Ethanol Conditioned Taste Aversion in High Drinking in the Dark Mice
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice and Husbandry
2.2. Experiments and Methods
2.2.1. Experiment 1. EtOH Conditioned Taste Aversion in Inbred HDID Mice
2.2.2. Experiment 2. Concentration-Dependent Initial Intake of EtOH: Low Concentrations
2.2.3. Experiment 3. Concentration-Dependent Initial Intake of EtOH: High Concentrations
2.2.4. Experiment 4. Concentration-Dependence of CTA with Thirst Motivation
2.2.5. Experiment 5: Effect of Number of Tubes of EtOH on Day 1
2.3. Statistical Analyses
3. Results
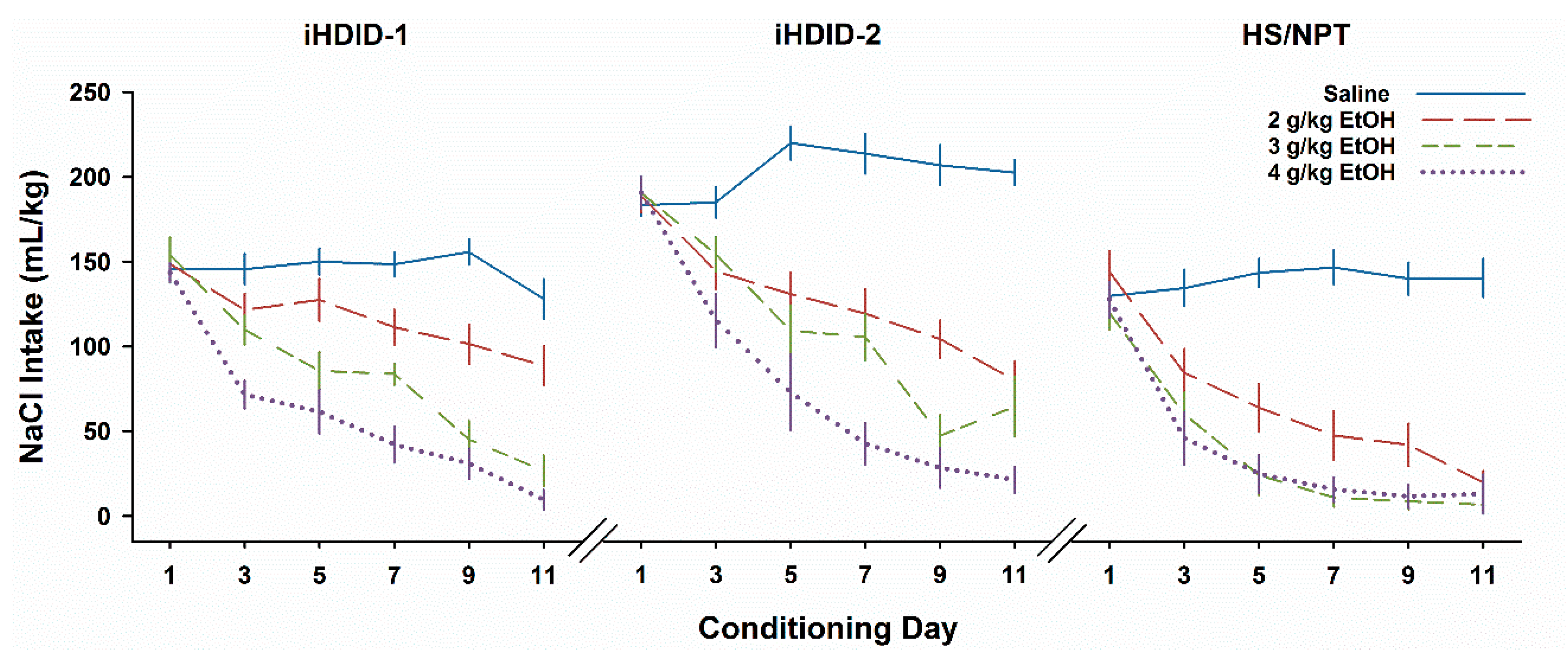
3.1. Experiment 1. Insensitivity to EtOH CTA in iHDID-1 and iHDID-2 Mice
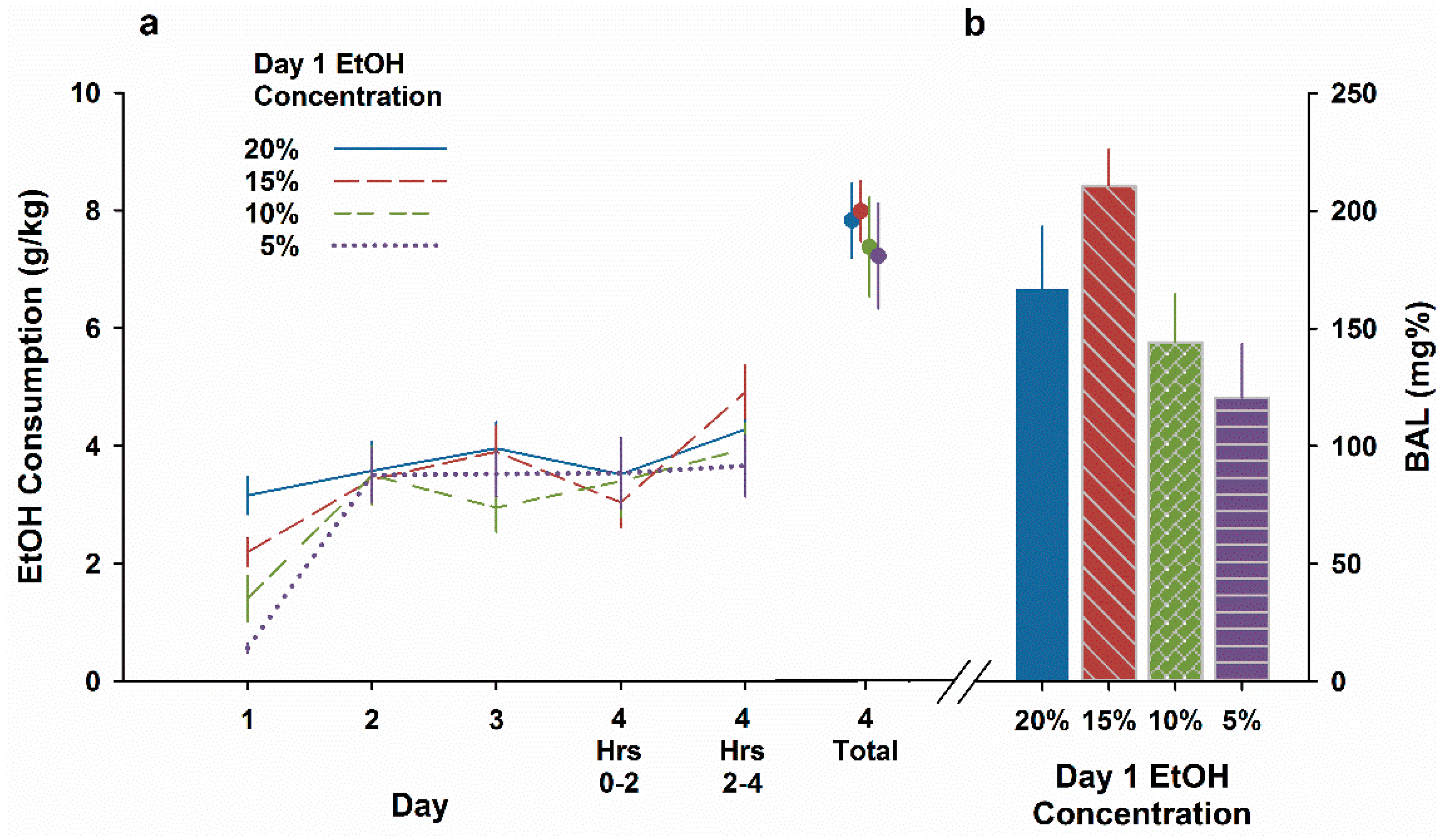
3.2. Experiment 2. No Evidence of a CTA after Exposure to Lower EtOH Concentrations
3.3. Experiment 3. EtOH CTA after Ingestion of Higher EtOH Concentrations
3.4. Experiment 4. CTA with Thirst Motivation in HDID-1 and HDID-2 but not HS Mice
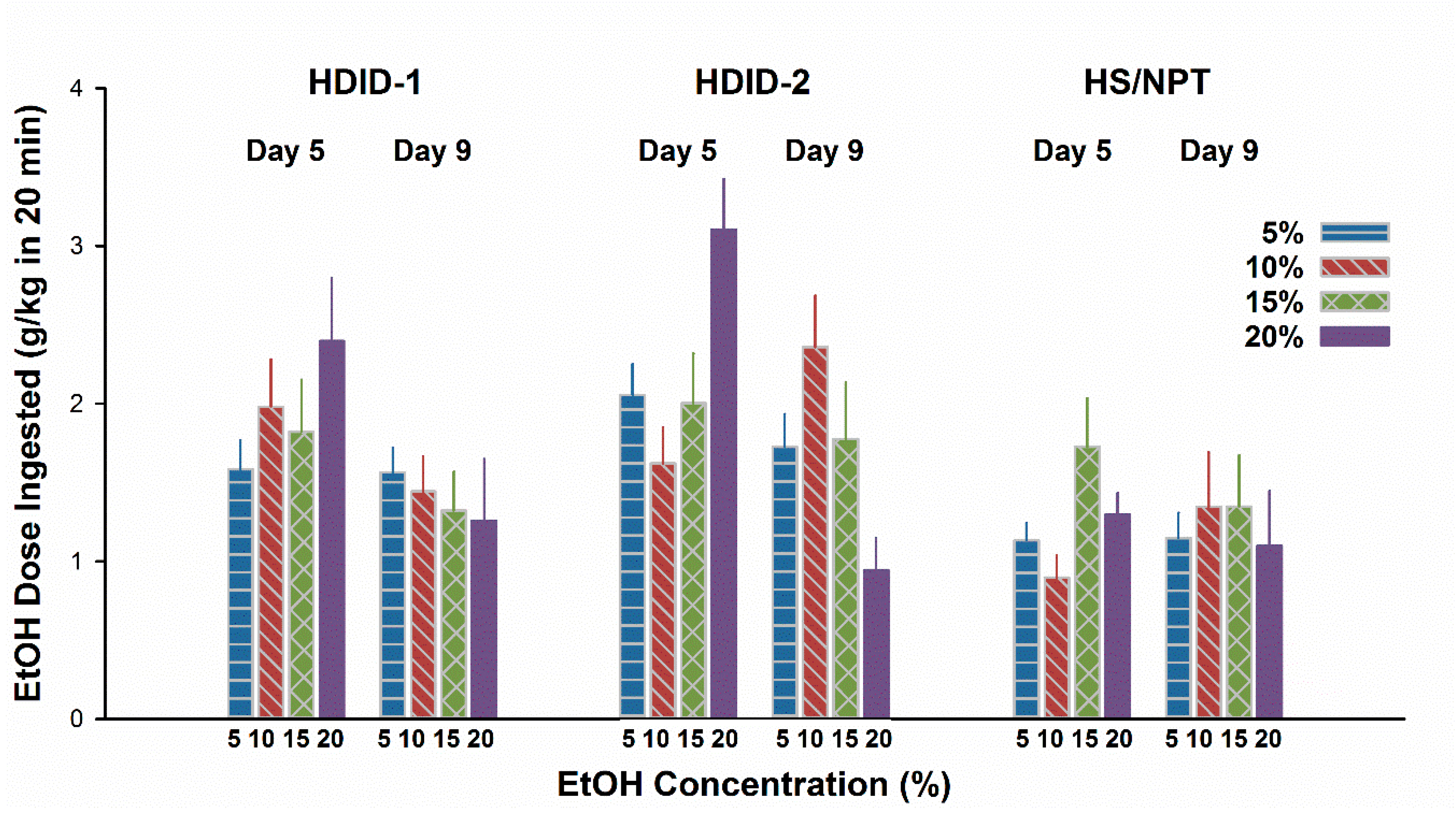
3.5. Experiment 5. Multiple Bottle Drinking Leads to a CTA
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Olsson, C.A.; Romaniuk, H.; Salinger, J.; Staiger, P.K.; Bonomo, Y.; Hulbert, C.; Patton, G.C. Drinking Patterns of Adolescents Who Develop Alcohol Use Disorders: Results from the Victorian Adolescent Health Cohort Study. BMJ Open 2016, 6, e010455. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.samhsa.gov/data/sites/default/files/NSDUH-FFR1-2016/NSDUH-FFR1-2016.htm (accessed on 26 December 2018).
- Available online: https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/alcohol-facts-and-statistics (accessed on 26 December 2018).
- National Institute on Alcohol Abuse and Alcoholism (NIAAA). NIAAA Council Approves Definition of Binge Drinking; DHHS-NIH: Bethesda, MD, USA, 2004; p. 3.
- Rolland, B.; Naassila, M. Binge Drinking: Current Diagnostic and Therapeutic Issues. CNS Drugs 2017, 31, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Samson, H.H.; Files, F.J.; Denning, C.; Marvin, S. Comparison of alcohol-preferring and nonpreferring selectively bred rat lines. I. Ethanol initiation and limited access operant self- administration. Alcohol. Clin. Exp. Res. 1998, 22, 2133–2146. [Google Scholar] [CrossRef] [PubMed]
- Oberlin, B.; Best, C.; Matson, L.; Henderson, A.; Grahame, N. Derivation and characterization of replicate high- and low-alcohol preferring lines of mice and a high-drinking crossed HAP line. Behav. Genet. 2011, 41, 288–302. [Google Scholar] [CrossRef] [PubMed]
- Crabbe, J.C. The genetic complexity of alcohol drinking in rodents. In The Neurobiology of Alcohol Dependence; Noronha, A.B.C., Cui, C., Harris, R.A., Crabbe, J.C., Eds.; Elsevier/Academic Press: San Diego, CA, USA, 2014; pp. 359–375. [Google Scholar]
- Petkov, P.M.; Ding, Y.; Cassell, M.A.; Zhang, W.; Wagner, G.; Sargent, E.E.; Asquith, S.; Crew, V.; Johnson, K.A.; Robinson, P.; et al. An efficient SNP system for mouse genome scanning and elucidating strain relationships. Genome Res. 2004, 14, 1806–1811. [Google Scholar] [CrossRef] [PubMed]
- Dole, V.P.; Gentry, R.T. Toward an analogue of alcoholism in mice: Scale factors in the model. Proc. Natl. Acad. Sci. USA 1984, 81, 3543–3546. [Google Scholar] [CrossRef] [PubMed]
- Esser, M.B.; Hedden, S.L.; Kanny, D.; Brewer, R.D.; Gfroerer, J.C.; Naimi, T.S. Prevalence of alcohol dependence among US adult drinkers, 2009–2011. Prev. Chronic Dis. 2014, 11, E206. [Google Scholar] [CrossRef] [PubMed]
- Humphreys, K.; Bickel, W.K. Toward a neuroscience of long-term recovery from addiction. JAMA Psychiatry 2018, 75, 875–876. [Google Scholar] [CrossRef]
- Verendeev, A.; Riley, A.L. The role of the aversive effects of drugs in self-administration: Assessing the balance of reward and aversion in drug-taking behavior. Behav. Pharmacol. 2013, 24, 363–374. [Google Scholar] [CrossRef]
- Revusky, S. Some laboratory paradigms for chemical aversion treatment of alcoholism. J. Behav. Ther. Exp. Psychiatry 1973, 4, 15–17. [Google Scholar] [CrossRef]
- Nathan, P.E. Aversion therapy in the treatment of alcoholism: Success and failure. Ann. N. Y. Acad. Sci. 1985, 443, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Riley, A.L. The paradox of drug taking: The role of the aversive effects of drugs. Physiol. Behav. 2011, 103, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Elkins, R.L. Separation of taste-aversion-prone and taste-aversion-resistant rats through selective breeding: Implications for individual differences in conditionability and aversion-therapy alcoholism treatment. Behav. Neurosci. 1986, 100, 121–124. [Google Scholar] [CrossRef] [PubMed]
- Orr, T.E.; Whitford-Stoddard, J.L.; Elkins, R.L. Taste-aversion-prone (TAP) rats and taste-aversion-resistant (TAR) rats differ in ethanol self-administration, but not in ethanol clearance or general consumption. Alcohol 2004, 33, 1–7. [Google Scholar] [PubMed]
- Orr, T.E.; Walters, P.A.; Elkins, R.L. Differences in free-choice ethanol acceptance between taste aversion-prone and taste aversion-resistant rats. Alcohol. Clin. Exp. Res. 1997, 21, 1491–1496. [Google Scholar] [CrossRef] [PubMed]
- Elkins, R.L.; Walters, P.A.; Orr, T.E. Continued development and unconditioned stimulus characterization of selectively bred lines of taste aversion prone and resistant rats. Alcohol. Clin. Exp. Res. 1992, 16, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Green, A.S.; Grahame, N.J. Ethanol drinking in rodents: Is free-choice drinking related to the reinforcing effects of ethanol? Alcohol 2008, 42, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Spierling, S.R.; Kreisler, A.D.; Williams, C.A.; Fang, S.Y.; Pucci, S.N.; Kines, K.T.; Zorrilla, E.P. Intermittent, extended access to preferred food leads to escalated food reinforcement and cyclic whole-body metabolism in rats: Sex differences and individual vulnerability. Physiol. Behav. 2018, 192, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Zorrilla, E.P.; Inoue, K.; Fekete, E.M.; Tabarin, A.; Valdez, G.R.; Koob, G.F. Measuring meals: Structure of prandial food and water intake of rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 288, R1450–R1467. [Google Scholar] [CrossRef] [PubMed]
- Linsenbardt, D.N.; Boehm, S.L. Alterations in the rate of binge ethanol consumption: Implications for preclinical studies in mice. Addict. Biol. 2014, 19, 812–825. [Google Scholar] [CrossRef]
- Rhodes, J.S.; Best, K.; Belknap, J.K.; Finn, D.A.; Crabbe, J.C. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol. Behav. 2005, 84, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Thiele, T.E.; Navarro, M. “Drinking in the dark” (DID) procedures: A model of binge-like ethanol drinking in non-dependent mice. Alcohol 2014, 48, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Fritz, B.M.; Boehm, S.L. Rodent models and mechanisms of voluntary binge-like ethanol consumption: Examples, opportunities, and strategies for preclinical research. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 65, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Jeanblanc, J.; Rolland, B.; Gierski, F.; Martinetti, M.P.; Naassila, M. Animal models of binge drinking, current challenges to improve face validity. Neurosci. Biobehav. Rev. 2018. [Google Scholar] [CrossRef] [PubMed]
- Crabbe, J.C.; Metten, P.; Rhodes, J.S.; Yu, C.-H.; Brown, L.L.; Phillips, T.J.; Finn, D.A. A line of mice selected for high blood ethanol concentrations shows drinking in the dark to intoxication. Biol. Psychiatry 2009, 65, 662–670. [Google Scholar] [CrossRef]
- Crabbe, J.C.; Metten, P.; Belknap, J.K.; Spence, S.E.; Cameron, A.J.; Schlumbohm, J.P.; Huang, L.C.; Barkley-Levenson, A.M.; Ford, M.M.; Phillips, T.J. Progress in a replicated selection for elevated blood ethanol concentrations in HDID mice. Genes Brain Behav. 2014, 13, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Barkley-Levenson, A.M.; Crabbe, J.C. Distinct ethanol drinking microstructures in two replicate lines of mice selected for drinking to intoxication. Genes Brain Behav. 2015, 14, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Crabbe, J.C. Using Signatures of Directional Selection to Guide Discovery. In Molecular-Genetic and Statistical Techniques for Behavioral and Neural Research, 1st ed.; Gerlai, R.T., Ed.; Elsevier/Academic Press: San Diego, CA, USA, 2018; pp. 243–263. [Google Scholar]
- Barkley-Levenson, A.M.; Crabbe, J.C. High drinking in the dark mice: A genetic model of drinking to intoxication. Alcohol 2014, 48, 217–223. [Google Scholar] [CrossRef]
- Cunningham, C.L.; Phillips, T.J. Genetic Basis of Ethanol Reward. In Molecular Biology of Drug Addiction; Maldonado, R., Ed.; Humana Press, Inc.: Totowa, NJ, USA, 2003; pp. 263–294. [Google Scholar]
- Stephens, D.N.; Duka, T.; Crombag, H.S.; Cunningham, C.L.; Heilig, M.; Crabbe, J.C. Reward sensitivity: Issues of measurement, and achieving consilience between human and animal phenotypes. Addict. Biol. 2010, 15, 145–168. [Google Scholar] [CrossRef]
- Barkley-Levenson, A.M.; Cunningham, C.L.; Smitasin, P.J.; Crabbe, J.C. Rewarding and aversive effects of ethanol in High Drinking in the Dark selectively bred mice. Addict. Biol. 2015, 20, 80–90. [Google Scholar] [CrossRef]
- Broadbent, J.; Muccino, K.J.; Cunningham, C.L. Ethanol-induced conditioned taste aversion in 15 inbred mouse strains. Behav. Neurosci. 2002, 116, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Barkley-Levenson, A.M.; Crabbe, J.C. Ethanol drinking microstructure of a High Drinking in the Dark selected mouse line. Alcohol. Clin. Exp. Res. 2012, 36, 1330–1339. [Google Scholar] [CrossRef] [PubMed]
- Belknap, J.K.; Coleman, R.R.; Foster, K. Alcohol consumption and sensory threshold differences between C57BL/6J and DBA/2J mice. Physiol. Psychol. 1978, 6, 71–74. [Google Scholar] [CrossRef]
- McClearn, G.E. Genetics and motivation of the mouse. In Nebraska Symposium on Motivation; Arnold, W.J., Ed.; University of Nebraska Press: Lincoln, Nebraska, 1968; pp. 47–83. [Google Scholar]
- Tordoff, M.G.; Bachmanov, A.A. Influence of the number of alcohol and water bottles on murine alcohol intake. Alcohol. Clin. Exp. Res. 2003, 27, 600–606. [Google Scholar] [CrossRef] [PubMed]
- Finn, D.A.; Belknap, J.K.; Cronise, K.; Yoneyama, N.; Murillo, A.; Crabbe, J.C. A procedure to produce high alcohol intake in mice. Psychopharmacology 2005, 178, 471–480. [Google Scholar] [CrossRef] [PubMed]
- Ryabinin, A.E.; Sharpe, A.L.; Tsivokovskaia, N.O.; Weitemier, A.Z. Ethanol self-administration during the circadian dark phase. Alcohol. Clin. Exp. Res. 2003, 27, S122. [Google Scholar]
- National Research Council. Guide for the Care and Use of Laboratory Animals; National Academies Press: Washington, DC, USA, 2010. [Google Scholar]
- Pardoe, I. Applied Regression Modeling, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Falconer, D.S.; Mackay, T.F.C. Introduction to Quantitative Genetics, 4th ed.; Longman: Harlow, UK, 1996. [Google Scholar]
- Lynch, M.; Walsh, B. Genetics and Analysis of Quantitative Traits; Sinauer Associates: Sunderland, MD, USA, 1998. [Google Scholar]
- Eisen, E.J.; Hanrahan, J.P.; Legates, J.E. Effects of population size and selection intensity on correlated responses to selection for postweaning gain in mice. Genetics 1973, 74, 157–170. [Google Scholar]
- Sharpe, A.L.; Tsivkovskaia, N.O.; Ryabinin, A.E. Ataxia and c-Fos expression in mice drinking ethanol in a limited access session. Alcohol. Clin. Exp. Res. 2005, 29, 1419–1426. [Google Scholar] [CrossRef] [PubMed]
- McClearn, G.E.; Rodgers, D.A. Differences in alcohol preference among inbred strains of mice. Q. J. Stud. Alcohol 1959, 20, 691–695. [Google Scholar]
- Thomas, K. Selection and avoidance of alcohol solutions by two strains of inbred mice and derived generations. Q. J. Stud. Alcohol 1969, 30, 849–861. [Google Scholar]
- Crabbe, J.C.; Spence, S.E.; Brown, L.L.; Metten, P. Alcohol preference drinking in a mouse line selectively bred for high drinking in the dark. Alcohol 2011, 45, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Tordoff, M.G.; Bachmanov, A.A. Mouse taste preference tests: Why only two bottles? Chem. Sens. 2003, 28, 315–324. [Google Scholar] [CrossRef]
- Tordoff, M.G. Obesity by choice: The powerful influence of nutrient availability on nutrient intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2002, 282, R1536–R1539. [Google Scholar] [CrossRef] [PubMed]
- Wahlsten, D. Insensitivity of the analysis of variance to hereditary-environment interaction. Behav. Brain Sci. 1990, 13, 109–120. [Google Scholar] [CrossRef]
- Morales, M.; Schatz, K.C.; Anderson, R.I.; Spear, L.P.; Varlinskaya, E.I. Conditioned taste aversion to ethanol in a social context: Impact of age and sex. Behav. Brain Res. 2014, 261, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Schramm-Sapyta, N.L.; Francis, R.; MacDonald, A.; Keistler, C.; O’Neill, L.; Kuhn, C.M. Effect of sex on ethanol consumption and conditioned taste aversion in adolescent and adult rats. Psychopharmacology 2014, 231, 1831–1839. [Google Scholar] [CrossRef] [PubMed]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Crabbe, J.C.; Metten, P.; Savarese, A.M.; Ozburn, A.R.; Schlumbohm, J.P.; Spence, S.E.; Hack, W.R. Ethanol Conditioned Taste Aversion in High Drinking in the Dark Mice. Brain Sci. 2019, 9, 2. https://doi.org/10.3390/brainsci9010002
Crabbe JC, Metten P, Savarese AM, Ozburn AR, Schlumbohm JP, Spence SE, Hack WR. Ethanol Conditioned Taste Aversion in High Drinking in the Dark Mice. Brain Sciences. 2019; 9(1):2. https://doi.org/10.3390/brainsci9010002
Chicago/Turabian StyleCrabbe, John C., Pamela Metten, Antonia M. Savarese, Angela R. Ozburn, Jason P. Schlumbohm, Stephanie E. Spence, and Wyatt R. Hack. 2019. "Ethanol Conditioned Taste Aversion in High Drinking in the Dark Mice" Brain Sciences 9, no. 1: 2. https://doi.org/10.3390/brainsci9010002
APA StyleCrabbe, J. C., Metten, P., Savarese, A. M., Ozburn, A. R., Schlumbohm, J. P., Spence, S. E., & Hack, W. R. (2019). Ethanol Conditioned Taste Aversion in High Drinking in the Dark Mice. Brain Sciences, 9(1), 2. https://doi.org/10.3390/brainsci9010002




