRef] [PubMed]
- Hashemirad, F.; Zoghi, M.; Fitzgerald, P.B.; Jaberzadeh, S. The effect of anodal transcranial direct current stimulation on motor sequence learning in healthy individuals: A systematic review and meta-analysis. Brain Cogn. 2016, 102, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Horvath, J.C.; Carter, O.; Forte, J.D. No significant effect of transcranial direct current stimulation (tDCS) found on simple motor reaction time comparing 15 different simulation protocols. Neuropsychologia 2016, 91, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Nitsche, M.A.; Doemkes, S.; Karakose, T.; Antal, A.; Liebetanz, D.; Lang, N.; Tergau, F.; Paulus, W. Shaping the effects of transcranial direct current stimulation of the human motor cortex. J. Neurophysiol. 2007, 97, 3109–3117. [Google Scholar] [CrossRef] [PubMed]
- Boros, K.; Poreisz, C.; Münchau, A.; Paulus, W.; Nitsche, M.A. Premotor transcranial direct current stimulation (tDCS) affects primary motor excitability in humans. Eur. J. Neurosci. 2008, 27, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Elbert, T.; Lutzenberger, W.; Rockstroh, B.; Birbaumer, N. The influence of low-level transcortical DC-currents on response speed in humans. Int. J. Neurosci. 1981, 14, 101–114. [Google Scholar] [CrossRef] [PubMed]
- Leff, D.R.; Orihuela-Espina, F.; Elwell, C.E.; Athanasiou, T.; Delpy, D.T.; Darzi, A.W.; Yang, G.-Z. Assessment of the cerebral cortex during motor task behaviours in adults: A systematic review of functional near infrared spectroscopy (fNIRS) studies. NeuroImage 2011, 54, 2922–2936. [Google Scholar] [CrossRef]
- Grafton, S.T.; Hazeltine, E.; Ivry, R. Functional mapping of sequence learning in normal humans. J. Cogn. Neurosci. 1995, 7, 497–510. [Google Scholar] [CrossRef]
- Dayan, E.; Cohen, L.G. Neuroplasticity subserving motor skill learning. Neuron 2011, 72, 443–454. [Google Scholar] [CrossRef] [PubMed]
- Marshall, L.; Mölle, M.; Siebner, H.R.; Born, J. Bifrontal transcranial direct current stimulation slows reaction time in a working memory task. BMC Neurosci. 2005, 6, 23. [Google Scholar] [CrossRef] [PubMed]
- Friehs, M.A.; Frings, C. Pimping inhibition: Anodal tDCS enhances stop-signal reaction time. J. Exp. Psychol. Hum. Percept. Perform. 2018, 44, 1933. [Google Scholar] [CrossRef]
- Krause, V.; Bashir, S.; Pollok, B.; Caipa, A.; Schnitzler, A.; Pascual-Leone, A. 1 Hz rTMS of the left posterior parietal cortex (PPC) modifies sensorimotor timing. Neuropsychologia 2012, 50, 3729–3735. [Google Scholar] [CrossRef]
- Heinen, K.; Sagliano, L.; Candini, M.; Husain, M.; Cappelletti, M.; Zokaei, N. Cathodal transcranial direct current stimulation over posterior parietal cortex enhances distinct aspects of visual working memory. Neuropsychologia 2016, 87, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Keitel, A.; Øfsteng, H.; Krause, V.; Pollok, B. Anodal transcranial direct current stimulation (tDCS) over the right primary motor cortex (M1) impairs implicit motor sequence learning of the ipsilateral hand. Front. Hum. Neurosci. 2018, 12, 289. [Google Scholar] [CrossRef]
- Doyon, J.; Benali, H. Reorganization and plasticity in the adult brain during learning of motor skills. Curr. Opin. Neurobiol. 2005, 15, 161–167. [Google Scholar] [CrossRef]
- Honda, M.; Marie-Pierre, D.; Ibanez, V.; Pascual-Leone, A.; Zhuang, P.; Hallett, M. Dynamic cortical involvement in implicit and explicit motor sequence learning: A PET study. Brain 1998, 121, 2159–2173. [Google Scholar] [CrossRef] [PubMed]
- Bloom, J.S.; Hynd, G.W. The role of the corpus callosum in interhemispheric transfer of information: Excitation or inhibition? Neuropsychol. Rev. 2005, 15, 59–71. [Google Scholar] [CrossRef]
- Daselaar, S.M.; Rombouts, S.A.; Veltman, D.J.; Raaijmakers, J.G.; Jonker, C. Similar network activated by young and old adults during the acquisition of a motor sequence. Neurobiol. Aging 2003, 24, 1013–1019. [Google Scholar] [CrossRef]
- Bischoff-Grethe, A.; Goedert, K.M.; Willingham, D.T.; Grafton, S.T. Neural substrates of response-based sequence learning using fMRI. J. Cogn. Neurosci. 2004, 16, 127–138. [Google Scholar] [CrossRef] [PubMed]




| Baseline RT | Block | Group | |||||
|---|---|---|---|---|---|---|---|
| M1 | DLPFC | PPC | Sham | χ2 | p | ||
| 10% MVC | Seq.R | 20.42 | 25.88 | 24.17 | 27.54 | 1.710 | 0.635 |
| Seq.L | 18.79 | 30.63 | 21.46 | 27.13 | 5.281 | 0.152 | |
| Ran.R | 24.13 | 22.46 | 25.17 | 26.25 | 0.479 | 0.924 | |
| Ran.L | 20.46 | 28.50 | 23.46 | 25.58 | 2.118 | 0.548 | |
| 15% MVC | Seq.R | 20.50 | 21.54 | 29.17 | 26.79 | 3.170 | 0.366 |
| Seq.L | 18.67 | 26.92 | 26.00 | 26.42 | 2.804 | 0.423 | |
| Ran.R | 15.92 | 26.50 | 28.79 | 26.79 | 6.206 | 0.102 | |
| Ran.L | 17.88 | 28.75 | 25.54 | 25.83 | 3.969 | 0.265 | |
| 20% MVC | Seq.R | 19.83 | 22.83 | 27.50 | 27.83 | 2.735 | 0.434 |
| Seq.L | 19.08 | 24.92 | 26.92 | 27.08 | 2.573 | 0.462 | |
| Ran.R | 20.42 | 28.42 | 22.00 | 27.17 | 2.778 | 0.427 | |
| Ran.L | 23.33 | 25.08 | 23.88 | 25.71 | 0.217 | 0.975 | |
| 25% MVC | Seq.R | 23.63 | 24.38 | 25.21 | 24.79 | 0.084 | 0.994 |
| Seq.L | 23.25 | 24.92 | 25.42 | 24.42 | 0.158 | 0.984 | |
| Ran.R | 19.58 | 27.58 | 22.83 | 28.00 | 2.982 | 0.394 | |
| Ran.L | 24.42 | 23.50 | 26.08 | 24.00 | 0.230 | 0.973 | |
| 30% MVC | Seq.R | 17.58 | 24.54 | 28.38 | 27.5 | 4.400 | 0.221 |
| Seq.L | 16.58 | 27.13 | 24.75 | 29.54 | 5.819 | 0.121 | |
| Ran.R | 23.50 | 25.88 | 23.83 | 24.79 | 0.209 | 0.976 | |
| Ran.L | 24.92 | 22.25 | 24.92 | 25.92 | 0.454 | 0.929 | |
| 35% MVC | Seq.R | 18.71 | 23.92 | 30.17 | 25.21 | 4.072 | 0.254 |
| Seq.L | 19.54 | 25.50 | 26.13 | 26.83 | 2.062 | 0.560 | |
| Ran.R | 20.96 | 25.50 | 23.38 | 28.17 | 1.730 | 0.630 | |
| Ran.L | 22.29 | 24.79 | 25.46 | 25.46 | 0.416 | 0.937 | |
| 40% MVC | Seq.R | 21.42 | 22.67 | 27.88 | 26.04 | 1.631 | 0.652 |
| Seq.L | 19.25 | 27.13 | 25.17 | 26.46 | 2.372 | 0.499 | |
| Ran.R | 17.00 | 28.04 | 24.58 | 28.38 | 5.132 | 0.162 | |
| Ran.L | 22.08 | 22.54 | 28.04 | 25.33 | 1.403 | 0.705 | |
| Ratio RT (Pre-Post/Pre) × 100 | Sequence Block | Random Block | ||
|---|---|---|---|---|
| Right Hand (Seq.R) | Left Hand (Seq.L) | Right Hand (Ran.R) | Left Hand (Ran.L) | |
| 10% MVC | H (3) = 0.65, p = 0.883 η2 = 0.053 | H (3) = 2.42, p = 0.49 η2 = 0.013 | H (3) = 0.834, p = 0.841 η2 = 0.049 | H (3) = 1.72, p = 0.632 η2 = 0.029 |
| 15% MVC | H (3) = 9.27, p = 0.026 * η2 = 0.143 | H (3) = 8.79, p = 0.032 * η2 = 0.132 | H (3) = 8.31, p = 0.04 * η2 = 0.121 | H (3) = 7.2, p = 0.066 η2 = 0.095 |
| 20% MVC | H (3) = 4.59, p = 0.204 η2 = 0.036 | H (3) = 5.5, p = 0.138 η2 = 0.057 | H (3) = 4.34, p = 0.226 η2 = 0.03 | H (3) = 1.36, p = 0.714 η2 = 0.037 |
| 25% MVC | H (3) = 4.01, p = 0.261 η2 = 0.023 | H (3) = 0.821, p = 0.845 η2 = 0.05 | H (3) = 1.84, p = 0.606 η2 = 0.026 | H (3) = 0.014, p = 1.000 η2 = 0.068 |
| 30% MVC | H (3) = 8.23, p = 0.041 * η2 = 0.119 | H (3) = 9.5, p = 0.023 * η2 = 0.148 | H (3) = 0.49, p = 0.92 η2 = 0.057 | H (3) = 0.275, p = 0.965 η2 = 0.062 |
| 35% MVC | H (3) = 4.81, p = 0.186 η2 = 0.041 | H (3) = 4.73, p = 0.192 η2 = 0.039 | H (3) = 2.07, p = 0.55 η2 = 0.021 | H (3) = 3.36, p = 0.339 η2 = 0.008 |
| 40% MVC | H (3) = 4.68, p = 0.196 η2 = 0.038 | H (3) = 4.24, p = 0.236 η2 = 0.028 | H (3) = 8.57, p = 0.035 * η2 = 0.127 | H (3) = 1.92, p = 0.587 η2 = 0.025 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashemirad, F.; Zoghi, M.; Fitzgerald, P.B.; Hashemirad, M.; Jaberzadeh, S. Site Dependency of Anodal Transcranial Direct-Current Stimulation on Reaction Time and Transfer of Learning during a Sequential Visual Isometric Pinch Task. Brain Sci. 2024, 14, 408. https://doi.org/10.3390/brainsci14040408
Hashemirad F, Zoghi M, Fitzgerald PB, Hashemirad M, Jaberzadeh S. Site Dependency of Anodal Transcranial Direct-Current Stimulation on Reaction Time and Transfer of Learning during a Sequential Visual Isometric Pinch Task. Brain Sciences. 2024; 14(4):408. https://doi.org/10.3390/brainsci14040408
Chicago/Turabian StyleHashemirad, Fahimeh, Maryam Zoghi, Paul B. Fitzgerald, Masoumeh Hashemirad, and Shapour Jaberzadeh. 2024. "Site Dependency of Anodal Transcranial Direct-Current Stimulation on Reaction Time and Transfer of Learning during a Sequential Visual Isometric Pinch Task" Brain Sciences 14, no. 4: 408. https://doi.org/10.3390/brainsci14040408






