A High-Fat Meal, or Intraperitoneal Administration of a Fat Emulsion, Increases Extracellular Dopamine in the Nucleus Accumbens
Abstract
:1. Introduction
2. Results
2.1. Experiment 1: A High-Fat Meal Increases Extracellular NAc DA Levels More than a Less Palatable Low-Fat Meal
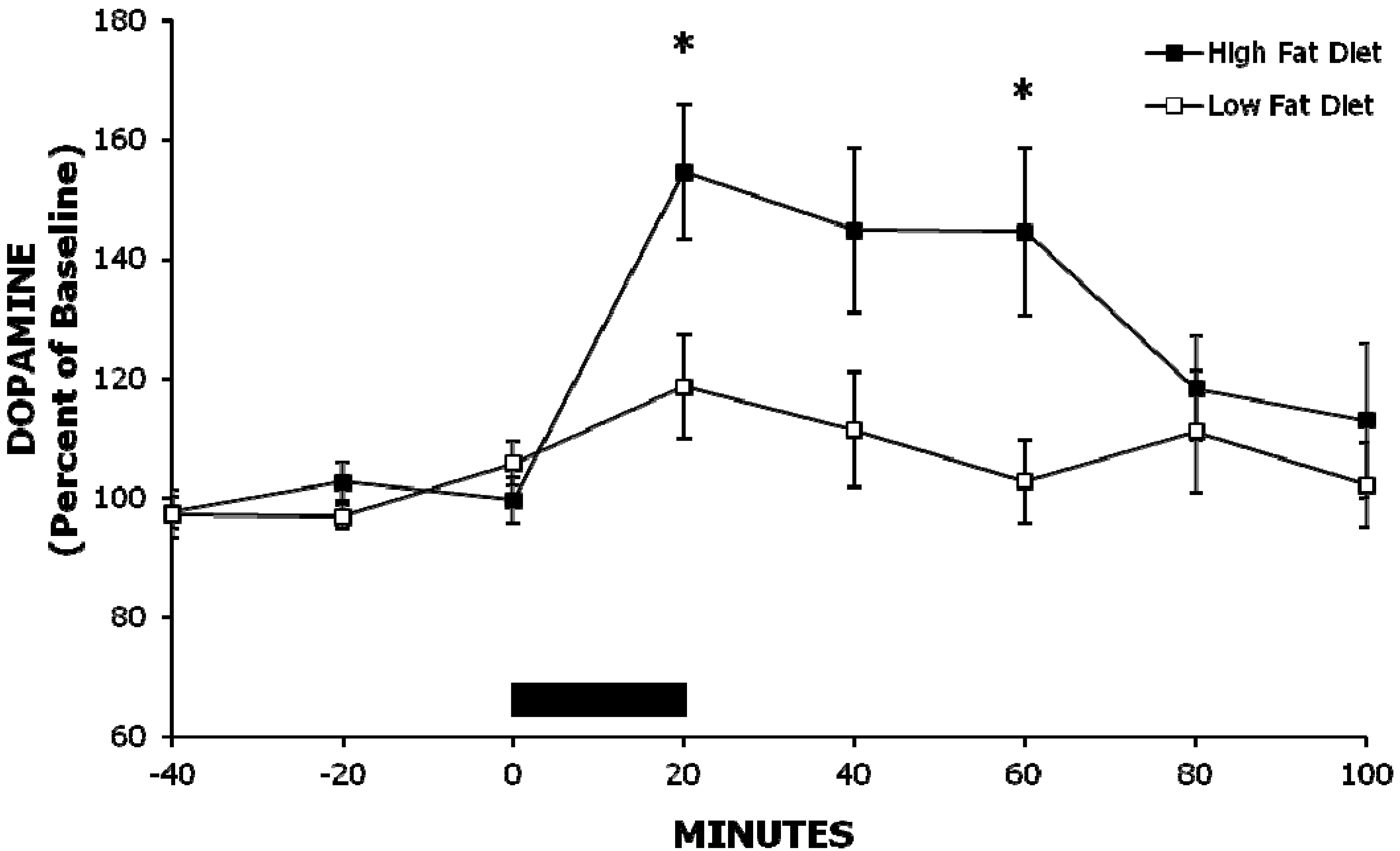
| Experiment 1 | Baseline | 20 min | 40 min | 60 min |
| DOPAC | ||||
| High-Fat Meal | 99 ± 2% | 124 ± 4% | 125 ± 5% | 122 ± 7% |
| Low-Fat Meal | 102 ± 1% | 113 ± 5% | 115 ± 5% | 112 ± 6% |
| HVA | ||||
| High-Fat Meal | 102 ± 3% | 117 ± 4% | 126 ± 4% | 130 ± 7% |
| Low-Fat Meal | 100 ± 2% | 112 ± 5% | 119 ± 6% | 120 ± 6% |
| Experiment 2 | Baseline | 60 min | 120 min | 180 min |
| DOPAC | ||||
| Intralipid | 99 ± 1% | 112 ± 7% | 100 ± 5% | 98 ± 3% |
| Glucose | 97 ± 1% | 93 ± 5% | 86 ± 3% | 82 ± 6% |
| Saline | 101 ± 2% | 94 ± 5% | 108 ± 12% | 103 ± 10% |
| HVA | ||||
| Intralipid | 99 ± 2% | 113 ± 8% | 103 ± 4% | 101 ± 5% |
| Glucose | 98 ± 1% | 95 ± 6% | 90 ± 2% | 92 ± 3% |
| Saline | 98 ± 3% | 97 ± 6% | 105 ± 8% | 103 ± 7% |
2.2. Experiment 2: Injection of Intralipid Increases Extracellular NAc DA Levels Compared to Glucose or Saline
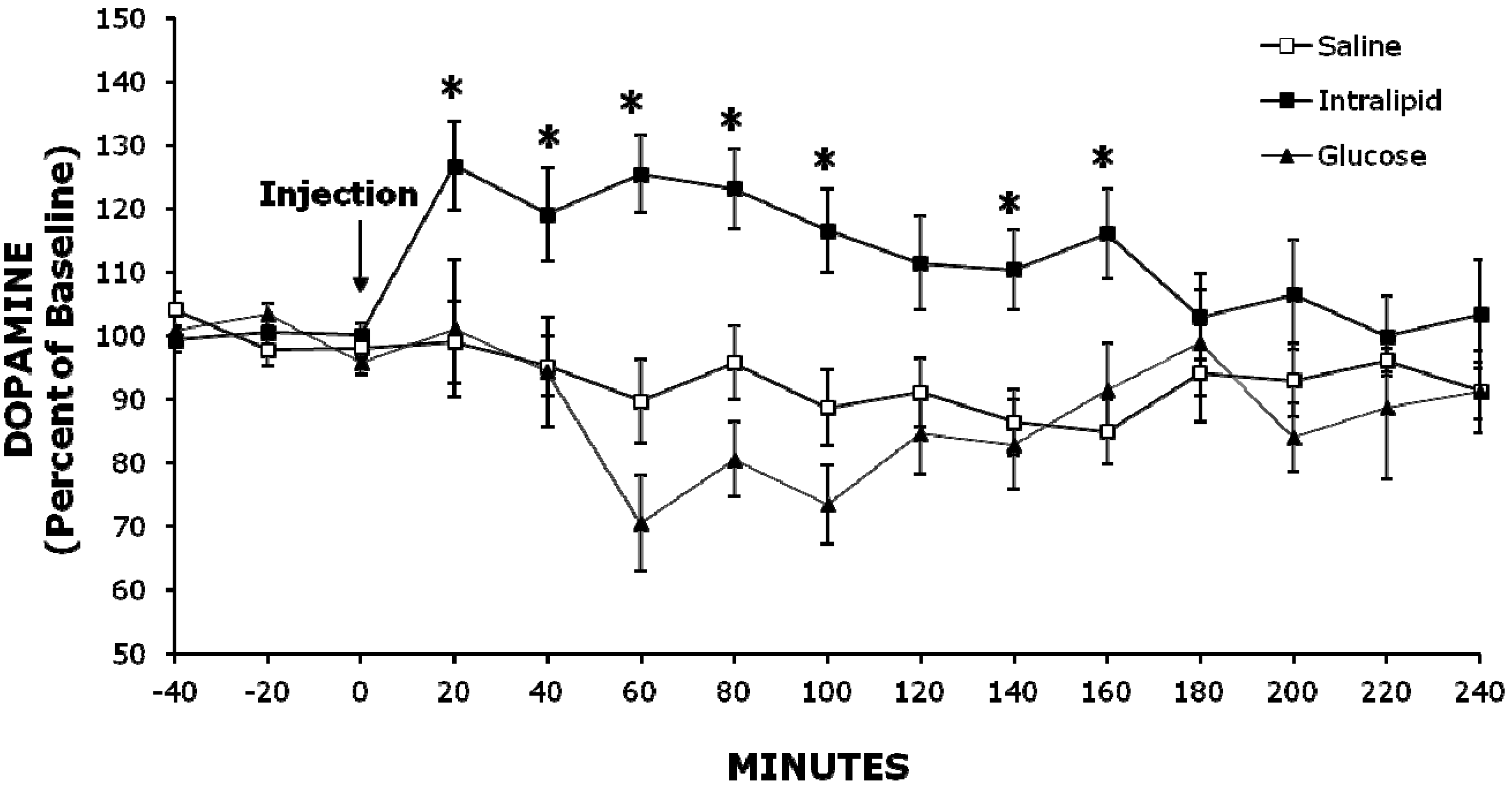
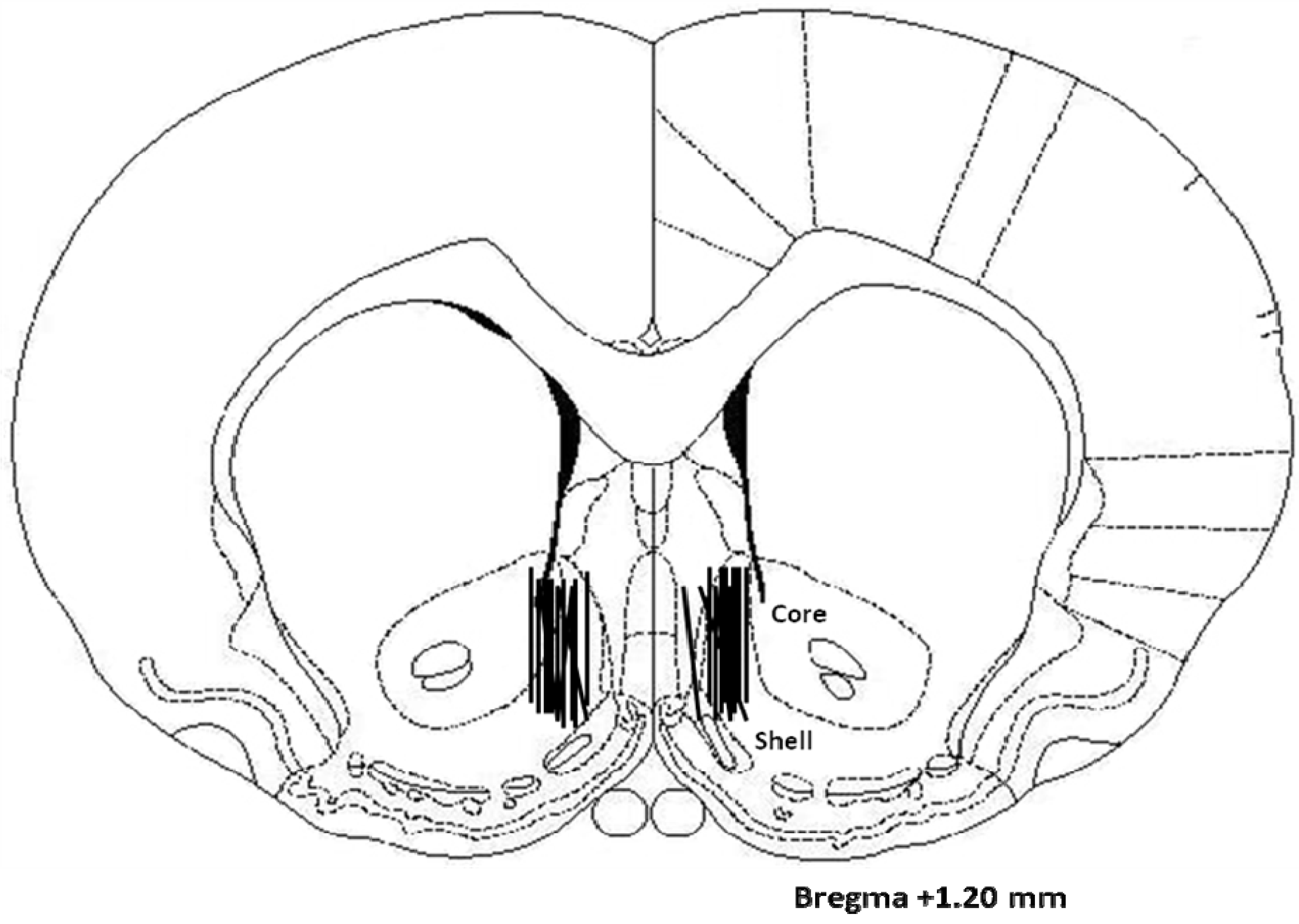
2.3. Histology
3. Discussion
3.1. Role of Calories in Fat-Induced Increase in Extracellular Levels of NAc DA and Hyperphagia
3.2. Role of Palatability in Fat-Induced Increase in Extracellular NAc DA Levels and Hyperphagia
3.3. Role of Circulating Lipids in Fat-Induced Increases in Extracellular NAc DA Levels and Hyperphagia
4. Experimental Procedure
4.1. Subjects
4.2. Surgery
4.3. Microdialysis and DA Assays
4.4. Diets
4.5. Test Procedures
4.6. Histology
4.7. Data Analysis
5. Conclusion
Abbreviations
| DA | dopamine |
| DOPAC | 3,4-dihydroxyphenlyacetic acid |
| HVA | homovanillic acid |
| NAc | nucleus accumbens |
| TG | triglycerides |
Acknowledgments
Conflict of Interest
References
- Leibowitz, S.F.; Wortley, K.E. Hypothalamic control of energy balance: different peptides, different functions. Peptides 2004, 25, 473–504. [Google Scholar] [CrossRef]
- Clegg, D.J.; Air, E.L.; Woods, S.C.; Seeley, R.J. Eating elicited by orexin-a, but not melanin-concentrating hormone, is opioid mediated. Endocrinology 2002, 143, 2995–3000. [Google Scholar]
- Naleid, A.M.; Grace, M.K.; Chimukangara, M.; Billington, C.J.; Levine, A.S. Paraventricular opioids alter intake of high-fat but not high-sucrose diet depending on diet preference in a binge model of feeding. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R99–R105. [Google Scholar] [CrossRef]
- Tempel, D.L.; Leibowitz, K.J.; Leibowitz, S.F. Effects of PVN galanin on macronutrient selection. Peptides 1988, 9, 309–314. [Google Scholar] [CrossRef]
- Chang, G.Q.; Karatayev, O.; Ahsan, R.; Gaysinskaya, V.; Marwil, Z.; Leibowitz, S.F. Dietary fat stimulates endogenous enkephalin and dynorphin in the paraventricular nucleus: Role of circulating triglycerides. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E561–E570. [Google Scholar]
- Leibowitz, S.F.; Dourmashkin, J.T.; Chang, G.Q.; Hill, J.O.; Gayles, E.C.; Fried, S.K.; Wang, J. Acute high-fat diet paradigms link galanin to triglycerides and their transport and metabolism in muscle. Brain Res. 1008, 168–178. [Google Scholar]
- Wortley, K.E.; Chang, G.Q.; Davydova, Z.; Leibowitz, S.F. Peptides that regulate food intake: Orexin gene expression is increased during states of hypertriglyceridemia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R1454–R1465. [Google Scholar]
- Gaysinskaya, V.A.; Karatayev, O.; Chang, G.Q.; Leibowitz, S.F. Increased caloric intake after a high-fat preload: Relation to circulating triglycerides and orexigenic peptides. Physiol. Behav. 2007, 91, 142–153. [Google Scholar] [CrossRef]
- Chang, G.Q.; Karatayev, O.; Davydova, Z.; Leibowitz, S.F. Circulating triglycerides impact on orexigenic peptides and neuronal activity in hypothalamus. Endocrinology 2004, 145, 3904–3912. [Google Scholar] [CrossRef]
- Bromberg-Martin, E.; Matsumoto, M.; Hikosaka, O. Dopamine in motivational control:Rewarding, aversive, and altering. Neuron 2010, 68, 815–834. [Google Scholar] [CrossRef]
- Di Chiara, G.; Bassareo, V. Reward system and addiction: what dopamine does and doesn’t do. Curr. Opin. Pharmacol. 2007, 7, 233. [Google Scholar] [CrossRef]
- Ikemoto, S. Dopamine reward circuitry: Two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex. Brain Res. Rev. 2007, 56, 27–78. [Google Scholar] [CrossRef]
- Robinson, T.E.; Berridge, K.C. The psychology and neurobiology of addiction: An incentive-sensitization view. Addiction 2000, 95 Suppl. 2, S91–S117. [Google Scholar]
- Schultz, W.; Dayan, P.; Montague, P.R. A neural substrate of prediction and reward. Science 1997, 275, 1593–1599. [Google Scholar]
- Salamone, J.D.; Correa, M.; Farrar, A.; Mingote, S.M. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl.) 2007, 191, 461–482. [Google Scholar] [CrossRef]
- Wise, R.A. Role of brain dopamine in food reward and reinforcement. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2006, 361, 1149–1158. [Google Scholar] [CrossRef]
- Avena, N.M.; Rada, P.; Moise, N.; Hoebel, B.G. Sucrose sham feeding on a binge schedule releases accumbens dopamine repeatedly and eliminates the acetylcholine satiety response. Neuroscience 2006, 139, 813–820. [Google Scholar] [CrossRef]
- Pal, G.K.; Thombre, D.P. Modulation of feeding and drinking by dopamine in caudate and accumbens nuclei in rats. Indian J. Exp. Biol. 1993, 31, 750–754. [Google Scholar]
- Rada, P.; Avena, N.M.; Hoebel, B.G. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience 2005, 134, 737–744. [Google Scholar] [CrossRef]
- Swanson, C.J.; Heath, S.; Stratford, T.R.; Kelley, A.E. Differential behavioral responses to dopaminergic stimulation of nucleus accumbens subregions in the rat. Pharmacol. Biochem. Behav. 1997, 58, 933–945. [Google Scholar] [CrossRef]
- Avena, N.M.; Rada, P.; Hoebel, B.G. Underweight rats have enhanced dopamine release and blunted acetylcholine response in the nucleus accumbens while bingeing on sucrose. Neuroscience 2008, 156, 865–871. [Google Scholar] [CrossRef]
- Wilson, C.; Nomikos, G.G.; Collu, M.; Fibiger, H.C. Dopaminergic correlates of motivated behavior: Importance of drive. J. Neurosci. 1995, 15, 5169–5178. [Google Scholar]
- Rada, P.; Mark, G.P.; Hoebel, B.G. Galanin in the hypothalamus raises dopamine and lowers acetylcholine release in the nucleus accumbens: a possible mechanism for hypothalamic initiation of feeding behavior. Brain Res. 1998, 798, 1–6. [Google Scholar] [CrossRef]
- Rada, P.; Barson, J.R.; Leibowitz, S.F.; Hoebel, B.G. Opioids in the hypothalamus control dopamine and acetylcholine levels in the nucleus accumbens. Brain Res. 1312, 1–9. [Google Scholar]
- Geiger, B.M.; Haburcak, M.; Avena, N.M.; Moyer, M.C.; Hoebel, B.G.; Pothos, E.N. Deficits of mesolimbic dopamine neurotransmission in rat dietary obesity. Neuroscience 2009, 159, 1193–1199. [Google Scholar] [CrossRef]
- Huang, X.F.; Yu, Y.; Zavitsanou, K.; Han, M.; Storlien, L. Differential expression of dopamine D2 and D4 receptor and tyrosine hydroxylase mRNA in mice prone, or resistant, to chronic high-fat diet-induced obesity. Brain Res. Mol. Brain Res. 2005, 135, 150–161. [Google Scholar]
- Liang, N.C.; Hajnal, A.; Norgren, R. Sham feeding corn oil increases accumbens dopamine in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006, 291, R1236–R1239. [Google Scholar] [CrossRef]
- South, T.; Huang, X.F. High-fat diet exposure increases dopamine D2 receptor and decreases dopamine transporter receptor binding density in the nucleus accumbens and caudate putamen of mice. Neurochem. Res. 2008, 33, 598–605. [Google Scholar] [CrossRef]
- Hajnal, A.; Smith, G.P.; Norgren, R. Oral sucrose stimulation increases accumbens dopamine in the rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R31–R37. [Google Scholar]
- Bassareo, V.; Di Chiara, G. Differential influence of associative and nonassociative learning mechanisms on the responsiveness of prefrontal and accumbal dopamine transmission to food stimuli in rats fed ad libitum. J. Neurosci. 1997, 17, 851–861. [Google Scholar]
- Rada, P.; Mark, G.P.; Pothos, E.; Hoebel, B.G. Systemic morphine simultaneously decreases extracellular acetylcholine and increases dopamine in the nucleus accumbens of freely moving rats. Neuropharmacology 1991, 30, 1133–1136. [Google Scholar] [CrossRef]
- Shor-Posner, G.; Brennan, G.; Ian, C.; Jasaitis, R.; Madhu, K.; Leibowitz, S.F. Meal patterns of macronutrient intake in rats with particular dietary preferences. Am. J. Physiol. 1994, 266, R1395–R1402. [Google Scholar]
- Ren, X.; Ferreira, J.G.; Zhou, L.; Shammah-Lagnado, S.J.; Yeckel, C.W.; de Araujo, I.E. Nutrient selection in the absence of taste receptor signaling. J. Neurosci. 2010, 30, 8012–8023. [Google Scholar]
- Avena, N.M.; Rada, P.; Hoebel, B.G. Evidence for sugar addiction: behavioral and neurochemical effects of intermittent, excessive sugar intake. Neurosci. Biobehav. Rev. 2008, 32, 20–39. [Google Scholar] [CrossRef]
- Surina-Baumgartner, D.M.; Arnold, M.; Moses, A.; Langhans, W. Metabolic effects of a fat- and carbohydrate-rich meal in rats. Physiol. Behav. 1996, 59, 973–981. [Google Scholar] [CrossRef]
- Zimmer, L.; Vancassel, S.; Cantagrel, S.; Breton, P.; Delamanche, S.; Guilloteau, D.; Durand, G.; Chalon, S. The dopamine mesocorticolimbic pathway is affected by deficiency in n-3 polyunsaturated fatty acids. Am. J. Clin. Nutr. 2002, 75, 662–667. [Google Scholar]
- Ackroff, K.; Lucas, F.; Sclafani, A. Flavor preference conditioning as a function of fat source. Physiol. Behav. 2005, 85, 448–460. [Google Scholar] [CrossRef]
- Touzani, K.; Bodnar, R.; Sclafani, A. Activation of dopamine D1-like receptors in nucleus accumbens is critical for the acquisition, but not the expression, of nutrient-conditioned flavor preferences in rats. Eur. J. Neurosci. 2008, 27, 1525–1533. [Google Scholar] [CrossRef]
- Hernandez, L.; Stanley, B.G.; Hoebel, B.G. A small, removable microdialysis probe. Life Sci. 1986, 39, 2629–2637. [Google Scholar] [CrossRef]
- Dourmashkin, J.T.; Chang, G.Q.; Hill, J.O.; Gayles, E.C.; Fried, S.K.; Leibowitz, S.F. Model for predicting and phenotyping at normal weight the long-term propensity for obesity in Sprague-Dawley rats. Physiol. Behav. 2006, 87, 666–678. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Academic Press: New York, NY, USA, 2005. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Rada, P.; Avena, N.M.; Barson, J.R.; Hoebel, B.G.; Leibowitz, S.F. A High-Fat Meal, or Intraperitoneal Administration of a Fat Emulsion, Increases Extracellular Dopamine in the Nucleus Accumbens. Brain Sci. 2012, 2, 242-253. https://doi.org/10.3390/brainsci2020242
Rada P, Avena NM, Barson JR, Hoebel BG, Leibowitz SF. A High-Fat Meal, or Intraperitoneal Administration of a Fat Emulsion, Increases Extracellular Dopamine in the Nucleus Accumbens. Brain Sciences. 2012; 2(2):242-253. https://doi.org/10.3390/brainsci2020242
Chicago/Turabian StyleRada, Pedro, Nicole M. Avena, Jessica R. Barson, Bartley G. Hoebel, and Sarah F. Leibowitz. 2012. "A High-Fat Meal, or Intraperitoneal Administration of a Fat Emulsion, Increases Extracellular Dopamine in the Nucleus Accumbens" Brain Sciences 2, no. 2: 242-253. https://doi.org/10.3390/brainsci2020242




