Ethanol Reinforcement Elicits Novel Response Inhibition Behavior in a Rat Model of Ethanol Dependence
Abstract
:1. Introduction
2. Methods
2.1. Animals
2.2. Materials and Apparatus
2.3. Chronic Intermittent Exposure Ethanol Vapor (CIE)
2.4. Blood Ethanol Concentrations
2.5. Differential Reinforcement of Low-Rate of Responding
2.6. Progressive-Ratio Schedule
2.7. Locomotor Activity
3. Data Analysis
3.1. Mathematical Analysis of Interresponse Time Data: Modified Temporal Regulation Model
- Response threshold θ = (N × c)/15, where N and c are the shape and scale parameters for the gamma distribution. (N × c) is the mean of timed IRTs. θ < 1 indicates lower accuracy of timed IRTs.
- The proportion of timed IRTs (p) is expressed as a fraction of all IRTs that were obtained from an individual rat. A larger p value indicates greater response inhibition capacity.
- The proportion of burst IRTs (q × (1 – p)) is expressed as a fraction of all IRTs that were obtained from an individual rat. Burst IRTs are a component of non-timed IRTs. A greater proportion of burst IRTs indicates lower response inhibition capacity.
- Rate-of-decay, L, which is the exponent fitting the burst IRTs under DRL15. A steeper decay may result from a larger drop in burst responding.
3.2. Parametric Data
4. Results
4.1. Reinforcement Efficacy of Sucrose during Acute Withdrawal and Prolonged Abstinence from CIE
4.2. Interresponse Time Distribution and Mathematical Modeling Data for Rats that Responded to Sucrose Reinforcers
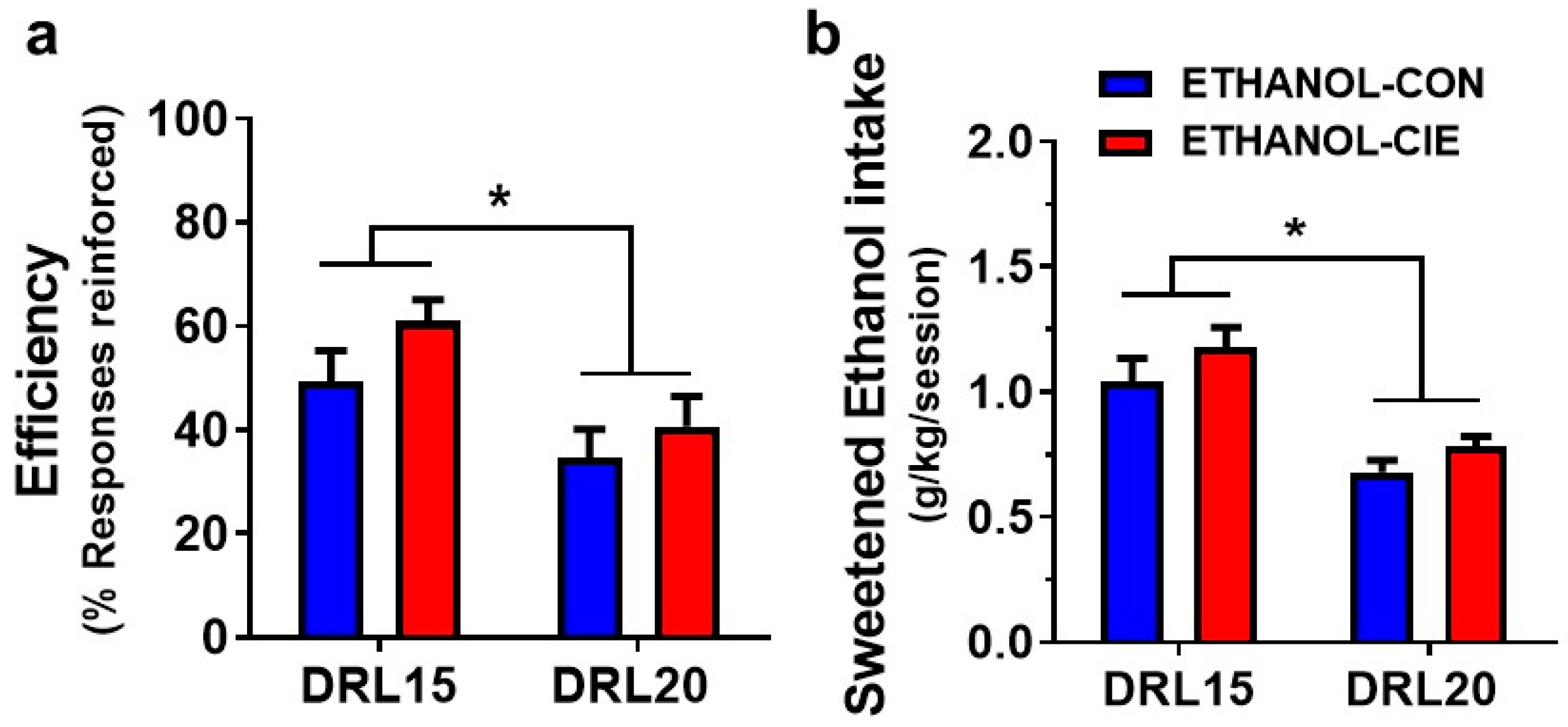
4.3. Reinforcement Efficacy with Ethanol during Acute Withdrawal and Prolonged Abstinence
4.4. Interresponse Time Distribution and Mathematical Modeling Data for Rats that Responded to Ethanol Reinforcers
4.5. Response Inhibition and Reinforcer Intake during an Unexpected DRL Challenge
4.6. Progressive-Ratio Schedule Using Ethanol Reinforcer
4.7. Locomotor Activity during DRL Responding
5. Discussion
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Center for Behavioral Health Statistics and Quality. Behavioral Health Trends in the United States: Results from the 2014 National Survey on Drug Use and Health. Available online: http://www.samhsa.gov/data/ (accessed on 12 June 2018).
- Berking, M.; Margraf, M.; Ebert, D.; Wupperman, P.; Hofmann, S.G.; Junghanns, K. Deficits in emotion-regulation skills predict alcohol use during and after cognitive-behavioral therapy for alcohol dependence. J. Consult. Clin. Psychol. 2011, 79, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Yeh, P.H.; Gazdzinski, S.; Durazzo, T.C.; Sjöstrand, K.; Meyerhoff, D.J. Hierarchical linear modeling (HLM) of longitudinal brain structural and cognitive changes in alcohol-dependent individuals during sobriety. Drug Alcohol Depend. 2007, 91, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Martelli, C.; Petillion, A.; Brunet-Lecomte, M.; Miranda Marcos, R.; Chanraud, S.; Amirouche, A.; Letierce, A.; Kostogianni, N.; Lemaitre, H.; Aubin, H.-J.; et al. Neuropsychological Impairment in Detoxified Alcohol-Dependent Subjects with Preserved Psychosocial Functioning. Front. Psychiatry 2017, 8, 193. [Google Scholar] [CrossRef] [PubMed]
- Aharonovich, E.; Brooks, A.C.; Nunes, E.V.; Hasin, D.S. Cognitive deficits in marijuana users: Effects on motivational enhancement therapy plus cognitive behavioral therapy treatment outcome. Drug Alcohol Depend. 2008, 95, 279–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnsten, A.F.; Li, B.M. Neurobiology of executive functions: Catecholamine influences on prefrontal cortical functions. Biol. Psychiatry 2005, 57, 1377–1384. [Google Scholar] [CrossRef] [PubMed]
- St Onge, J.R.; Floresco, S.B. Prefrontal cortical contribution to risk-based decision making. Cereb. Cortex 2010, 20, 1816–1828. [Google Scholar] [CrossRef] [PubMed]
- Arnsten, A.F. Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology: An important role for prefrontal cortex dysfunction. CNS Drugs 2009, 23 (Suppl. 1), 33–41. [Google Scholar] [CrossRef] [PubMed]
- Dick, D.M.; Smith, G.; Olausson, P.; Mitchell, S.H.; Leeman, R.F.; O’malley, S.S.; Sher, K. Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addict. Biol. 2010, 15, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Verdejo-Garcia, A.; Lawrence, A.J.; Clark, L. Impulsivity as a vulnerability marker for substance-use disorders: Review of findings from high-risk research, problem gamblers and genetic association studies. Neurosci. Biobehav. Rev. 2008, 32, 777–810. [Google Scholar] [CrossRef] [PubMed]
- Dawes, M.A.; Tarter, R.E.; Kirisci, L. Behavioral self-regulation: Correlates and 2 year follow-ups for boys at risk for substance abuse. Drug Alcohol Depend. 1997, 45, 165–176. [Google Scholar] [CrossRef]
- Nigg, J.T.; Wong, M.M.; Martel, M.M.; Jester, J.M.; Puttler, L.I.; Glass, J.M.; Adams, K.; Fitzgerald, H.E.; Zucker, R.A. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J. Am. Acad. Child Adolesc. Psychiatry 2006, 45, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Krueger, R.F.; Hicks, B.M.; Patrick, C.J.; Carlson, S.R.; Iacono, W.G.; McGue, M. Etiologic connections among substance dependence, antisocial behavior, and personality: Modeling the externalizing spectrum. J. Abnorm. Psychol. 2002, 111, 411–424. [Google Scholar] [CrossRef] [PubMed]
- Jentsch, J.D.; Taylor, J.R. Impulsivity resulting from frontostriatal dysfunction in drug abuse: Implications for the control of behavior by reward-related stimuli. Psychopharmacology 1999, 146, 373–390. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, R.Z.; Volkow, N.D. Drug addiction and its underlying neurobiological basis: Neuroimaging evidence for the involvement of the frontal cortex. Am. J. Psychiatry 2002, 159, 1642–1652. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Roige, S.; Baro, V.; Trick, L.; Pena-Oliver, Y.; Stephens, D.N.; Duka, T. Exaggerated waiting impulsivity associated with human binge drinking, and high alcohol consumption in mice. Neuropsychopharmacology 2014, 39, 2919–2927. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Le Moal, M. Drug abuse: Hedonic homeostatic dysregulation. Science 1997, 278, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Winstanley, C.A.; Olausson, P.; Taylor, J.R.; Jentsch, J.D. Insight into the relationship between impulsivity and substance abuse from studies using animal models. Alcohol. Clin. Exp. Res. 2010, 34, 1306–1318. [Google Scholar] [CrossRef] [PubMed]
- Grant, J.E.; Chamberlain, S.R. Impulsive action and impulsive choice across substance and behavioral addictions: Cause or consequence? Addict. Behav. 2014, 39, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- Linsenbardt, D.N.; Smoker, M.P.; Janetsian-Fritz, S.S.; Lapish, C.C. Impulsivity in rodents with a genetic predisposition for excessive alcohol consumption is associated with a lack of a prospective strategy. Cogn. Affect. Behav. Neurosci. 2017, 17, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Beckwith, S.W.; Czachowski, C.L. Alcohol-Preferring P Rats Exhibit Elevated Motor Impulsivity Concomitant with Operant Responding and Self-Administration of Alcohol. Alcohol. Clin. Exp. Res. 2016, 40, 1100–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steinmetz, J.E.; Blankenship, M.R.; Green, J.T.; Smith, G.B.; Finn, P.R. Evaluation of behavioral disinhibition in P/NP and HAD1/LAD1 rats. Prog. Neuropsychopharmacol. Biol. Psychiatry 2000, 24, 1025–1039. [Google Scholar] [CrossRef]
- Pena-Oliver, Y.; Giuliano, C.; Economidou, D.; Goodlett, C.R.; Robbins, T.W.; Dalley, J.W.; Everitt, B.J. Alcohol-Preferring Rats Show Goal Oriented Behaviour to Food Incentives but Are Neither Sign-Trackers Nor Impulsive. PLoS ONE 2015, 10, e0131016. [Google Scholar]
- Mejia-Toiber, J.; Boutros, N.; Markou, A.; Semenova, S. Impulsive choice and anxiety-like behavior in adult rats exposed to chronic intermittent ethanol during adolescence and adulthood. Behav. Brain Res. 2014, 266, 19–28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somkuwar, S.S.; Fannon, M.J.; Nguyen, T.B.; Mandyam, C. Hyper-oligodendrogenesis at the vascular niche and reduced blood-brain barrier integrity in the prefrontal cortex during protracted abstinence. Neuroscience 2017, 362, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Somkuwar, S.S.; Fannon, M.J.; Staples, M.C.; Zamora-Martinez, E.R.; Navarro, A.I.; Kim, A.; Quigley, J.A.; Edwards, S.; Mandyam, C.D. Alcohol dependence-induced regulation of the proliferation and survival of adult brain progenitors is associated with altered BDNF-TrkB signaling. Brain Struct. Funct. 2016, 221, 4319–4335. [Google Scholar] [CrossRef] [PubMed]
- Somkuwar, S.S.; Fannon-Pavlich, M.J.; Ghofranian, A.; Quigley, J.A.; Dutta, R.R.; Galinato, M.H.; Mandyam, C.D. Wheel running reduces ethanol seeking by increasing neuronal activation and reducing oligodendroglial/neuroinflammatory factors in the medial prefrontal cortex. Brain Behav. Immun. 2016, 58, 357–368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- George, O.; Sanders, C.; Freiling, J.; Grigoryan, E.; Vu, S.; Allen, C.D.; Crawford, E.; Mandyam, C.D.; Koob, G.F. Recruitment of medial prefrontal cortex neurons during alcohol withdrawal predicts cognitive impairment and excessive alcohol drinking. Proc. Natl. Acad. Sci. USA 2012, 109, 18156–18161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro, A.I.; Mandyam, C.D. Protracted abstinence from chronic ethanol exposure alters the structure of neurons and expression of oligodendrocytes and myelin in the medial prefrontal cortex. Neuroscience 2015, 293, 35–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, H.N.; Chan, S.H.; Crawford, E.F.; Lee, Y.K.; Funk, C.K.; Koob, G.F.; Mandyam, C.D. Permanent impairment of birth and survival of cortical and hippocampal proliferating cells following excessive drinking during alcohol dependence. Neurobiol. Dis. 2009, 36, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trantham-Davidson, H.; Chandler, L.J. Alcohol-induced alterations in dopamine modulation of prefrontal activity. Alcohol 2015, 49, 773–779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trantham-Davidson, H.; Burnett, E.J.; Gass, J.T.; Lopez, M.F.; Mulholland, P.J.; Centanni, S.W.; Floresco, S.B.; Chandler, J. Chronic alcohol disrupts dopamine receptor activity and the cognitive function of the medial prefrontal cortex. J. Neurosci. 2014, 34, 3706–3718. [Google Scholar] [CrossRef] [PubMed]
- Sokolowski, J.D.; Salamone, J.D. Effects of dopamine depletions in the medial prefrontal cortex on DRL performance and motor activity in the rat. Brain Res. 1994, 642, 20–28. [Google Scholar] [CrossRef]
- Simon, N.W.; Beas, B.S.; Montgomery, K.S.; Haberman, R.P.; Bizon, J.L.; Setlow, B. Prefrontal cortical-striatal dopamine receptor mRNA expression predicts distinct forms of impulsivity. Eur. J. Neurosci. 2013, 37, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Somkuwar, S.S.; Kantak, K.M.; Bardo, M.T.; Dwoskin, L.P. Adolescent methylphenidate treatment differentially alters adult impulsivity and hyperactivity in the Spontaneously Hypertensive Rat model of ADHD. Pharmacol. Biochem. Behav. 2016, 141, 66–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Somkuwar, S.S.; Darna, M.; Kantak, K.M.; Dwoskin, L.P. Adolescence methylphenidate treatment in a rodent model of attention deficit/hyperactivity disorder: Dopamine transporter function and cellular distribution in adulthood. Biochem. Pharmacol. 2013, 86, 309–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irimia, C.; Wiskerke, J.; Natividad, L.A.; Polis, I.Y.; Vries, T.J.; Pattij, T.; Parsons, L.H. Increased impulsivity in rats as a result of repeated cycles of alcohol intoxication and abstinence. Addict. Biol. 2015, 20, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Ji, D.; Gilpin, N.W.; Richardson, H.N.; Rivier, C.L.; Koob, G.F. Effects of naltrexone, duloxetine, and a corticotropin-releasing factor type 1 receptor antagonist on binge-like alcohol drinking in rats. Behav. Pharmacol. 2008, 19, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spierling, S.R.; Mattock, M.; Zorrilla, E.P. Modeling hypohedonia following repeated social defeat: Individual vulnerability and dopaminergic involvement. Physiol. Behav. 2017, 177, 99–106. [Google Scholar] [CrossRef] [PubMed]
- Gilpin, N.W.; Richardson, H.N.; Cole, M.; Koob, G.F. Vapor inhalation of alcohol in rats. Curr. Protoc. Neurosci. 2008, 9, 9–29. [Google Scholar]
- O’Dell, L.E.; Roberts, A.J.; Smith, R.T.; Koob, G.F. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol. Clin. Exp. Res. 2004, 28, 1676–1682. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F.; Arends, M.A.; Le Moal, M. Drugs, Addiction, and the Brain; Academic Press: Boston, MA, USA, 2014. [Google Scholar]
- Courtney, K.E.; Polich, J. Binge drinking in young adults: Data, definitions, and determinants. Psychol. Bull. 2009, 135, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Ernst, A.J.; Dempster, J.P.; Yee, R.; Dennis, C.; Nakano, L. Alcohol toxicity, blood alcohol concentration and body water in young and adult rats. J. Stud. Alcohol. 1976, 37, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Gilpin, N.W.; Richardson, H.N.; Lumeng, L.; Koob, G.F. Dependence-induced alcohol drinking by alcohol-preferring (P) rats and outbred Wistar rats. Alcohol. Clin. Exp. Res. 2008, 32, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Sanabria, F.; Killeen, P.R. Evidence for impulsivity in the Spontaneously Hypertensive Rat drawn from complementary response-withholding tasks. Behav. Brain. Funct. 2008, 4, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richards, J.B.; Sabol, K.E.; Seiden, L.S. DRL interresponse-time distributions: Quantification by peak deviation analysis. J. Exp. Anal. Behav. 1993, 60, 361–385. [Google Scholar] [CrossRef] [PubMed]
- Somkuwar, S.S.; Vendruscolo, L.F.; Fannon, M.J.; Schmeichel, B.E.; Nguyen, T.B.; Guevara, J.; Sidhu, H.; Contet, C.; Zorrilla, E.P.; Mandyam, C.D. Abstinence from prolonged ethanol exposure affects plasma corticosterone, glucocorticoid receptor signaling and stress-related behaviors. Psychoneuroendocrinology 2017, 84, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Richardson, H.N.; Lee, S.Y.; O’Dell, L.E.; Koob, G.F.; Rivier, C.L. Alcohol self-administration acutely stimulates the hypothalamic-pituitary-adrenal axis, but alcohol dependence leads to a dampened neuroendocrine state. Eur. J. Neurosci. 2008, 28, 1641–1653. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, N.R.; Roberts, D.C. Progressive ratio schedules in drug self-administration studies in rats: A method to evaluate reinforcing efficacy. J. Neurosci. Methods 1996, 66, 1–11. [Google Scholar] [CrossRef]
- Wee, S.; Mandyam, C.D.; Lekic, D.M.; Koob, G.F. Alpha 1-noradrenergic system role in increased motivation for cocaine intake in rats with prolonged access. Eur. Neuropsychopharmacol. 2008, 18, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Engelmann, A.J.; Aparicio, M.B.; Kim, A.; Sobieraj, J.C.; Yuan, C.J.; Grant, Y.; Mandyam, C.D. Chronic wheel running reduces maladaptive patterns of methamphetamine intake: Regulation by attenuation of methamphetamine-induced neuronal nitric oxide synthase. Brain Struct. Funct. 2014, 219, 657–672. [Google Scholar] [CrossRef] [PubMed]
- Hodos, W. Progressive ratio as a measure of reward strength. Science 1961, 134, 943–944. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.C.; Covarrubias, P.; Terry, J.; Sanabria, F. The effect of methylphenidate and rearing environment on behavioral inhibition in adult male rats. Psychopharmacology 2012, 219, 353–362. [Google Scholar] [CrossRef] [PubMed]
- Stephens, D.N.; Voet, B. Differential effects of anxiolytic and non-anxiolytic benzodiazepine receptor ligands on performance of a differential reinforcement of low rate (DRL) schedule. Behav. Pharmacol. 1994, 5, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Richards, J.B.; Seiden, L.S. A quantitative interresponse-time analysis of DRL performance differentiates similar effects of the antidepressant desipramine and the novel anxiolytic gepirone. J. Exp. Anal. Behav. 1991, 56, 173–192. [Google Scholar] [CrossRef] [PubMed]
- McClure, G.Y.; McMillan, D.E. Effects of drugs on response duration differentiation. VI: Differential effects under differential reinforcement of low rates of responding schedules. J. Pharmacol. Exp. Ther. 1997, 281, 1368–1380. [Google Scholar] [PubMed]
- Bouton, M.E. Learning and Behavior: A Contemporary Synthesis; Sinauer Associates: Sunderland, MA, USA, 2007. [Google Scholar]
- Weiss, F.; Lorang, M.T.; Bloom, F.E.; Koob, G.F. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: Genetic and motivational determinants. J. Pharmacol. Exp. Ther. 1993, 267, 250–258. [Google Scholar] [PubMed]
- Lewis, M.J.; June, H.L. Neurobehavioral studies of ethanol reward and activation. Alcohol 1990, 7, 213–219. [Google Scholar] [CrossRef]
- Waller, M.B.; Murphy, J.M.; McBride, W.J.; Lumeng, L.; Li, T.K. Effect of low dose ethanol on spontaneous motor activity in alcohol-preferring and -nonpreferring lines of rats. Pharmacol. Biochem. Behav. 1986, 24, 617–623. [Google Scholar] [CrossRef]
- Karlsson, O.; Roman, E. Dose-dependent effects of alcohol administration on behavioral profiles in the MCSF test. Alcohol 2016, 50, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Alijan-Pour, J.; Abrari, K. Ethanol disrupts reactivated contextual conditioned fear memory: Behavioral and histological perspectives. Cell J. 2012, 13, 265–274. [Google Scholar] [PubMed]
- Pian, J.P.; Criado, J.R.; Ehlers, C.L. Differential effects of acute alcohol on prepulse inhibition and event-related potentials in adolescent and adult Wistar rats. Alcohol. Clin. Exp. Res. 2008, 32, 2062–2073. [Google Scholar] [CrossRef] [PubMed]
- Kozawa, S.; Yukawa, N.; Liu, J.; Shimamoto, A.; Kakizaki, E.; Fujimiya, T. Effect of chronic ethanol administration on disposition of ethanol and its metabolites in rat. Alcohol 2007, 41, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Hopf, F.W.; Chang, S.J.; Sparta, D.R.; Bowers, M.S.; Bonci, A. Motivation for alcohol becomes resistant to quinine adulteration after 3 to 4 months of intermittent alcohol self-administration. Alcohol. Clin. Exp. Res. 2010, 34, 1565–1573. [Google Scholar] [CrossRef] [PubMed]
- Avila, I.; Reilly, M.P.; Sanabria, F.; Posadas-Sánchez, D.; Chavez, C.L.; Banerjee, N.; Killeen, P.; Castañeda, E. Modeling operant behavior in the Parkinsonian rat. Behav. Brain Res. 2009, 198, 298–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koob, G.F.; Volkow, N.D. Neurobiology of addiction: A neurocircuitry analysis. Lancet Psychiatry 2016, 3, 760–773. [Google Scholar] [CrossRef]
- Everitt, B.J.; Belin, D.; Economidou, D.; Pelloux, Y.; Dalley, J.W.; Robbins, T.W. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos. Trans. R. Soc. Lond B Biol. Sci. 2008, 363, 3125–3135. [Google Scholar] [CrossRef] [PubMed]
- Chudasama, Y.; Robbins, T.W. Functions of frontostriatal systems in cognition: Comparative neuropsychopharmacological studies in rats, monkeys and humans. Biol. Psychol. 2006, 73, 19–38. [Google Scholar] [CrossRef] [PubMed]
- Everitt, B.J.; Wolf, M.E. Psychomotor stimulant addiction: A neural systems perspective. J. Neurosci. 2002, 22, 3312–3320. [Google Scholar] [CrossRef] [PubMed]
- McFarland, K.; Lapish, C.C.; Kalivas, P.W. Prefrontal glutamate release into the core of the nucleus accumbens mediates cocaine-induced reinstatement of drug-seeking behavior. J. Neurosci. 2003, 23, 3531–3537. [Google Scholar] [CrossRef] [PubMed]
- Amodeo, L.R.; Kneiber, D.; Wills, D.N.; Ehlers, C.L. Alcohol drinking during adolescence increases consumptive responses to alcohol in adulthood in Wistar rats. Alcohol 2017, 59, 43–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pandey, S.C.; Sakharkar, A.J.; Tang, L.; Zhang, H. Potential role of adolescent alcohol exposure-induced amygdaloid histone modifications in anxiety and alcohol intake during adulthood. Neurobiol. Dis. 2015, 82, 607–619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimbrough, A.; Kim, S.; Cole, M.; Brennan, M.; George, O. Intermittent Access to Ethanol Drinking Facilitates the Transition to Excessive Drinking After Chronic Intermittent Ethanol Vapor Exposure. Alcohol. Clin. Exp. Res. 2017, 41, 1502–1509. [Google Scholar] [CrossRef] [PubMed]
- Vendruscolo, L.F.; Barbier, E.; Schlosburg, J.E.; Misra, K.K.; Whitfield, T.W.; Logrip, M.L.; Rivier, C.; Repunte-Canonigo, V.; Zorrilla, E.P.; Sanna, P.P.; et al. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J. Neurosci. 2012, 32, 7563–7571. [Google Scholar] [CrossRef] [PubMed]
- Wise, R.A. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia 1973, 29, 203–210. [Google Scholar] [CrossRef] [PubMed]
- Risher, M.L.; Fleming, R.L.; Boutros, N.; Semenova, S.; Wilson, W.A.; Levin, E.D.; Markou, A.; Swartzwelder, H.S.; Acheson, S.K. Long-term effects of chronic intermittent ethanol exposure in adolescent and adult rats: Radial-arm maze performance and operant food reinforced responding. PLoS ONE 2013, 8, e62940. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.M.; Koob, G.F. The gamma-aminobutyric acid-B receptor agonist baclofen attenuates responding for ethanol in ethanol-dependent rats. Alcohol. Clin. Exp. Res. 2007, 31, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Leventhal, A.M.; Kahler, C.W.; Ray, L.A.; Stone, K.; Young, D.; Chelminski, I.; Zimmerman, M. Anhedonia and amotivation in psychiatric outpatients with fully remitted stimulant use disorder. Am. J. Addict. 2008, 17, 218–223. [Google Scholar] [CrossRef] [PubMed]
- Criado, J.R.; Liu, T.; Ehlers, C.L.; Mathé, A.A. Prolonged chronic ethanol exposure alters neuropeptide Y and corticotropin-releasing factor levels in the brain of adult Wistar rats. Pharmacol. Biochem. Behav. 2011, 99, 104–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merlo Pich, E.; Lorang, M.; Yeganeh, M.; De Fonseca, F.R.; Raber, J.; Koob, G.F.; Weiss, F. Increase of extracellular corticotropin-releasing factor-like immunoreactivity levels in the amygdala of awake rats during restraint stress and ethanol withdrawal as measured by microdialysis. J. Neurosci. 1995, 15, 5439–5447. [Google Scholar] [CrossRef] [PubMed]
- Valdez, G.R.; Roberts, A.J.; Chan, K.; Davis, H.; Brennan, M.; Zorrilla, E.P.; Koob, G.F. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: Regulation by corticotropin-releasing factor. Alcohol. Clin. Exp. Res. 2002, 26, 1494–1501. [Google Scholar] [CrossRef] [PubMed]
- Britton, K.T.; Koob, G.F. Effects of corticotropin releasing factor, desipramine and haloperidol on a DRL schedule of reinforcement. Pharmacol. Biochem. Behav. 1989, 32, 967–970. [Google Scholar] [CrossRef]
- Littlefield, A.K.; Sher, K.J.; Wood, P.K. A personality-based description of maturing out of alcohol problems: Extension with a five-factor model and robustness to modeling challenges. Addict. Behav. 2010, 35, 948–954. [Google Scholar] [CrossRef] [PubMed]
- Roberts, B.W.; Walton, K.E.; Viechtbauer, W. Patterns of mean-level change in personality traits across the life course: A meta-analysis of longitudinal studies. Psychol. Bull. 2006, 132, 1–25. [Google Scholar] [CrossRef] [PubMed]






| GROUPS | SUCROSE-CON | SUCROSE-CIE | ETHANOL-CON | ETHANOL-CIE |
|---|---|---|---|---|
| Prevapor | 447 ± 12.4 | 411 ± 6.36 | 393 ± 6.84 | 434 ± 9.07 |
| Week 1 (Vapor 1) | 470 ± 13.0 | 462 ± 8.11 | 407 ± 7.02 | 457 ± 12.1 |
| Week 2 (Vapor 2) | NA | 477 ± 8.50 | 421 ± 7.87 | 464 ± 14.1 |
| Week 3 (Vapor 3) | 516 ± 15.2 | 493 ± 10.3 | 429 ± 8.89 | 478 ± 13.2 |
| Week 4 (Vapor 4) | 500 ± 21.5 | 507 ± 12.1 | 449 ± 9.29 | 506 ± 15.2 |
| Week 5 (Vapor 5) | 531 ± 15.1 | 523 ± 12.3 | 470 ± 9.60 | 507 ± 16.1 |
| Week 6 (Vapor 6) | 555 ± 12.8 # | NA | 488 ± 10.2 | 517 ± 15.3 |
| Week 7 (Abstinence 1) | 569 ± 13.1 # | 548 ± 13.5 | 499 ± 10.9 | 505 ± 19.1 |
| Week 8 (Abstinence 2) | 581 ± 12.8 # | 563 ± 15.4 | 510 ± 11.8 | 525 ± 16.7 |
| Week 9 (Abstinence 3) | 597 ± 15.7 # | 585 ± 16.6 | 506 ± 13.9 | 534 ± 17.4 |
| Week 10 (Abstinence 4) | 601 ± 15.1 # | 589 ± 17.8 | 540 ± 14.4 | 570 ± 18.4 |
| Week 11 (Abstinence 5) | 612 ± 14.6 # | 606 ± 20.2 | 555 ± 14.1 | 601 ± 19.6 |
| Groups | Responses | Response Distribution | CON | CIE | t Ratio |
|---|---|---|---|---|---|
| SUCROSE | Proportion of timed responses | Proportion gamma; p | 0.67 ± 0.04 | 0.57 ± 0.07 | t(143) = 0.05 |
| Accuracy of timed responses | Response threshold; θ = (N × c)/15 | 1.17 ± 0.05 | 1.11 ± 0.03 | t(143) = 0.03 | |
| Proportion of burst responses | Proportion short exponent; (q × (1 – p)) | 0.16 ± .03 | 0.29 ± .07 | t(143) = 0.07 | |
| Rate of decay of burst responses | Rate of decay for short exponent L | 5.80 ± 2.22 | 4.42 ± 2.86 | t(143) = 0.77 | |
| ETHANOL | Proportion of timed responses | Proportion gamma; p | 0.78 ± 0.05 | 0.77 ± 2.86 | t(132) = 0.00 |
| Accuracy of timed responses | Response threshold; θ = (N × c)/15 | 1.12 ± 0.11 | 1.07 ± 0.10 | t(132) = 0.03 | |
| Proportion of burst responses | Proportion short exponent; (q × (1 – p)) | 0.07 ± 0.02 | 0.11 ± 0.04 | t(132) = 0.02 |
| Reinforcers Earned | ETHANOL-CON | ETHANOL-CIE |
|---|---|---|
| Lever responses | 33.6 ± 14.8 | 38.5 ± 8.55 |
| Ethanol reinforcers earned | 4.00 ± 1.33 | 5.25 ± 0.88 |
| Group | Prevapor (Week 0) | Acute Withdrawal (Week 5) | Prolonged Abstinence (Week 11) |
|---|---|---|---|
| SUCROSE-CON | 65.0 ± 14.8 | 86.3 ± 12.9 | 50.5 ± 6.17 |
| SUCROSE-CIE | 84.1 ± 25.2 | 90.1 ± 26.7 | 66.0 ± 24.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Somkuwar, S.S.; Quach, L.W.; Quigley, J.A.; Purohit, D.C.; Fannon, M.J.; Koob, G.F.; Mandyam, C.D. Ethanol Reinforcement Elicits Novel Response Inhibition Behavior in a Rat Model of Ethanol Dependence. Brain Sci. 2018, 8, 119. https://doi.org/10.3390/brainsci8070119
Somkuwar SS, Quach LW, Quigley JA, Purohit DC, Fannon MJ, Koob GF, Mandyam CD. Ethanol Reinforcement Elicits Novel Response Inhibition Behavior in a Rat Model of Ethanol Dependence. Brain Sciences. 2018; 8(7):119. https://doi.org/10.3390/brainsci8070119
Chicago/Turabian StyleSomkuwar, Sucharita S., Leon W. Quach, Jacqueline A. Quigley, Dvijen C. Purohit, McKenzie J. Fannon, George F. Koob, and Chitra D. Mandyam. 2018. "Ethanol Reinforcement Elicits Novel Response Inhibition Behavior in a Rat Model of Ethanol Dependence" Brain Sciences 8, no. 7: 119. https://doi.org/10.3390/brainsci8070119
APA StyleSomkuwar, S. S., Quach, L. W., Quigley, J. A., Purohit, D. C., Fannon, M. J., Koob, G. F., & Mandyam, C. D. (2018). Ethanol Reinforcement Elicits Novel Response Inhibition Behavior in a Rat Model of Ethanol Dependence. Brain Sciences, 8(7), 119. https://doi.org/10.3390/brainsci8070119





