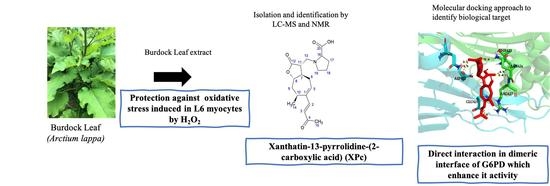
A Novel Sesquiterpene Lactone Xanthatin-13-(pyrrolidine-2-carboxylic acid) Isolated from Burdock Leaf Up-Regulates Cells’ Oxidative Stress Defense Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Plant Extracts
2.3. Preparative UPLC-HRMS-MS to Obtain a Pure Extract of the Active Molecule
2.4. NMR Analysis
2.5. In Vitro Test Protection against H2O2 on L6 Cells
2.6. Real-Time RT-PCR (qPCR)
2.7. Molecular Docking Simulation
2.7.1. Preparation of the G6PD (2BHL) Protein Receptor
- -
- Polar hydrogen atoms, as they are involved in hydrogen bonding.
- -
- Partial charges on the atoms to simulate electrostatic interactions.
2.7.2. Preparation of the Ligand Input File
2.7.3. Docking Simulation
2.8. Statistical Analysis
3. Results
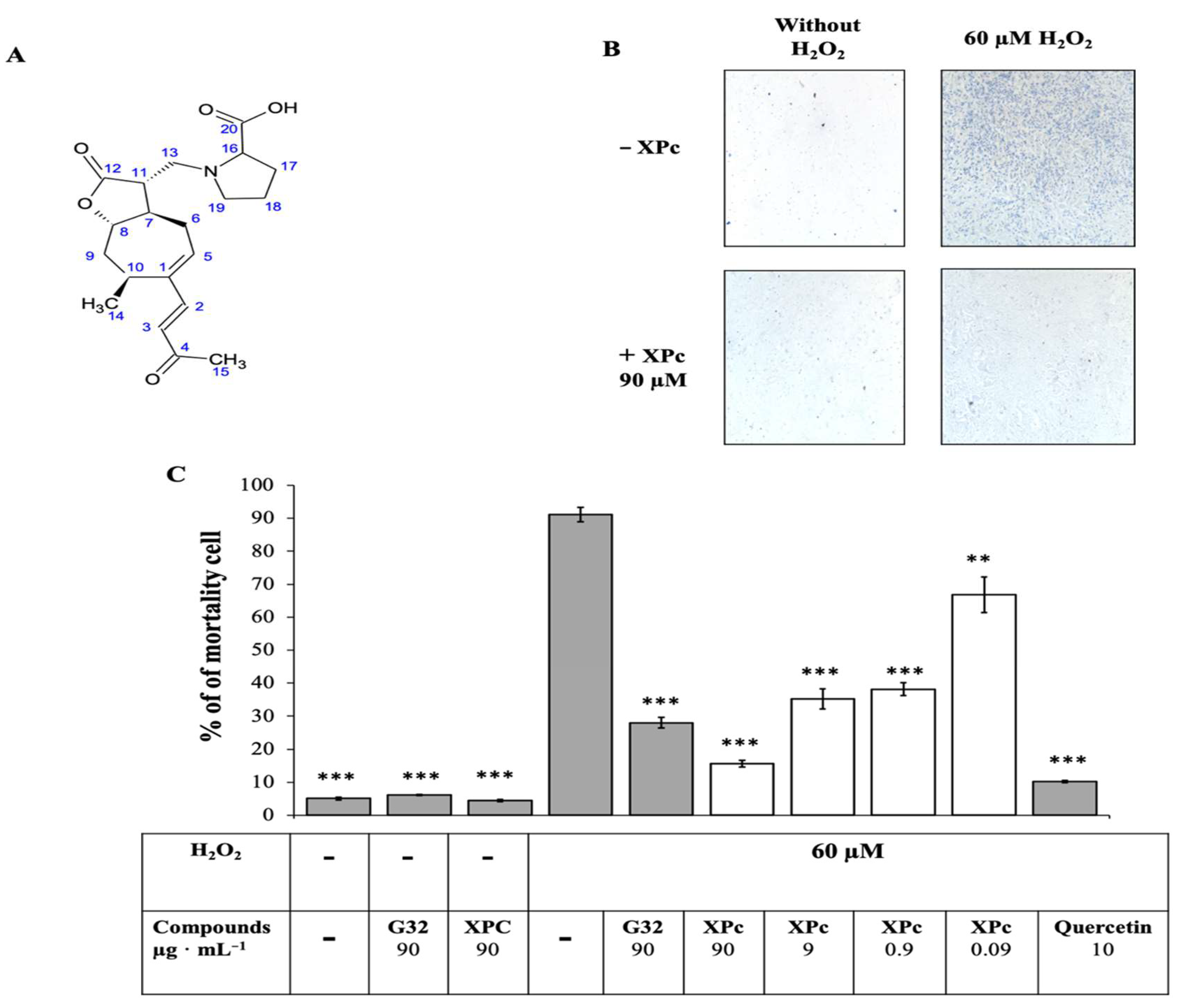
3.1. In Vitro Test Protection of L6 Cells against H2O2 to Screen Eight Asteraceae Extracts
3.2. Isolation and Identification of the Protective Compound
3.2.1. Isolation of Active Compound
3.2.2. Structural Elucidation
3.3. Research of XPc Biological Targets
3.3.1. RT qPCR
3.3.2. Molecular Docking
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Burton, G.J.; Jauniaux, E. Oxidative Stress. Best Pract. Res. Clin. Obstet. Gynaecol. 2011, 25, 287–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive Oxygen Species (ROS) Homeostasis and Redox Regulation in Cellular Signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittler, R. ROS Are Good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Krzywanski, D.M.; Dickinson, D.A.; Iles, K.E.; Wigley, A.F.; Franklin, C.C.; Liu, R.-M.; Kavanagh, T.J.; Forman, H.J. Variable Regulation of Glutamate Cysteine Ligase Subunit Proteins Affects Glutathione Biosynthesis in Response to Oxidative Stress. Arch. Biochem. Biophys. 2004, 423, 116–125. [Google Scholar] [CrossRef]
- Lu, S.C. Regulation of Glutathione Synthesis. Mol. Asp. Med. 2009, 30, 42–59. [Google Scholar] [CrossRef] [Green Version]
- Kurata, M.; Suzuki, M.; Agar, N.S. Glutathione Regeneration in Mammalian Erythrocytes. Comp. Haematol. Int. 2000, 10, 59–67. [Google Scholar] [CrossRef]
- Pandolfi, P.P.; Sonati, F.; Rivi, R.; Mason, P.; Grosveld, F.; Luzzatto, L. Targeted Disruption of the Housekeeping Gene Encoding Glucose 6-Phosphate Dehydrogenase (G6PD): G6PD Is Dispensable for Pentose Synthesis but Essential for Defense against Oxidative Stress. EMBO J. 1995, 14, 5209–5215. [Google Scholar] [CrossRef]
- Yang, H.-C.; Wu, Y.-H.; Yen, W.-C.; Liu, H.-Y.; Hwang, T.-L.; Stern, A.; Chiu, D.T.-Y. The Redox Role of G6PD in Cell Growth, Cell Death, and Cancer. Cells 2019, 8, 1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furukawa, S.; Fujita, T.; Shimabukuro, M.; Iwaki, M.; Yamada, Y.; Nakajima, Y.; Nakayama, O.; Makishima, M.; Matsuda, M.; Shimomura, I. Increased Oxidative Stress in Obesity and Its Impact on Metabolic Syndrome. J. Clin. Invest. 2004, 114, 1752–1761. [Google Scholar] [CrossRef] [PubMed]
- García-Sánchez, A.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. The Role of Oxidative Stress in Physiopathology and Pharmacological Treatment with Pro- and Antioxidant Properties in Chronic Diseases. Oxidative Med. Cell. Longev. 2020, 2020, 2082145. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-J.; Yen, C.-H.; Huang, Y.-C.; Lee, B.-J.; Hsia, S.; Lin, P.-T. Relationships between Inflammation, Adiponectin, and Oxidative Stress in Metabolic Syndrome. PLoS ONE 2012, 7, e45693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacher, P.; Beckman, J.S.; Liaudet, L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Awwad, A.; Poucheret, P.; Idres, A.Y.; Bidel, L.; Tousch, D. The Bitter Asteraceae: An Interesting Approach to Delay the Metabolic Syndrome Progression. NFS J. 2020, 18, 29–38. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxidative Med. Cell. Longev. 2016, 2016, e7432797. [Google Scholar] [CrossRef] [Green Version]
- Song, E.-K.; Park, H.; Kim, H.-S. Additive Effect of Walnut and Chokeberry on Regulation of Antioxidant Enzyme Gene Expression and Attenuation of Lipid Peroxidation in D-Galactose-Induced Aging-Mouse Model. Nutr. Res. 2019, 70, 60–69. [Google Scholar] [CrossRef]
- Alonso, M.R.; Anesini, C.A.; Martino, R.F. Anti-inflammatory Activity. In Sesquiterpene Lactones; Sülsen, V.P., Martino, V.S., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 325–346. ISBN 978-3-319-78273-7. [Google Scholar]
- Bork, P.M.; Schmitz, M.L.; Kuhnt, M.; Escher, C.; Heinrich, M. Sesquiterpene Lactone Containing Mexican Indian Medicinal Plants and Pure Sesquiterpene Lactones as Potent Inhibitors of Transcription Factor NF-ΚB. FEBS Lett. 1997, 402, 85–90. [Google Scholar] [CrossRef] [Green Version]
- Rüngeler, P.; Castro, V.; Mora, G.; Gören, N.; Vichnewski, W.; Pahl, H.L.; Merfort, I.; Schmidt, T.J. Inhibition of Transcription Factor NF-ΚB by Sesquiterpene Lactones: A Proposed Molecular Mechanism of Action. Bioorganic Med. Chem. 1999, 7, 2343–2352. [Google Scholar] [CrossRef]
- Umemura, K.; Itoh, T.; Hamada, N.; Fujita, Y.; Akao, Y.; Nozawa, Y.; Matsuura, N.; Iinuma, M.; Ito, M. Preconditioning by Sesquiterpene Lactone Enhances H2O2-Induced Nrf2/ARE Activation. Biochem. Biophys. Res. Commun. 2008, 368, 948–954. [Google Scholar] [CrossRef]
- Azay-Milhau, J.; Ferrare, K.; Leroy, J.; Aubaterre, J.; Tournier, M.; Lajoix, A.-D.; Tousch, D. Antihyperglycemic Effect of a Natural Chicoric Acid Extract of Chicory (Cichorium Intybus L.): A Comparative in Vitro Study with the Effects of Caffeic and Ferulic Acids. J. Ethnopharmacol. 2013, 150, 755–760. [Google Scholar] [CrossRef]
- Ferrare, K.; Bidel, L.P.R.; Awwad, A.; Poucheret, P.; Cazals, G.; Lazennec, F.; Azay-Milhau, J.; Tournier, M.; Lajoix, A.-D.; Tousch, D. Increase in Insulin Sensitivity by the Association of Chicoric Acid and Chlorogenic Acid Contained in a Natural Chicoric Acid Extract (NCRAE) of Chicory (Cichorium Intybus L.) for an Antidiabetic Effect. J. Ethnopharmacol. 2018, 215, 241–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El Khatib, N.; Morel, S.; Hugon, G.; Rapior, S.; Carnac, G.; Saint, N. Identification of a Sesquiterpene Lactone from Arctium Lappa Leaves with Antioxidant Activity in Primary Human Muscle Cells. Molecules 2021, 26, 1328. [Google Scholar] [CrossRef] [PubMed]
- Awwad, A.; Poucheret, P.; Idres, Y.A.; Tshibangu, D.S.T.; Servent, A.; Ferrare, K.; Lazennec, F.; Bidel, L.P.R.; Cazals, G.; Tousch, D. In Vitro Tests for a Rapid Evaluation of Antidiabetic Potential of Plant Species Containing Caffeic Acid Derivatives: A Validation by Two Well-Known Antidiabetic Plants, Ocimum Gratissimum L. Leaf and Musanga Cecropioides R. Br. Ex Tedlie (Mu) Stem Bark. Molecules 2021, 26, 5566. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Cosconati, S.; Forli, S.; Perryman, A.L.; Harris, R.; Goodsell, D.S.; Olson, A.J. Virtual Screening with AutoDock: Theory and Practice. Expert Opin. Drug Discov. 2010, 5, 597–607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koes, D.R.; Baumgartner, M.P.; Camacho, C.J. Lessons Learned in Empirical Scoring with Smina from the CSAR 2011 Benchmarking Exercise. J. Chem. Inf. Model. 2013, 53, 1893–1904. [Google Scholar] [CrossRef] [PubMed]
- Berman, H.M. The Protein Data Bank. Nucleic Acids Res. 2000, 28, 235–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An Open Chemical Toolbox. J. Cheminform 2011, 3, 33. [Google Scholar] [CrossRef] [Green Version]
- Zhi, X.; Jiang, L.; Li, T.; Song, L.; Wu, L.; Cao, H.; Yang, C. Natural Product-Based Semisynthesis and Biological Evaluation of Thiol/Amino-Michael Adducts of Xanthatin Derived from Xanthium Strumarium as Potential Pesticidal Agents. Bioorganic Chem. 2020, 97, 103696. [Google Scholar] [CrossRef]
- Ursini, M.V.; Parrella, A.; Rosa, G.; Salzano, S.; Martini, G. Enhanced Expression of Glucose-6-Phosphate Dehydrogenase in Human Cells Sustaining Oxidative Stress. Biochem. J. 1997, 323 Pt 3, 801–806. [Google Scholar] [CrossRef] [Green Version]
- Saddala, M.S.; Lennikov, A.; Huang, H. Discovery of Small-Molecule Activators for Glucose-6-Phosphate Dehydrogenase (G6PD) Using Machine Learning Approaches. IJMS 2020, 21, 1523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raub, A.G.; Hwang, S.; Horikoshi, N.; Cunningham, A.D.; Rahighi, S.; Wakatsuki, S.; Mochly-Rosen, D. Small-Molecule Activators of Glucose-6-Phosphate Dehydrogenase (G6PD) Bridging the Dimer Interface. ChemMedChem 2019, 14, 1321–1324. [Google Scholar] [CrossRef] [PubMed]
- Bonsignore, A.; Cancedda, R.; Lorenzoni, I.; Cosulich, M.E.; De Flora, A. Human Erythrocyte Glucose 6-Phosphate Dehydrogenase. Physical Properties. Biochem. Biophys. Res. Commun. 1971, 43, 94–101. [Google Scholar] [CrossRef]
- Wu, B.; Fukuo, K.; Suzuki, K.; Yoshino, G.; Kazumi, T. Relationships of Systemic Oxidative Stress to Body Fat Distribution, Adipokines and Inflammatory Markers in Healthy Middle-Aged Women. Endocr. J. 2009, 56, 773–782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonomini, F.; Rodella, L.F.; Rezzani, R. Metabolic Syndrome, Aging and Involvement of Oxidative Stress. Aging Dis. 2015, 6, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roberts, C.K.; Sindhu, K.K. Oxidative Stress and Metabolic Syndrome. Life Sci. 2009, 84, 705–712. [Google Scholar] [CrossRef]
- Liu, R.; Shi, D.; Zhang, J.; Li, X.; Han, X.; Yao, X.; Fang, J. Xanthatin Promotes Apoptosis via Inhibiting Thioredoxin Reductase and Eliciting Oxidative Stress. Mol. Pharm. 2018, 15, 3285–3296. [Google Scholar] [CrossRef]
- Ramírez-Erosa, I.; Huang, Y.; Hickie, R.A.; Sutherland, R.G.; Barl, B. Xanthatin and Xanthinosin from the Burs of Xanthium Strumarium L. as Potential Anticancer AgentsThis Article Is One of a Selection of Papers Published in This Special Issue (Part 2 of 2) on the Safety and Efficacy of Natural Health Products. Can. J. Physiol. Pharmacol. 2007, 85, 1160–1172. [Google Scholar] [CrossRef]
- Kim, I.-T.; Park, Y.-M.; Won, J.-H.; Jung, H.-J.; Park, H.-J.; Choi, J.-W.; Lee, K.-T. Methanol Extract of Xanthium Strumarium L. Possesses Anti-Inflammatory and Anti-Nociceptive Activities. Biol. Pharm. Bull. 2005, 28, 94–100. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Ruan, J.; Yan, L.; Li, W.; Wu, Y.; Tao, L.; Zhang, F.; Zheng, S.; Wang, A.; Lu, Y. Xanthatin Induces Cell Cycle Arrest at G2/M Checkpoint and Apoptosis via Disrupting NF-ΚB Pathway in A549 Non-Small-Cell Lung Cancer Cells. Molecules 2012, 17, 3736–3750. [Google Scholar] [CrossRef] [Green Version]
- Merfort, I. Perspectives on Sesquiterpene Lactones in Inflammation and Cancer. CDT 2011, 12, 1560–1573. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Mendoza, N.; Morales-González, Á.; Madrigal-Santillán, E.O.; Madrigal-Bujaidar, E.; Álvarez-González, I.; García-Melo, L.F.; Anguiano-Robledo, L.; Fregoso-Aguilar, T.; Morales-Gonzalez, J.A. Antioxidant and Adaptative Response Mediated by Nrf2 during Physical Exercise. Antioxidants 2019, 8, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soto, M.E.; Soria-Castro, E.; Guarner Lans, V.; Muruato Ontiveros, E.; Iván Hernández Mejía, B.; Jorge Martínez Hernandez, H.; Barragán García, R.; Herrera, V.; Pérez-Torres, I. Analysis of Oxidative Stress Enzymes and Structural and Functional Proteins on Human Aortic Tissue from Different Aortopathies. Oxidative Med. Cell. Longev. 2014, 2014, 760694. [Google Scholar] [CrossRef] [Green Version]
- He, F.; Antonucci, L.; Karin, M. NRF2 as a Regulator of Cell Metabolism and Inflammation in Cancer. Carcinogenesis 2020, 41, 405–416. [Google Scholar] [CrossRef]
- Finkel, T.; Holbrook, N.J. Oxidants, Oxidative Stress and the Biology of Ageing. Nature 2000, 408, 239–247. [Google Scholar] [CrossRef]
- Garcia, A.A.; Koperniku, A.; Ferreira, J.C.B.; Mochly-Rosen, D. Treatment Strategies for Glucose-6-Phosphate Dehydrogenase Deficiency: Past and Future Perspectives. Trends Pharmacol. Sci. 2021, 42, 829–844. [Google Scholar] [CrossRef]
- Dong, J.; Sulik, K.K.; Chen, S. Nrf2-Mediated Transcriptional Induction of Antioxidant Response in Mouse Embryos Exposed to Ethanol in Vivo: Implications for the Prevention of Fetal Alcohol Spectrum Disorders. Antioxid. Redox Signal. 2008, 10, 2023–2033. [Google Scholar] [CrossRef] [Green Version]
- Formisano, C.; Sanna, C.; Ballero, M.; Chianese, G.; Sirignano, C.; Rigano, D.; Millán, E.; Muñoz, E.; Taglialatela-Scafati, O. Anti-Inflammatory Sesquiterpene Lactones from Onopordum Illyricum L. (Asteraceae), an Italian Medicinal Plant. Fitoterapia 2017, 116, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Menegon, S.; Columbano, A.; Giordano, S. The Dual Roles of NRF2 in Cancer. Trends Mol. Med. 2016, 22, 578–593. [Google Scholar] [CrossRef]
- Lau, A.; Villeneuve, N.; Sun, Z.; Wong, P.; Zhang, D. Dual Roles of Nrf2 in Cancer. Pharmacol. Res. 2008, 58, 262–270. [Google Scholar] [CrossRef]
- Sadeghi, M.R.; Jeddi, F.; Soozangar, N.; Somi, M.H.; Samadi, N. The Role of Nrf2-Keap1 Axis in Colorectal Cancer, Progression, and Chemoresistance. Tumour Biol. 2017, 39, 101042831770551. [Google Scholar] [CrossRef] [Green Version]
- Salvemini, F.; Franzé, A.; Iervolino, A.; Filosa, S.; Salzano, S.; Ursini, M.V. Enhanced Glutathione Levels and Oxidoresistance Mediated by Increased Glucose-6-Phosphate Dehydrogenase Expression. J. Biol. Chem. 1999, 274, 2750–2757. [Google Scholar] [CrossRef] [Green Version]
- Frederiks, W.M.; Bosch, K.S.; De Jong, J.S.S.G.; Van Noorden, C.J.F. Post-Translational Regulation of Glucose-6-Phosphate Dehydrogenase Activity in (Pre)Neoplastic Lesions in Rat Liver. J Histochem Cytochem. 2003, 51, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Hwang, S.; Mruk, K.; Rahighi, S.; Raub, A.G.; Chen, C.-H.; Dorn, L.E.; Horikoshi, N.; Wakatsuki, S.; Chen, J.K.; Mochly-Rosen, D. Correcting Glucose-6-Phosphate Dehydrogenase Deficiency with a Small-Molecule Activator. Nat. Commun. 2018, 9, 4045. [Google Scholar] [CrossRef]
- Nóbrega-Pereira, S.; Fernandez-Marcos, P.J.; Brioche, T.; Gomez-Cabrera, M.C.; Salvador-Pascual, A.; Flores, J.M.; Viña, J.; Serrano, M. G6PD Protects from Oxidative Damage and Improves Healthspan in Mice. Nat. Commun. 2016, 7, 10894. [Google Scholar] [CrossRef] [Green Version]
- Bermúdez-Muñoz, J.M.; Celaya, A.M.; Hijazo-Pechero, S.; Wang, J.; Serrano, M.; Varela-Nieto, I. G6PD Overexpression Protects from Oxidative Stress and Age-related Hearing Loss. Aging Cell 2020, 19. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.C. Glutathione Synthesis. Biochim. Et Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 3143–3153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.S.; Chang, L.S.; Anderson, M.E.; Meister, A. Catalytic and Regulatory Properties of the Heavy Subunit of Rat Kidney Gamma-Glutamylcysteine Synthetase. J. Biol. Chem. 1993, 268, 19675–19680. [Google Scholar] [CrossRef]
- Yang, Y.; Chen, Y.; Johansson, E.; Schneider, S.N.; Shertzer, H.G.; Nebert, D.W.; Dalton, T.P. Interaction between the Catalytic and Modifier Subunits of Glutamate-Cysteine Ligase. Biochem. Pharmacol. 2007, 74, 372–381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Won, Y.-K.; Ong, C.-N.; Shen, H.-M. Anti-Cancer Potential of Sesquiterpene Lactones: Bioactivity and Molecular Mechanisms. CMCACA 2005, 5, 239–249. [Google Scholar] [CrossRef]




| SOD | G6PD | GPx | |
|---|---|---|---|
| ΔΔCt ± SD | −0.14 ± 0.11 | −0.75 ± 0.12 | 0.63 ± 0.21 |
| 2−ΔΔCt ± SD | 1.10 ± 0.09 | 1.69 ± 0.15 | 0.66 ± 0.14 |
| Pose | Affinity (Kcal·mole−1) | Amino Acids of Monomer 1 | Amino Acids of Monomer 2 |
|---|---|---|---|
| Pose A | −6.4 | ASN-426 | THR-423 |
| ASP-421 | ASN-426 | ||
| GLU-419 | ARG-427 | ||
| Pose B | −5.8 | ASN-426 | ASP-421 |
| ASP-421 | ASN-426 | ||
| ARG-427 | |||
| Pose C | −5.6 | ASN-426 | ASN-426 |
| ASP-421 | ARG-427 | ||
| Pose D | −5.1 | ASP-421 | ASP-421 |
| ASN-397 | ASN-426 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Idres, Y.A.; Tousch, D.; Cazals, G.; Lebrun, A.; Naceri, S.; Bidel, L.P.R.; Poucheret, P. A Novel Sesquiterpene Lactone Xanthatin-13-(pyrrolidine-2-carboxylic acid) Isolated from Burdock Leaf Up-Regulates Cells’ Oxidative Stress Defense Pathway. Antioxidants 2021, 10, 1617. https://doi.org/10.3390/antiox10101617
Idres YA, Tousch D, Cazals G, Lebrun A, Naceri S, Bidel LPR, Poucheret P. A Novel Sesquiterpene Lactone Xanthatin-13-(pyrrolidine-2-carboxylic acid) Isolated from Burdock Leaf Up-Regulates Cells’ Oxidative Stress Defense Pathway. Antioxidants. 2021; 10(10):1617. https://doi.org/10.3390/antiox10101617
Chicago/Turabian StyleIdres, Yanis A., Didier Tousch, Guillaume Cazals, Aurélien Lebrun, Sarah Naceri, Luc P. R. Bidel, and Patrick Poucheret. 2021. "A Novel Sesquiterpene Lactone Xanthatin-13-(pyrrolidine-2-carboxylic acid) Isolated from Burdock Leaf Up-Regulates Cells’ Oxidative Stress Defense Pathway" Antioxidants 10, no. 10: 1617. https://doi.org/10.3390/antiox10101617