Go/No-Go Ratios Modulate Inhibition-Related Brain Activity: An Event-Related Potential Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
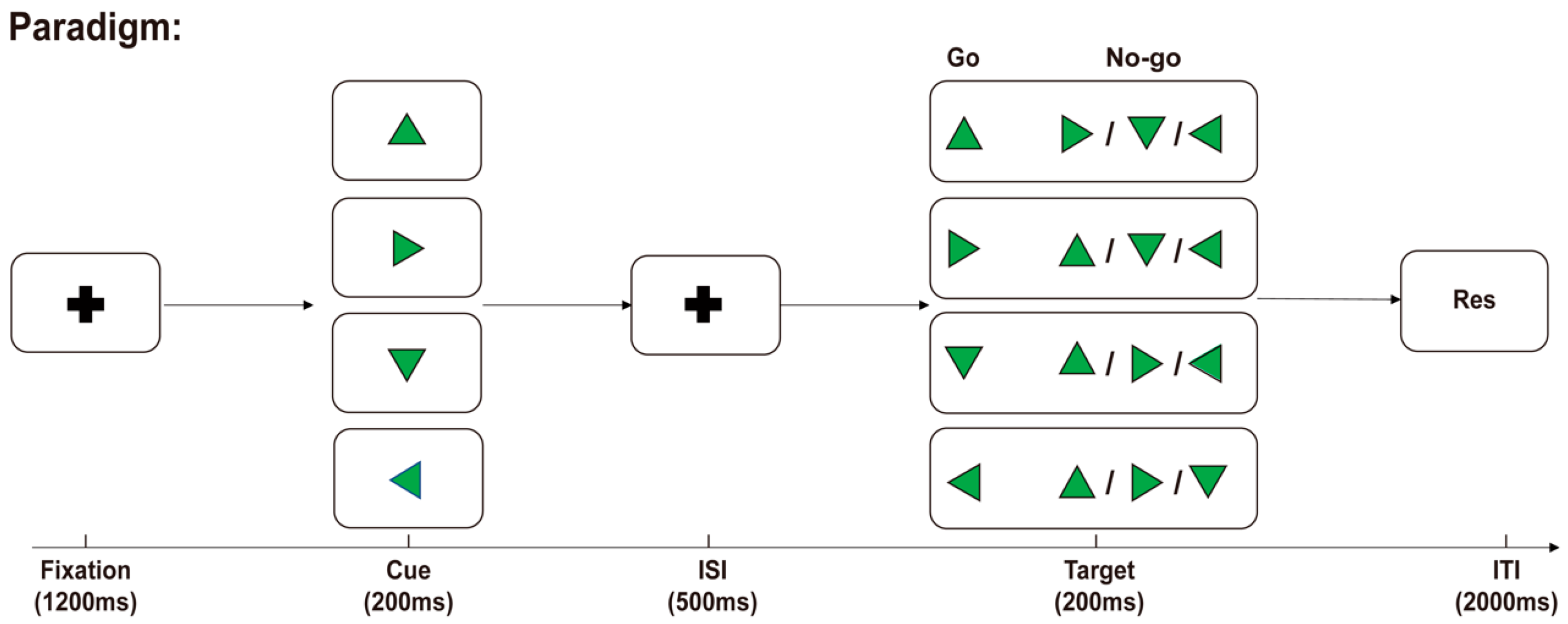
2.2. Stimuli and Procedures
2.3. EEG Recording and Preprocessing
2.4. Statistical Analysis
3. Results
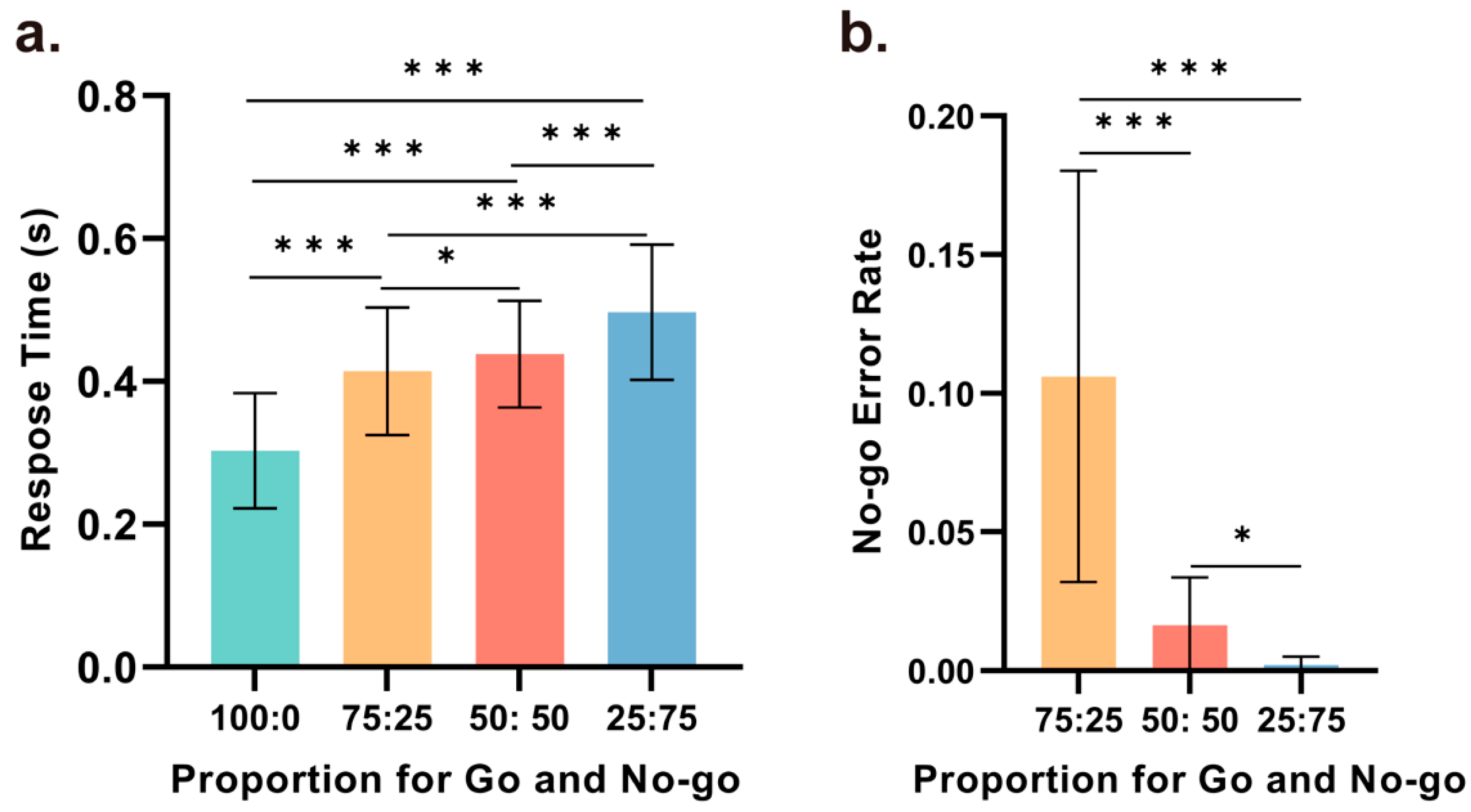
3.1. Behavioral Performance
3.2. ERP Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aron, A.R. The Neural Basis of Inhibition in Cognitive Control. Neuroscientist 2007, 13, 214–228. [Google Scholar] [CrossRef] [PubMed]
- Baumeister, R.F. Self-regulation, ego depletion, and inhibition. Neuropsychologia 2014, 65, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Migliaccio, R.; Tanguy, D.; Bouzigues, A.; Sezer, I.; Dubois, B.; Le Ber, I.; Batrancourt, B.; Godefroy, V.; Levy, R. Cognitive and behavioural inhibition deficits in neurodegenerative dementias. Cortex 2020, 131, 265–283. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, S.M.; Lee, J.; Williams, R.; Jones, A. Psychopathy and response inhibition: A meta-analysis of go/no-go and stop signal task performance. Neurosci. Biobehav. Rev. 2022, 142, 104868. [Google Scholar] [CrossRef] [PubMed]
- Raud, L.; Westerhausen, R.; Dooley, N.; Huster, R.J. Differences in unity: The go/no-go and stop signal tasks rely on different mechanisms. NeuroImage 2020, 210, 116582. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, D.J.; Pekar, J.J.; Mostofsky, S.H. Meta-analysis of go/no-go tasks demonstrating that fmri activation associated with response inhibition is task-dependent. Neuropsychologia 2008, 46, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Gavazzi, G.; Giovannelli, F.; Noferini, C.; Cincotta, M.; Cavaliere, C.; Salvatore, M.; Mascalchi, M.; Viggiano, M.P. Subregional prefrontal cortex recruitment as a function of inhibitory demand: An fMRI metanalysis. Neurosci. Biobehav. Rev. 2023, 152, 105285. [Google Scholar] [CrossRef] [PubMed]
- Kok, A.; Ramautar, J.R.; De Ruiter, M.B.; Band, G.P.H.; Ridderinkhof, K.R. ERP components associated with successful and unsuccessful stopping in a stop-signal task. Psychophysiology 2004, 41, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Kirby, N.H. Sequential effects in two-choice reaction time: Automatic facilitation or subjective expectancy? J. Exp. Psychol. Hum. Percept. Perform. 1976, 2, 567. [Google Scholar] [CrossRef]
- Pfefferbaum, A.; Ford, J.; Johnson, R.; Wenegrat, B.; Kopell, B.S. Manipulation of p3 latency: Speed vs. accuracy instructions t. Electroencephalogr. Clin. Neurophysiol. 1983, 55, 188–197. [Google Scholar] [CrossRef]
- Montare, A. Conditioning reaction time: Evidence for a process of conditioned automatization. Percept. Mot. Ski. 1992, 75, 755–770. [Google Scholar] [CrossRef] [PubMed]
- Friedman, N.P.; Robbins, T.W. The role of prefrontal cortex in cognitive control and executive function. Neuropsychopharmacology 2021, 47, 72–89. [Google Scholar] [CrossRef] [PubMed]
- Morein-Zamir, S.; Robbins, T.W. Fronto-striatal circuits in response-inhibition: Relevance to addiction. Brain Res. 2015, 1628, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Tsai, H.Y.; Cheng, H.N. The effect of age on n2 and p3 components: A meta-analysis of go/nogo tasks. Brain Cogn. 2019, 135, 103574. [Google Scholar] [CrossRef] [PubMed]
- Groom, M.J.; Cragg, L. Differential modulation of the n2 and p3 event-related potentials by response conflict and inhibition. Brain Cogn. 2015, 97, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Kropotov, J.D.; Ponomarev, V.A.; Pronina, M.; Jäncke, L. Functional indexes of reactive cognitive control: Erps in cued go/no-go tasks. Psychophysiology 2017, 54, 1899–1915. [Google Scholar] [CrossRef] [PubMed]
- Nakata, H.; Takezawa, M.; Kamijo, K.; Shibasaki, M. Modality differences in erp components between somatosensory and auditory go/no-go paradigms in prepubescent children. PLoS ONE 2021, 16, e0259653. [Google Scholar] [CrossRef] [PubMed]
- Albert, J.; López-Martín, S.; Hinojosa, J.A.; Carretié, L. Spatiotemporal characterization of response inhibition. Neuroimage 2013, 76, 272–281. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zheng, Y.; Smith, S.D.; Shic, F.; Moore, C.C.; Zheng, X.; Qi, Y.; Liu, Z.; Leckman, J.F. A preliminary study of movement intensity during a go/no-go task and its association with adhd outcomes and symptom severity. Child Adolesc. Psychiatry Ment. Health 2016, 10, 47. [Google Scholar] [CrossRef] [PubMed]
- Young, M.E.; Sutherland, S.C.; McCoy, A.W. Optimal go/no-go ratios to maximize false alarms. Behav. Res. Methods 2017, 50, 1020–1029. [Google Scholar] [CrossRef]
- Hester, R.L.; Murphy, K.; Foxe, J.J.; Foxe, D.M.; Javitt, D.C.; Garavan, H. Predicting success: Patterns of cortical activation and deactivation prior to response inhibition. J. Cogn. Neurosci. 2004, 16, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Moutoussis, M.; Fearon, P.; El-Deredy, W.; Dolan, R.J.; Friston, K.J. Bayesian inferences about the self (and others): A review. Conscious. Cogn. 2014, 25, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Bubic, A.; Yves von Cramon, D.; Schubotz, R.I. Prediction, cognition and the brain. Front. Hum. Neurosci. 2010, 4, 25. [Google Scholar] [CrossRef] [PubMed]
- Kveraga, K.; Ghuman, A.S.; Bar, M. Top-down predictions in the cognitive brain. Brain Cogn. 2007, 65, 145–168. [Google Scholar] [CrossRef] [PubMed]
- Wulf, G.; Lewthwaite, R. Optimizing performance through intrinsic motivation and attention for learning: The optimal theory of motor learning. Psychon. Bull. Rev. 2016, 23, 1382–1414. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y. Selective attention on representations in working memory: Cognitive and neural mechanisms. PeerJ 2018, 6, e4585. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Crawford, J.D. Allocentric representations for target memory and reaching in human cortex. Ann. New York Acad. Sci. 2020, 1464, 142–155. [Google Scholar] [CrossRef] [PubMed]
- Meffert, H.; Hwang, S.; Nolan, Z.T.; Chen, G.; Blair, J.R. Segregating attention from response control when performing a motor inhibition task. Neuroimage 2016, 126, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Rezvanfard, M.; Golesorkhi, M.; Ghassemian, E.; Safaei, H.; Eghbali, A.N.; Alizadeh, H.; Ekhtiari, H. Evaluation of inhibition response behavior using the go/no-go paradigm in normal individuals: Effects of variations in the task design. Acta Neuropsychol. 2016, 14, 357–366. [Google Scholar] [CrossRef]
- Ertekin, E.; Üçok, A.; Keskin-Ergen, Y.; Devrim-Üçok, M. Deficits in go and nogo p3 potentials in patients with schizophrenia. Psychiatry Res. 2017, 254, 126–132. [Google Scholar] [CrossRef] [PubMed]
- Jonkman, L.M.; Lansbergen, M.; Stauder, J.E.A. Developmental differences in behavioral and event-related brain responses associated with response preparation and inhibition in a go/nogo task. Psychophysiology 2003, 40, 752–761. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.L.; Johnstone, S.J.; Barry, R.J. Movement-related potentials in the go/nogo task: The p3 reflects both cognitive and motor inhibition. Clin. Neurophysiol. 2008, 119, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Bosch, T.J.; Groth, C.; Eldridge, T.A.; Gnimpieba, E.Z.; Baugh, L.A.; Singh, A. Altered cerebellar oscillations in parkinson’s disease patients during cognitive and motor tasks. Neuroscience 2021, 475, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Nan, W.; Dias, A.P.B.; Rosa, A.C. Neurofeedback training for cognitive and motor function rehabilitation in chronic stroke: Two case reports. Front. Neurol. 2019, 10, 800. [Google Scholar] [CrossRef] [PubMed]
- Benikos, N.; Johnstone, S.J.; Roodenrys, S.J. Short-term training in the go/nogo task: Behavioural and neural changes depend on task demands. Int. J. Psychophysiol. 2013, 87, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Wascher, E.; Arnau, S.; Schneider, D.; Hoppe, K.; Getzmann, S.; Verleger, R. No effect of target probability on p3b amplitudes. Int. J. Psychophysiol. 2020, 153, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, P.D.; Falkenstein, M. Effects of task complexity on erp components in go/nogo tasks. Int. J. Psychophysiol. 2013, 87, 273–278. [Google Scholar] [CrossRef] [PubMed]
- Los, S.A. The role of response inhibition in temporal preparation: Evidence from a go/no-go task. Cognition 2013, 129, 328–344. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.; Saxe, R.; Tenenbaum, J. Bayesian theory of mind: Modeling joint belief-desire attribution. In Proceedings of the Annual Meeting of the Cognitive Science Society, Boston, MA, USA, 20–23 July 2011; Volume 33. [Google Scholar]
- Chater, N.; Oaksford, M.; Hahn, U.; Heit, E. Bayesian models of cognition. WIREs Cogn. Sci. 2010, 1, 811–823. [Google Scholar] [CrossRef] [PubMed]
- van de Schoot, R.; Depaoli, S.; King, R.; Kramer, B.; Märtens, K.; Tadesse, M.G.; Vannucci, M.; Gelman, A.; Veen, D.; Willemsen, J.; et al. Bayesian statistics and modelling. Nat. Rev. Methods Prim. 2021, 1, 1–26. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; An, W.; Yu, Y.; Wu, J.; Yang, J. Go/No-Go Ratios Modulate Inhibition-Related Brain Activity: An Event-Related Potential Study. Brain Sci. 2024, 14, 414. https://doi.org/10.3390/brainsci14050414
Zhang N, An W, Yu Y, Wu J, Yang J. Go/No-Go Ratios Modulate Inhibition-Related Brain Activity: An Event-Related Potential Study. Brain Sciences. 2024; 14(5):414. https://doi.org/10.3390/brainsci14050414
Chicago/Turabian StyleZhang, Nan, Weichao An, Yinghua Yu, Jinglong Wu, and Jiajia Yang. 2024. "Go/No-Go Ratios Modulate Inhibition-Related Brain Activity: An Event-Related Potential Study" Brain Sciences 14, no. 5: 414. https://doi.org/10.3390/brainsci14050414



