Cocaine Sensitization Increases Kyphosis and Modulates Neural Activity in Adult Nulliparous Rats
Abstract
:1. Introduction
2. Results and Discussion
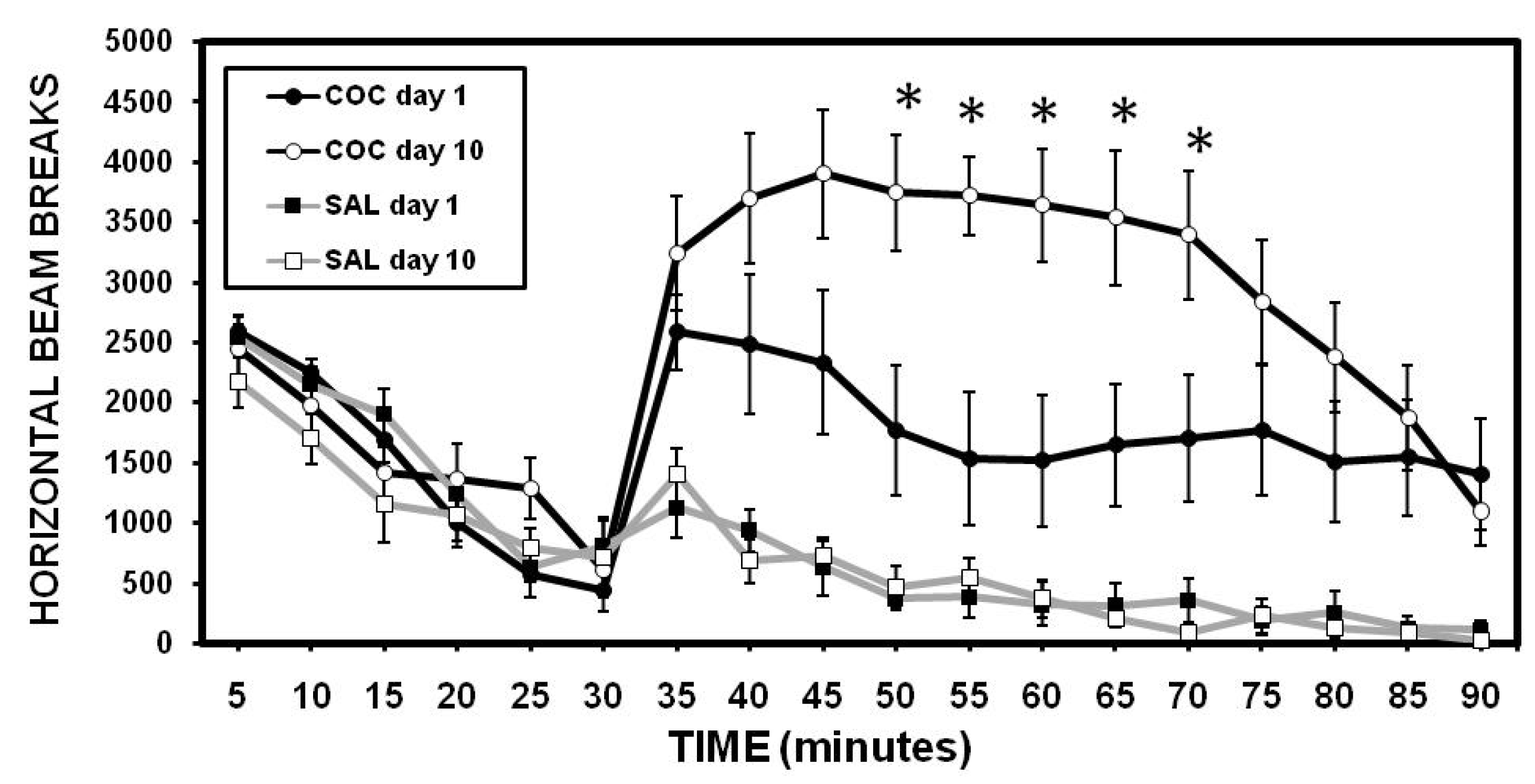
2.1. Sensitization to Cocaine
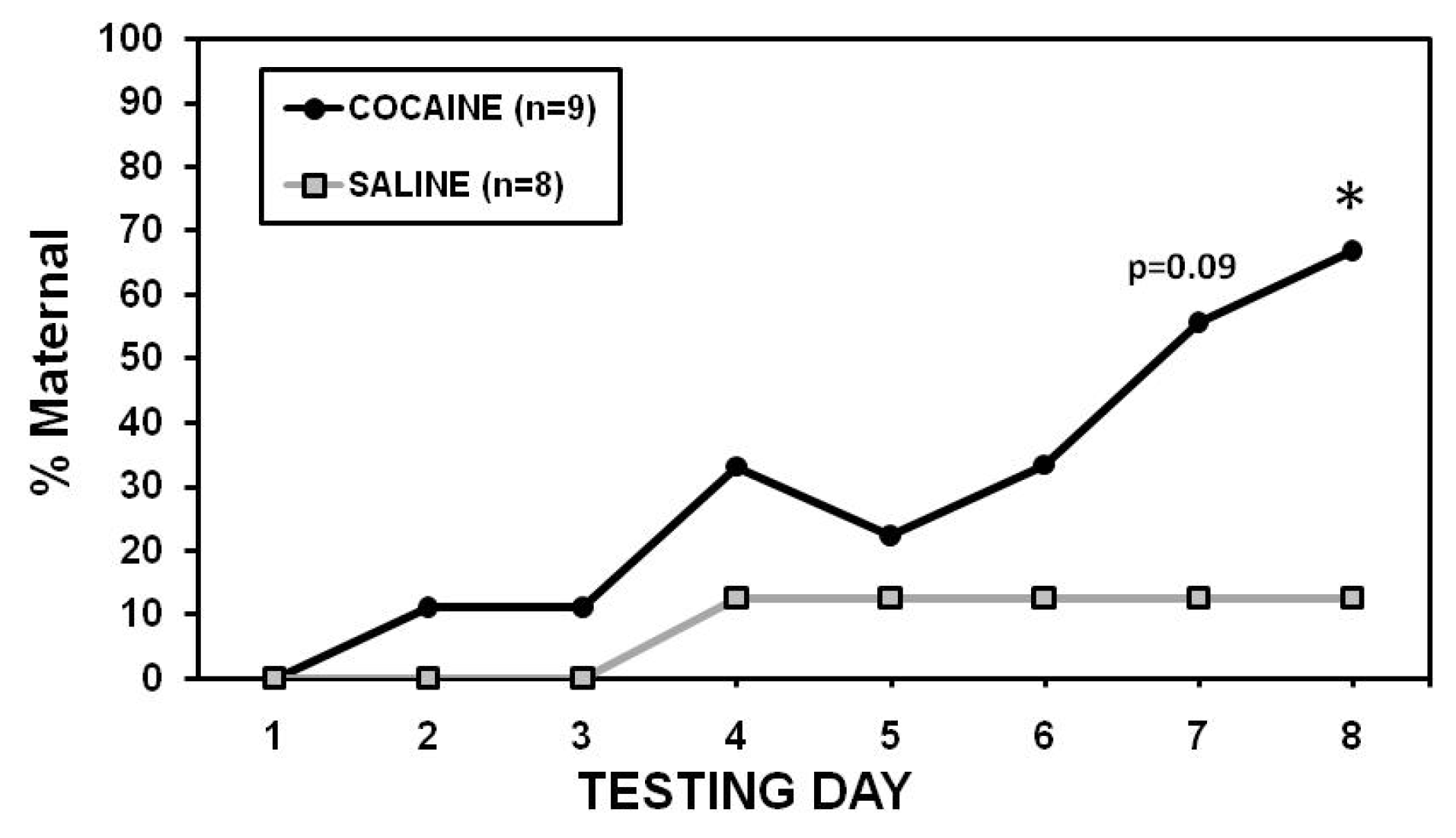
2.2. Maternal Care
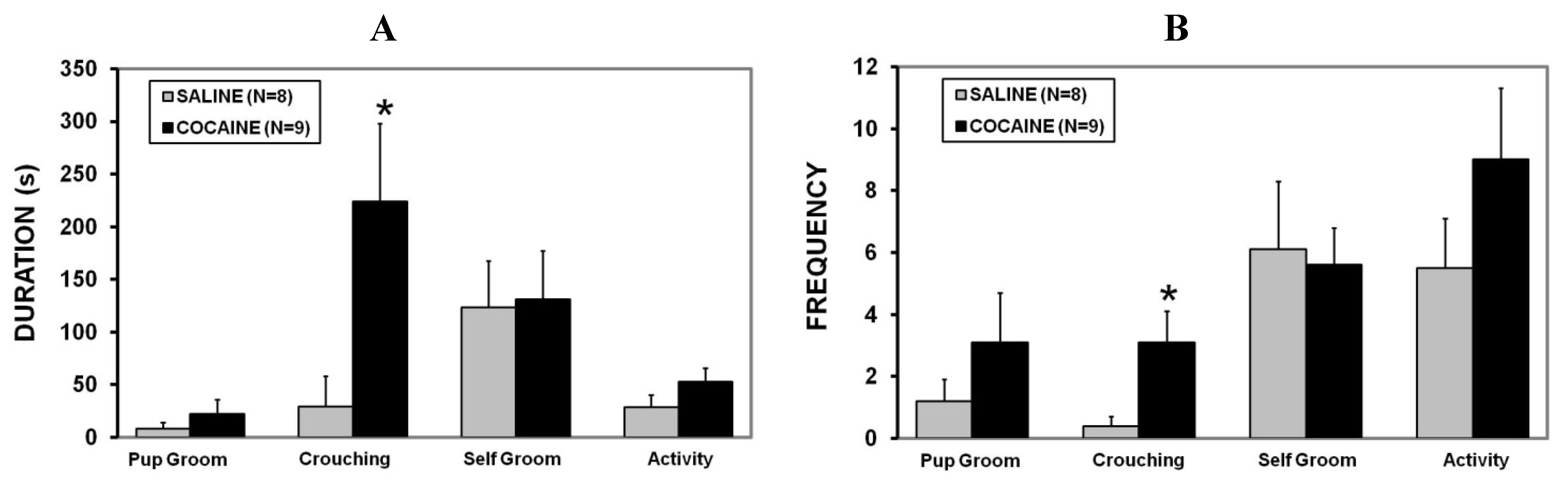
2.3. Maternal Aggression
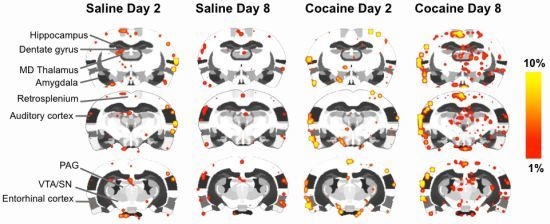
2.4. fMRI
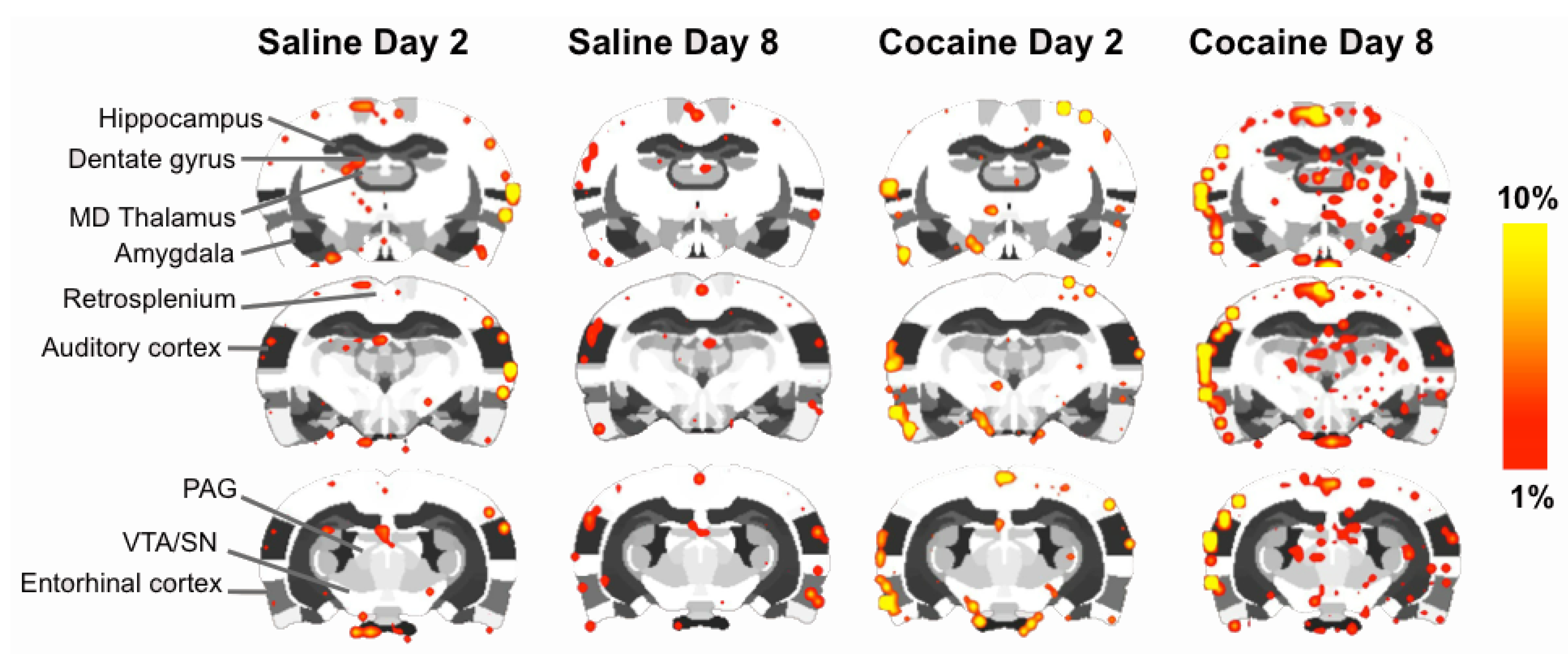
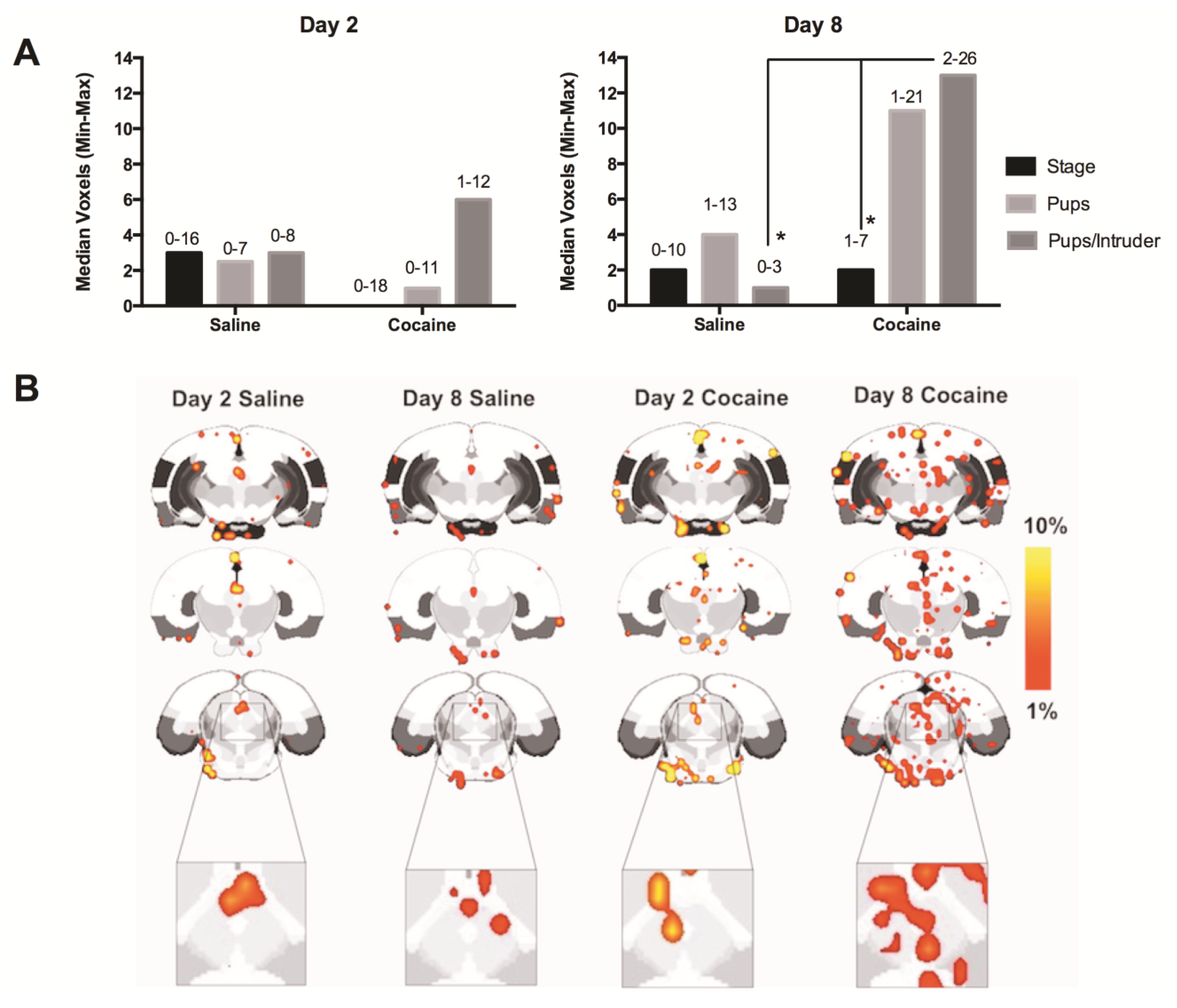
2.5. Discussion
3. Experimental Section
3.1. Animals
3.2. Cocaine Treatment and Sensitization
3.3. Maternal Behavior Sensitization
3.4. Behavioral Testing
3.5. Behavioral Statistics
3.6. Acclimatization Procedures and Preparations for Imaging
3.7. Magnetic Resonance Imaging Scanning Parameters
3.8. Imaging BOLD Responses
3.9. fMRI Statistical Analysis
4. Conclusions
Conflict of Interest
References
- Substance Abuse and Mental Health Services Administration, Results from the 2007 National Survey on Drug Use and Health: National Findings; Substance Abuse and Mental Health Services Administration: Rockville, MD, USA, 2007.
- Coyer, S.M. Mothers recovering from cocaine addiction: Factors affecting parenting skills. J. Obstet. Gynecol. Neonatal Nurs. 2001, 30, 71–79. [Google Scholar] [CrossRef]
- Coyer, S.M. Women in recovery discuss parenting while addicted to cocaine. MCN Am. J. Matern. Child Nurs. 2003, 28, 45–49. [Google Scholar] [CrossRef]
- Harmer, A.L.; Sanderson, J.; Mertin, P. Influence of negative childhood experiences on psychological functioning, social support, and parenting for mothers recovering from addiction. Child Abuse Negl. 1999, 23, 421–433. [Google Scholar]
- Johns, J.M.; Noonan, L.R.; Li, L.; Pedersen, C.A. Effects of chronic and acute cocaine treatment on the onset of maternal behavior and aggression in Sprague-Dawley rats. Behav. Neurosci. 1994, 108, 107–112. [Google Scholar] [CrossRef]
- Kinsley, C.H.; Turco, D.; Bauer, A.; Beverly, M.; Wellman, J.; Graham, A.L. Cocaine alters the onset and maintenance of maternal behavior in lactating rats. Pharmacol. Biochem. Behav. 1994, 47, 857–864. [Google Scholar] [CrossRef]
- Zimmerberg, B.; Gray, M.S. The effects of cocaine on maternal behaviors in the rat. Physiol. Behav. 1992, 52, 379–384. [Google Scholar] [CrossRef]
- Johns, J.M.; Nelson, C.J.; Meter, K.E.; Lubin, D.A.; Couch, C.D.; Ayers, A.; Walker, C.H. Dose-dependent effects of multiple acute cocaine injections on maternal behavior and aggression in Sprague-Dawley rats. Dev. Neurosci. 1998, 20, 525–532. [Google Scholar] [CrossRef]
- Febo, M.; Ferris, C.F. Development of cocaine sensitization before pregnancy affects subsequent maternal retrieval of pups and prefrontal cortical activity during nursing. Neuroscience 2007, 148, 400–412. [Google Scholar]
- Nephew, B.; Febo, M. Effect of cocaine sensitization prior to pregnancy on maternal care and aggression in the rat. Psychopharmacology 2010, 209, 127–135. [Google Scholar] [CrossRef]
- Petruzzi, S.; Cirulli, F.; Laviola, G. Prior cocaine exposure in different environments affects the behavioral responses of mouse dams. Pharmacol. Biochem. Behav. 1997, 56, 541–547. [Google Scholar]
- United States Census Bureau, Survey of Income and Program Participation; United States Census Bureau: Washington, DC, USA, 2010.
- United States Census Bureau, America’s Families and Living Arrangements; United States Census Bureau: Washington, DC, USA, 2010.
- Nephew, B.C.; Caffrey, M.K.; Felix-Ortiz, A.C.; Ferris, C.F.; Febo, M. Blood oxygen level-dependent signal responses in corticolimbic “emotions” circuitry of lactating rats facing intruder threat to pups. Eur. J. Neurosci. 2009, 30, 934–945. [Google Scholar]
- Cohen, J.; Bridges, R.S. Retention of maternal behavior in nulliparous and primiparous rats: Effects of duration of previous maternal experience. J. Comp. Physiol. Psychol. 1981, 95, 40–45. [Google Scholar]
- Bridges, R.S.; Zarrow, M.X.; Gandelman, R.; Denenberg, V.H. Differences in maternal responsiveness between lactating and sensitized rats. Dev. Psychobiol. 1972, 5, 123–127. [Google Scholar] [CrossRef]
- Stern, J.M.; Mackinnon, D.A. Postpartum, hormonal, and nonhormonal induction of maternal behavior in rats: Effects on T-maze retrieval of pups. Horm. Behav. 1976, 7, 305–316. [Google Scholar]
- Stern, J.M. Somatosensation and Maternal Care in Norway Rats. In Advances in the Study of Behavior; Rosenblatt, J.S., Hinde, R.A., Beer, C.G., Busnel, M.C., Eds.; Academic Press: New York, NY, USA, 1996; pp. 243–294. [Google Scholar]
- Numan, M. Motivational systems and the neural circuitry of maternal behavior in the rat. Dev. Psychobiol. 2007, 49, 12–21. [Google Scholar] [CrossRef]
- Vernotica, E.M.; Lisciotto, C.A.; Rosenblatt, J.S.; Morrell, J.I. Cocaine transiently impairs maternal behavior in the rat. Behav. Neurosci. 1996, 110, 315–323. [Google Scholar]
- Fiorino, D.F.; Phillips, A.G. Facilitation of sexual behavior in male rats following D-amphetamine-induced behavioral sensitization. Psychopharmacology 1999, 142, 200–208. [Google Scholar] [CrossRef]
- Ferris, C.F.; Kulkarni, P.; Sullivan, J.M., Jr.; Harder, J.A.; Messenger, T.L.; Febo, M. Pup suckling is more rewarding than cocaine: Evidence from functional magnetic resonance imaging and three-dimensional computational analysis. J. Neurosci. 2005, 25, 149–156. [Google Scholar]
- Mattson, B.J.; Williams, S.; Rosenblatt, J.S.; Morrell, J.I. Comparison of two positive reinforcing stimuli: Pups and cocaine throughout the postpartum period. Behav. Neurosci. 2001, 115, 683–694. [Google Scholar]
- Mattson, B.J.; Williams, S.E.; Rosenblatt, J.S.; Morrell, J.I. Preferences for cocaine- or pup-associated chambers differentiates otherwise behaviorally identical postpartum maternal rats. Psychopharmacolgy 2003, 167, 1–8. [Google Scholar]
- Bosch, O.J.; Neumann, I.D. Brain vasopressin is an important regulator of maternal behavior independent of dams’ trait anxiety. Proc. Natl. Acad. Sci. USA 2008, 105, 17139–17144. [Google Scholar]
- Nephew, B.C.; Bridges, R.S. Central actions of arginine vasopressin and a V1a receptor antagonist on maternal aggression, maternal behavior, and grooming in lactating rats. Pharmacol. Biochem. Behav. 2008, 91, 77–83. [Google Scholar]
- Nephew, B.C.; Bridges, R.S. Arginine vasopressin V1a receptor antagonist impairs maternal memory in rats. Physiol. Behav. 2008, 95, 182–186. [Google Scholar] [CrossRef]
- Post, R.M.; Contel, N.R.; Gold, P. Impaired behavioral sensitization to cocaine in vasopressin deficient rats. Life Sci. 1982, 31, 2745–2750. [Google Scholar]
- Van Ree, J.M.; Burbach-Bloemarts, E.M.; Wallace, M. Vasopressin neuropeptides and acquisition of heroin and cocaine self-administration in rats. Life Sci. 1988, 42, 1091–1099. [Google Scholar]
- Zhou, Y.; Bendor, J.T.; Yuferov, V.; Schlussman, S.D.; Ho, A.; Kreek, M.J. Amygdalar vasopressin mRNA increases in acute cocaine withdrawal: Evidence for opioid receptor modulation. Neuroscience 2005, 134, 1391–1397. [Google Scholar]
- Johns, J.M.; Lubin, D.A.; Walker, C.H.; Meter, K.E.; Mason, G.A. Chronic gestational cocaine treatment decreases oxytocin levels in the medial preoptic area, ventral tegmental area and hippocampus in Sprague-Dawley rats. Neuropeptides 1997, 31, 439–443. [Google Scholar] [CrossRef]
- Elliot, J.C.; Lubin, D.A.; Walker, C.H.; Johns, J.M. Acute cocaine alters oxytocin levels in the medial preoptic area and amygdala in lactating rat dams: Implications for cocaine-induced changes in maternal behavior and maternal aggression. Neuropeptides 2001, 35, 127–134. [Google Scholar] [CrossRef]
- Keverne, E.B. Central mechanisms underlying the neural and neuroendocrine determinants of maternal behaviour. Psychoneuroendocrinology 1988, 13, 127–141. [Google Scholar] [CrossRef]
- Kendrick, K.M.; da Costa, A.P.C.; Broad, K.D.; Ohkura, S.; Guevara, R.; Lévy, F.; Keverne, E.B. Neural control of maternal behaviour and olfactory recognition of offspring. Brain Res. Bull. 1997, 44, 383–395. [Google Scholar]
- Bridges, R.S.; Nephew, B.C.; Larry, R.S. Neuroendocrine Control: Maternal Behavior. In Encyclopedia of Neuroscience; Academic Press: Oxford, UK, 2009; pp. 333–342. [Google Scholar]
- Erskine, M.S.; Barfield, R.J.; Goldman, B.D. Postpartum aggression in rats: II. Dependence on maternal sensitivity to young and effects of experience with pregnancy and parturition. J. Comp. Physiol. Psychol. 1980, 94, 495–505. [Google Scholar] [CrossRef]
- Lonstein, J.S.; Simmons, D.A.; Swann, J.M.; Stern, J.M. Forebrain expression of c-fos due to active maternal behaviour in lactating rats. Neuroscience 1997, 82, 267–281. [Google Scholar] [CrossRef]
- Caffrey, M.K.; Nephew, B.C.; Febo, M. Central vasopressin V1a receptors modulate neural processing in mothers facing intruder threat to pups. Neuropharmacology 2010, 58, 107–116. [Google Scholar]
- Pawluski, J.L.; Vanderbyl, B.L.; Ragan, K.; Galea, L.A. First reproductive experience persistently affects spatial reference and working memory in the mother and these effects are not due to pregnancy or “mothering” alone. Behav. Brain Res. 2006, 175, 157–165. [Google Scholar] [CrossRef]
- Pawluski, J.L.; Walker, S.K.; Galea, L.A. Reproductive experience differentially affects spatial reference and working memory performance in the mother. Horm. Behav. 2006, 49, 143–149. [Google Scholar]
- Kinsley, C.H.; Madonia, L.; Gifford, G.W.; Tureski, K.; Griffin, G.R.; Lowry, C.; Williams, J.; Collins, J.; Mclearie, H.; Lambert, K.G. Motherhood improves learning and memory. Nature 1999, 402, 137–138. [Google Scholar]
- Pawluski, J.L.; Galea, L.A. Hippocampal morphology is differentially affected by reproductive experience in the mother. J. Neurobiol. 2006, 66, 71–81. [Google Scholar]
- Kinsley, C.H.; Trainer, R.; Stafisso-Sandoz, G.; Quadros, P.; Marcus, L.K.; Hearon, C.; Meyer, E.A.; Hester, N.; Morgan, M.; Kozub, F.J.; Lambert, K.G. Motherhood and the hormones of pregnancy modify concentrations of hippocampal neuronal dendritic spines. Horm. Behav. 2006, 49, 131–142. [Google Scholar] [CrossRef]
- Liu, D.; Diorio, J.; Tannenbaum, B.; Caldji, C.; Francis, D.; Freedman, A.; Sharma, S.; Pearson, D.; Plotsky, P.M.; Meaney, M.J. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science 1997, 277, 1659–1662. [Google Scholar] [CrossRef]
- Liu, D.; Diorio, J.; Day, J.C.; Francis, D.D.; Meaney, M.J. Maternal care, hippocampal synaptogenesis and cognitive development in rats. Nat. Neurosci. 2000, 3, 799–806. [Google Scholar]
- D’Amato, F.R.; Zanettini, C.; Sgobio, C.; Sarli, C.; Carone, V.; Moles, A.; Ammassari-Teule, M. Intensification of maternal care by double-mothering boosts cognitive function and hippocampal morphology in the adult offspring. Hippocampus 2011, 21, 298–308. [Google Scholar] [CrossRef]
- Frankfurt, M.; Salas-Ramirez, K.; Friedman, E.; Luine, V. Cocaine alters dendritic spine density in cortical and subcortical brain regions of the postpartum and virgin female rat. Synapse 2011, 65, 955–961. [Google Scholar] [CrossRef]
- Braun, K.; Poeggel, G. Recognition of Mother’s voice evokes metabolic activation in the medial prefrontal cortex and lateral thalamus of Octodon degus pups. Neuroscience 2001, 103, 861–864. [Google Scholar] [CrossRef]
- Lorberbaum, J.P.; Newman, J.D.; Horwitz, A.R.; Dubno, J.R.; Lydiard, R.B.; Hamner, M.B.; Bohning, D.E.; George, M.S. A potential role for thalamocingulate circuitry in human maternal behavior. Biol. Psychiatry 2002, 51, 431–445. [Google Scholar] [CrossRef]
- Strathearn, L.; Li, J.; Fonagy, P.; Montague, P.R. What’s in a smile? Maternal brain responses to infant facial cues. Pediatrics 2008, 122, 40–51. [Google Scholar] [CrossRef]
- Lonstein, J.S.; Stern, J.M. Site and behavioral specificity of periaqueductal gray lesions on postpartum sexual, maternal and aggressive behaviors in rats. Brain Res. 1998, 804, 21–35. [Google Scholar]
- Gammie, S.C.; Nelson, R.J. cFOS and pCREB activation and maternal aggression in mice. Brain Res. 2001, 898, 232–241. [Google Scholar] [CrossRef]
- Erskine, M.S.; Barfield, R.J.; Goldman, B.D. Intraspecific fighting during late pregnancy and lactation in rats and effects of litter removal. Behav. Biol. 1978, 23, 206–218. [Google Scholar]
- King, J.A.; Garelick, T.S.; Brevard, M.E.; Chen, W.; Messenger, T.L.; Duong, T.Q.; Ferris, C.F. Procedure for minimizing stress for fMRI studies in conscious rats. J. Neurosci. Methods 2005, 148, 154–160. [Google Scholar]
- Ferris, C.F. Functional magnetic resonance imaging and the neurobiology of vasopressin and oxytocin. Prog. Brain Res. 2008, 170, 305–320. [Google Scholar]
- Ferris, C.F.; Stolberg, T.; Kulkarni, P.; Murugavel, M.; Blanchard, R.; Blanchard, D.C.; Febo, M.; Brevard, M.; Simon, N.G. Imaging the neural circuitry and chemical control of aggressive motivation. BMC Neurosci. 2008, 9. [Google Scholar]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 3rd ed; Academic Press: Boston, MA, USA, 1997. [Google Scholar]
- Swanson, L.W. Brain Maps: Structure of the Rat Brain, 2nd ed; Elsevier Science: Boston, MA, USA, 1999. [Google Scholar]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Nephew, B.C.; Caffrey, M.K.; Felix-Ortiz, A.C.; Febo, M. Cocaine Sensitization Increases Kyphosis and Modulates Neural Activity in Adult Nulliparous Rats. Brain Sci. 2012, 2, 667-683. https://doi.org/10.3390/brainsci2040667
Nephew BC, Caffrey MK, Felix-Ortiz AC, Febo M. Cocaine Sensitization Increases Kyphosis and Modulates Neural Activity in Adult Nulliparous Rats. Brain Sciences. 2012; 2(4):667-683. https://doi.org/10.3390/brainsci2040667
Chicago/Turabian StyleNephew, Benjamin C., Martha K. Caffrey, Ada C. Felix-Ortiz, and Marcelo Febo. 2012. "Cocaine Sensitization Increases Kyphosis and Modulates Neural Activity in Adult Nulliparous Rats" Brain Sciences 2, no. 4: 667-683. https://doi.org/10.3390/brainsci2040667