Hydroxytyrosol-Fortified Foods Obtained by Supercritical Fluid Extraction of Olive Oil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Food Samples
2.2. Chemicals and Standards
2.3. Fortified Samples Preparation-Supercritical Fluid CO2 Extraction (SFE)
2.4. Instrumental Analysis
2.4.1. Sample Preparation for HPLC-UV/MS Analysis
2.4.2. Sample Preparation for Mass Spectrometric Analysis
2.4.3. HPLC-UV/MS
2.4.4. Mass Spectrometry
2.5. Antioxidant Capacity Assays
2.5.1. Sample Preparation
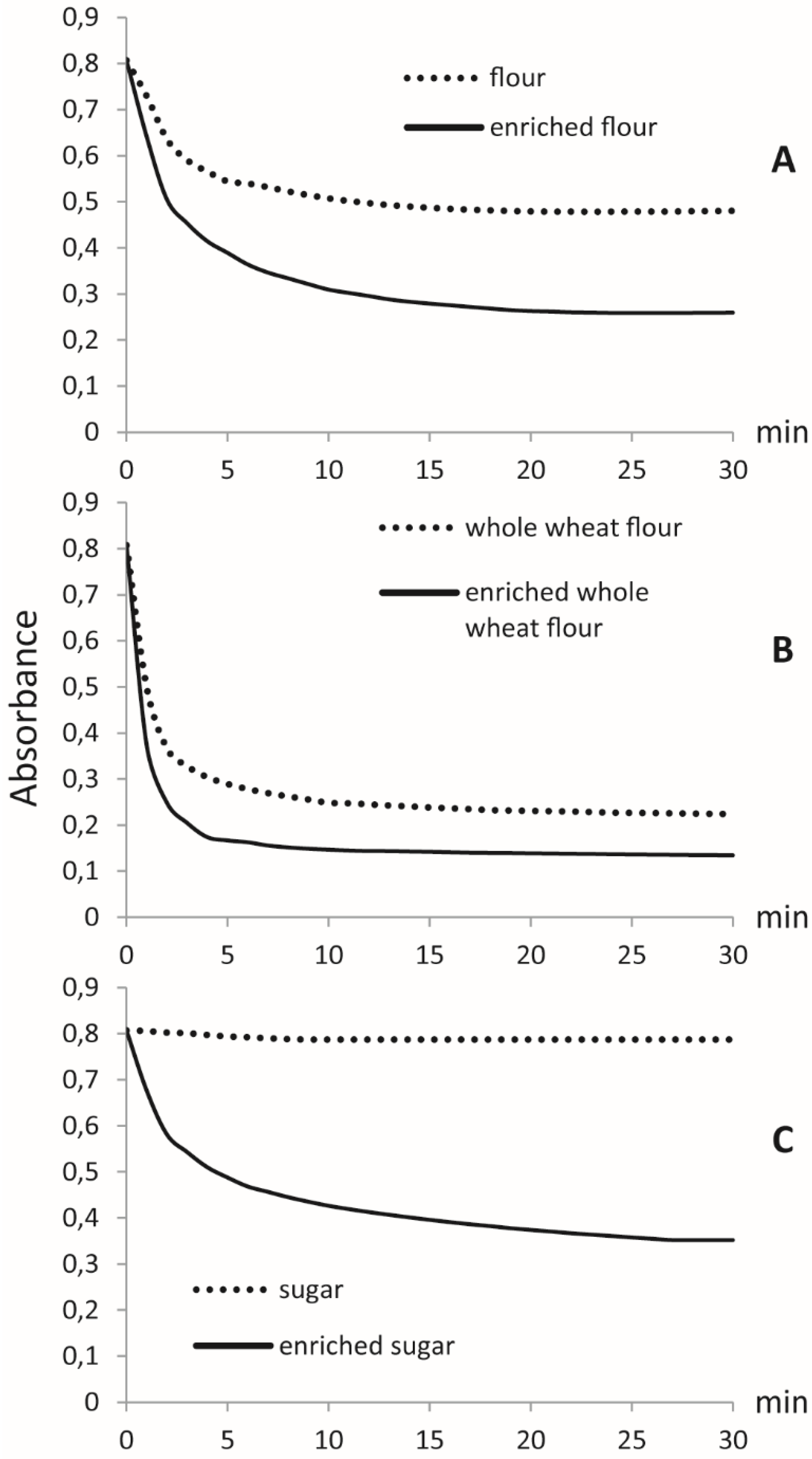
2.5.2. DPPH Radical Scavenging Activity
3. Results
Supercritical CO2 Fluid Extraction to Produce Fortified Foodstuff
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shahidi, F. Nutraceuticals and functional foods: Whole versus processed foods. Trends Food Sci. Tech. 2009, 20, 376–387. [Google Scholar] [CrossRef]
- Food and Agriculture Organization. FAO Term Portal: Entry: 170967—Collection: FAOTERM. 2005. Available online: http://www.fao.org/faoterm/viewentry/en/?entryId=170967 (accessed on 1 September 2020).
- Gul, K.; Singh, A.K.; Jabeen, R. Nutraceuticals and Functional Foods: The Foods for the Future World. Crit. Rev. Food Sci. Nutr. 2016, 56, 2617–2627. [Google Scholar] [CrossRef] [PubMed]
- Bartella, L.; Di Donna, L.; Napoli, A.; Sindona, G.; Mazzotti, F. High-throughput determination of vitamin E in extra virgin olive oil by paper spray tandem mass spectrometry. Anal. Bioanal. Chem. 2019, 411, 2885–2890. [Google Scholar] [CrossRef] [PubMed]
- Grand View Research. Market Research Report. 2016. Available online: https://www.grandviewresearch.com/industry-analysis/functional-food-market/ (accessed on 1 September 2020).
- Commission of the European Communities. Commission Regulation No 432/2012 of 16 May 2012 Establishing a List of Permitted Health Claims Made on Foods, Other than Those Referring to the Reduction of Disease Risk and to Children’s Development and Health. Official Journal of the European Union. 2012. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2012:136:0001:0040:EN:PDF/ (accessed on 1 September 2020).
- Bulotta, S.; Celano, M.; Lepore, S.M.; Montalcini, T.; Pujia, A.; Russo, D. Beneficial effects of the olive oil phenolic components oleuropein and hydroxytyrosol: Focus on protection against cardiovascular and metabolic diseases. J. Transl. Med. 2014, 12, 219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Fidalgo, S.; Sánchez De Ibargüen, L.; Cárdeno, A.; Alarcón De La Lastra, C. Influence of extra virgin olive oil diet enriched with hydroxytyrosol in a chronic DSS colitis model. Eur. J. Nutr. 2012, 51, 497–506. [Google Scholar] [CrossRef] [PubMed]
- Burattini, S.; Salucci, S.; Baldassarri, V.; Accorsi, A.; Piatti, E.; Madrona, A.; Espartero, J.L.; Candiracci, M.; Zappia, G.; Falcieri, E. Anti-apoptotic activity of hydroxytyrosol and hydroxytyrosyl laurate. Food Chem. Toxicol. 2013, 55, 248–256. [Google Scholar] [CrossRef]
- Takeda, Y.; Bui, V.N.; Iwasaki, K.; Kobayashi, T.; Ogawa, H.; Imai, K. Influence of olive-derived hydroxytyrosol on the toll-like receptor 4-dependent inflammatory response of mouse peritoneal macrophages. Biochem. Biophys. Res. Commun. 2014, 446, 1225–1230. [Google Scholar] [CrossRef]
- Bisignano, C.; Filocamo, A.; Ginestra, G.; Giofre’, S.V.; Navarra, M.; Romeo, R.; Mandalari, G. 3,4-DHPEA-EA from Olea Europaea L. is effective against standard and clinical isolates of Staphylococcus sp. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Auñon-Calles, D.; Canut, L.; Visioli, F. Toxicological evaluation of pure hydroxytyrosol. Food Chem. Toxicol. 2013, 55, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Baiano, A.; Gambacorta, G.; Terracone, C.; Previtali, M.A.; Lamacchia, C.; La Notte, E. Changes in phenolic content and antioxidant activity of italian extra-virgin olive oils during storage. J. Food Sci. 2009, 74, C177–C183. [Google Scholar] [CrossRef] [PubMed]
- Brenes, M.; García, A.; García, P.; Garrido, A. Acid hydrolysis of secoiridoid aglycons during storage of virgin olive oil. J. Agric. Food Chem. 2001, 49, 5609–5614. [Google Scholar] [CrossRef] [PubMed]
- Stefanoudaki, E.; Williams, M.; Harwood, J. Changes in virgin olive oil characteristics during different storage conditions. Eur. J. Lipid Sci. Technol. 2010, 112, 906–914. [Google Scholar] [CrossRef]
- Morelló, J.R.; Motilva, M.J.; Tovar, M.J.; Romero, M.P. Changes in commercial virgin olive oil (cv Arbequina) during storage, with special emphasis on the phenolic fraction. Food Chem. 2004, 85, 357–364. [Google Scholar] [CrossRef]
- Kuwajima, H.; Takai, Y.; Takaishi, K.; Inoue, K. Synthesis of 13C-labeled possible intermediates in the biosynthesis of phenylethanoid derivatives, cornoside and rengyosides. Chem. Pharm. Bull. 1998, 46, 581–586. [Google Scholar] [CrossRef] [Green Version]
- Bartella, L.; Mazzotti, F.; Napoli, A.; Sindona, G.; Di Donna, L. A comprehensive evaluation of tyrosol and hydroxytyrosol derivatives in extra virgin olive oil by microwave-assisted hydrolysis and HPLC-MS/MS. Anal. Bioanal. Chem. 2018, 410, 2193–2201. [Google Scholar] [CrossRef] [PubMed]
- Bartella, L.; Mazzotti, F.; Sindona, G.; Napoli, A.; Di Donna, L. Rapid determination of the free and total hydroxytyrosol and tyrosol content in extra virgin olive oil by stable isotope dilution analysis and paper spray tandem mass spectrometry. Food Chem. Toxicol. 2020, 136, 111110. [Google Scholar] [CrossRef] [PubMed]
- Pyrzynska, K.; Pȩkal, A. Application of free radical diphenylpicrylhydrazyl (DPPH) to estimate the antioxidant capacity of food samples. Anal. Methods 2013, 5, 4288–4295. [Google Scholar] [CrossRef]
- Del Valle, J.M.; Aguilera, J.M. Review: High pressure CO2 extraction. Fundamentals and applications in the food industry. Rev. Agaroquimica Tecnol. Aliment. 1999, 5, 1–24. [Google Scholar] [CrossRef]
- Raventòs, M.; Duarte, S.; Alarcón, R. Application and Possibilities of Supercritical CO2 Extraction in Food Processing Industry: An Overview. Food Sci. Technol. Int. 2002, 8, 269–284. [Google Scholar] [CrossRef]



| Amount of Hydroxytyrosol (µg) | Transfer Yield % | |
|---|---|---|
| Olive oil | 82.32 | n/a * |
| Flour | 82.24 | 99.90 |
| Whole-wheat flour | 81.20 | 98.64 |
| Sugar | 81.92 | 99.51 |
| Sample | % Inhibition |
|---|---|
| Flour Enriched flour | 38.80 64.38 |
| Whole wheat flour Enriched whole wheat flour | 74.65 86.11 |
| Sugar Enriched sugar | 2.55 58.28 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartella, L.; Mazzotti, F.; Talarico, I.R.; Santoro, I.; Di Donna, L. Hydroxytyrosol-Fortified Foods Obtained by Supercritical Fluid Extraction of Olive Oil. Antioxidants 2021, 10, 1619. https://doi.org/10.3390/antiox10101619
Bartella L, Mazzotti F, Talarico IR, Santoro I, Di Donna L. Hydroxytyrosol-Fortified Foods Obtained by Supercritical Fluid Extraction of Olive Oil. Antioxidants. 2021; 10(10):1619. https://doi.org/10.3390/antiox10101619
Chicago/Turabian StyleBartella, Lucia, Fabio Mazzotti, Ines Rosita Talarico, Ilaria Santoro, and Leonardo Di Donna. 2021. "Hydroxytyrosol-Fortified Foods Obtained by Supercritical Fluid Extraction of Olive Oil" Antioxidants 10, no. 10: 1619. https://doi.org/10.3390/antiox10101619





