Olive Leaf Extract Supplementation to Old Wistar Rats Attenuates Aging-Induced Sarcopenia and Increases Insulin Sensitivity in Adipose Tissue and Skeletal Muscle
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. In Vivo Study
2.2.1. Animals
2.2.2. Treatment
2.2.3. Incubation in Presence/Absence of Insulin (10−7 M) of Epididymal Adipose Tissue and Gastrocnemius Muscle Explants
2.2.4. Western Blot
2.2.5. RNA Extraction and Purification
2.2.6. Quantitative Real-Time PCR
2.2.7. Statistical Analysis
3. Results
3.1. Adipose Tissue Depots
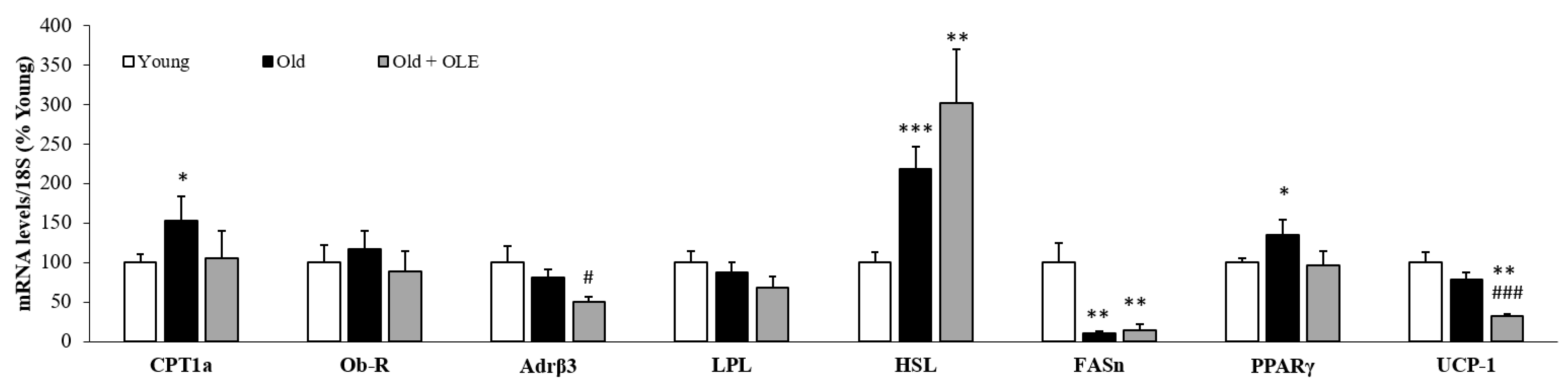
3.2. mRNA Levels of Lipid Metabolism Related Enzymes in White Adipose Tissue
3.3. mRNA Levels of Lipid Metabolism and Thermogenesis-Related Enzymes in Brown Adipose Tissue
3.4. Body Weight and Protein Content in Soleus and Gastrocnemius Muscles
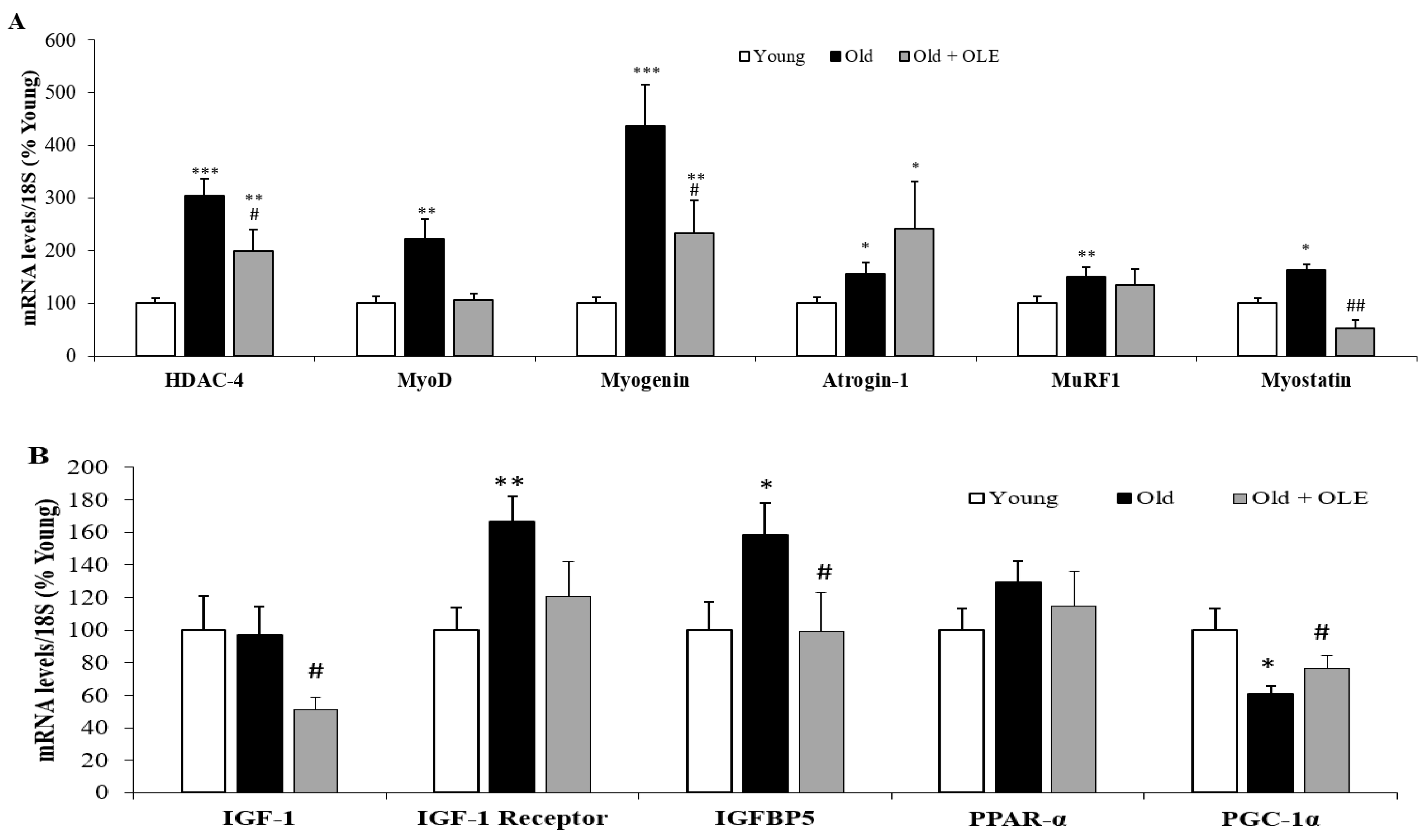
3.5. Gene Expression of Sarcopenia-Related Markers in Gastrocnemius Muscle
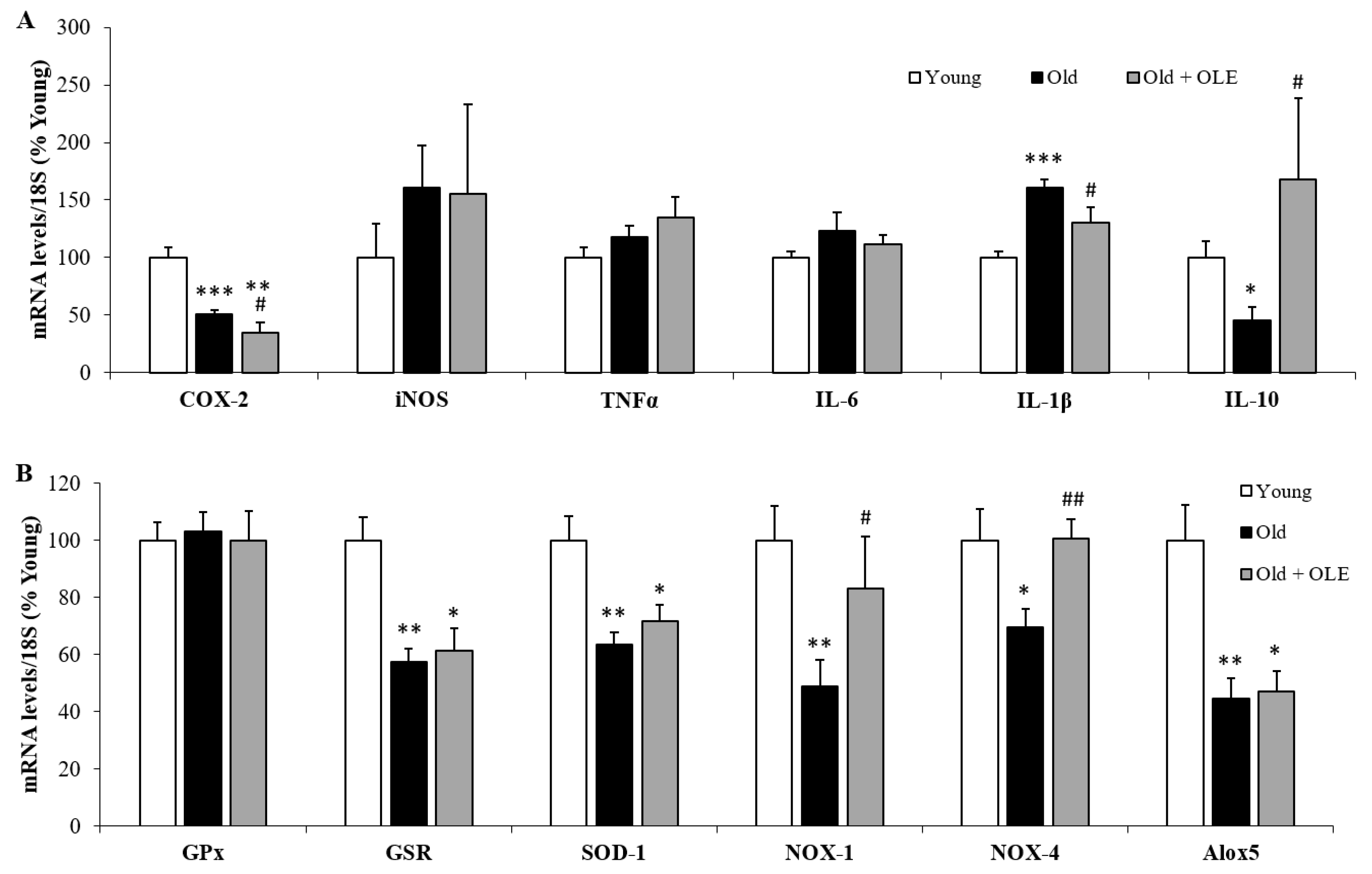
3.6. Gene Expression of Oxidative Stress and Inflammatory Markers in Gastrocnemius Muscle
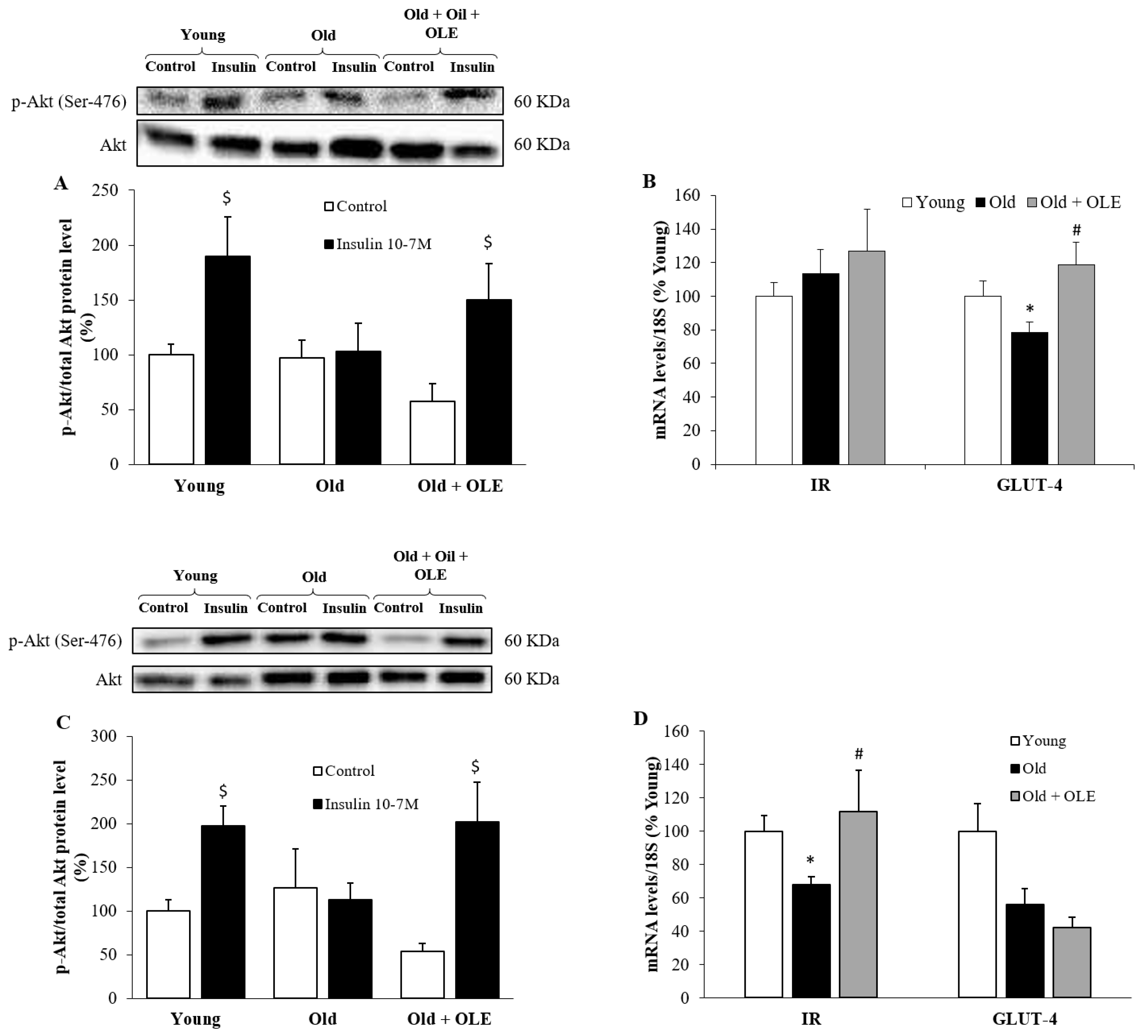
3.7. Insulin-Induced Activation of PI3K/Akt Pathway and mRNA Levels of IR and GLUT-4 in Visceral Adipose Tissue and Gastrocnemius Muscle
3.8. Gene Expression of Oxidative Stress and Inflammatory Markers in Visceral Epididymal Adipose Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jafari Nasabian, P.; Inglis, J.E.; Reilly, W.; Kelly, O.J.; Ilich, J.Z. Aging human body: Changes in bone, muscle and body fat with consequent changes in nutrient intake. J. Endocrinol. 2017, 234, R37–R51. [Google Scholar] [CrossRef] [Green Version]
- Huxley, R.; Mendis, S.; Zheleznyakov, E.; Reddy, S.; Chan, J. Body mass index, waist circumference and waist:hip ratio as predictors of cardiovascular risk--a review of the literature. Eur. J. Clin. Nutr. 2010, 64, 16–22. [Google Scholar] [CrossRef] [Green Version]
- Walston, J.D. Sarcopenia in older adults. Curr. Opin. Rheumatol. 2012, 24, 623–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priego, T.; Martín, A.I.; González-Hedström, D.; Granado, M.; López-Calderón, A. Chapter twenty—Role of hormones in sarcopenia. Vitam. Horm. 2021, 115, 535–570. [Google Scholar] [CrossRef]
- Chai, R.J.; Vukovic, J.; Dunlop, S.; Grounds, M.D.; Shavlakadze, T. Striking denervation of neuromuscular junctions without lumbar motoneuron loss in geriatric mouse muscle. PLoS ONE 2011, 6, e28090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verdijk, L.B.; Dirks, M.L.; Snijders, T.; Prompers, J.J.; Beelen, M.; Jonkers, R.A.; Thijssen, D.H.; Hopman, M.T.; Van Loon, L.J. Reduced satellite cell numbers with spinal cord injury and aging in humans. Med. Sci. Sports Exerc. 2012, 44, 2322–2330. [Google Scholar] [CrossRef] [PubMed]
- Dalle, S.; Rossmeislova, L.; Koppo, K. The role of inflammation in age-related sarcopenia. Front. Physiol. 2017, 8, 1045. [Google Scholar] [CrossRef] [Green Version]
- Ascenzi, F.; Barberi, L.; Dobrowolny, G.; Villa Nova Bacurau, A.; Nicoletti, C.; Rizzuto, E.; Rosenthal, N.; Scicchitano, B.M.; Musaro, A. Effects of IGF-1 isoforms on muscle growth and sarcopenia. Aging Cell 2019, 18, e12954. [Google Scholar] [CrossRef] [PubMed]
- Gumucio, J.P.; Mendias, C.L. Atrogin-1, MuRF-1, and sarcopenia. Endocrine 2013, 43, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Yarasheski, K.E.; Bhasin, S.; Sinha-Hikim, I.; Pak-Loduca, J.; Gonzalez-Cadavid, N.F. Serum myostatin-immunoreactive protein is increased in 60–92 year old women and men with muscle wasting. J. Nutr. Health Aging 2002, 6, 343–348. [Google Scholar]
- Elliott, B.; Renshaw, D.; Getting, S.; Mackenzie, R. The central role of myostatin in skeletal muscle and whole body homeostasis. Acta Physiol. 2012, 205, 324–340. [Google Scholar] [CrossRef]
- McPherron, A.C. The ups and downs of exercise and insulin sensitivity: A role for the myokine myostatin in glucose metabolism? Acta Physiol. 2016, 217, 6–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alway, S.E.; Lowe, D.A.; Chen, K.D. The effects of age and hindlimb supension on the levels of expression of the myogenic regulatory factors MyoD and myogenin in rat fast and slow skeletal muscles. Exp. Physiol. 2001, 86, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Martin, S.C.; Parkington, J.; Cadena, S.M.; Zhu, J.; Ibebunjo, C.; Summermatter, S.; Londraville, N.; Patora-Komisarska, K.; Widler, L.; et al. HDAC4 controls muscle homeostasis through deacetylation of myosin heavy chain, PGC-1alpha, and Hsc70. Cell Rep. 2019, 29, 749–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Hedström, D.; Priego, T.; López-Calderón, A.; Amor, S.; de la Fuente-Fernández, M.; Inarejos-García, A.M.; García-Villalón, A.L.; Martín, A.I.; Granado, M. Beneficial effects of a mixture of algae and extra virgin olive oils on the age-induced alterations of rodent skeletal muscle: Role of HDAC-4. Nutrients 2020, 13, 44. [Google Scholar] [CrossRef]
- Ibebunjo, C.; Chick, J.M.; Kendall, T.; Eash, J.K.; Li, C.; Zhang, Y.; Vickers, C.; Wu, Z.; Clarke, B.A.; Shi, J.; et al. Genomic and proteomic profiling reveals reduced mitochondrial function and disruption of the neuromuscular junction driving rat sarcopenia. Mol. Cell Biol. 2013, 33, 194–212. [Google Scholar] [CrossRef] [Green Version]
- Zahn, J.M.; Sonu, R.; Vogel, H.; Crane, E.; Mazan-Mamczarz, K.; Rabkin, R.; Davis, R.W.; Becker, K.G.; Owen, A.B.; Kim, S.K. Transcriptional profiling of aging in human muscle reveals a common aging signature. PLoS Genet. 2006, 2, e115. [Google Scholar] [CrossRef]
- Schwartz, R.S.; Shuman, W.P.; Bradbury, V.L.; Cain, K.C.; Fellingham, G.W.; Beard, J.C.; Kahn, S.E.; Stratton, J.R.; Cerqueira, M.D.; Abrass, I.B. Body fat distribution in healthy young and older men. J. Gerontol. 1990, 45, M181–M185. [Google Scholar] [CrossRef]
- Camell, C.D.; Sander, J.; Spadaro, O.; Lee, A.; Nguyen, K.Y.; Wing, A.; Goldberg, E.L.; Youm, Y.H.; Brown, C.W.; Elsworth, J.; et al. Inflammasome-driven catecholamine catabolism in macrophages blunts lipolysis during ageing. Nature 2017, 550, 119–123. [Google Scholar] [CrossRef] [PubMed]
- Zoico, E.; Rubele, S.; De Caro, A.; Nori, N.; Mazzali, G.; Fantin, F.; Rossi, A.; Zamboni, M. Brown and Beige Adipose Tissue and Aging. Front. Endocrinol. 2019, 10, 368. [Google Scholar] [CrossRef] [Green Version]
- McDonald, R.B.; Horwitz, B.A. Brown adipose tissue thermogenesis during aging and senescence. J. Bioenerg. Biomembr. 1999, 31, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, P.; Bouchard, B. The impact of aging on adipose function and adipokine synthesis. Front. Endocrinol. 2019, 10, 137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dziegielewska-Gesiak, S.; Stoltny, D.; Brozek, A.; Muc-Wierzgon, M.; Wysocka, E. Are insulin-resistance and oxidative stress cause or consequence of aging. Exp. Biol. Med. 2020, 245, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Park, M.H.; Kim, D.H.; Lee, E.K.; Kim, N.D.; Im, D.S.; Lee, J.; Yu, B.P.; Chung, H.Y. Age-related inflammation and insulin resistance: A review of their intricate interdependency. Arch. Pharm. Res. 2014, 37, 1507–1514. [Google Scholar] [CrossRef] [Green Version]
- Rudich, A.; Tirosh, A.; Potashnik, R.; Hemi, R.; Kanety, H.; Bashan, N. Prolonged oxidative stress impairs insulin-induced GLUT4 translocation in 3T3-L1 adipocytes. Diabetes 1998, 47, 1562–1569. [Google Scholar] [CrossRef]
- Stephens, J.M.; Lee, J.; Pilch, P.F. Tumor necrosis factor-alpha-induced insulin resistance in 3T3-L1 adipocytes is accompanied by a loss of insulin receptor substrate-1 and GLUT4 expression without a loss of insulin receptor-mediated signal transduction. J. Biol. Chem. 1997, 272, 971–976. [Google Scholar] [CrossRef] [Green Version]
- DeFronzo, R.A. Insulin resistance, hyperinsulinemia, and coronary artery disease: A complex metabolic web. J. Cardiovasc. Pharmacol. 1992, 20 (Suppl. 11), S1–S16. [Google Scholar] [CrossRef]
- Lara-Castro, C.; Garvey, W.T. Intracellular lipid accumulation in liver and muscle and the insulin resistance syndrome. Endocrinol. Metab. Clin. N. Am. 2008, 37, 841–856. [Google Scholar] [CrossRef] [Green Version]
- Shou, J.; Chen, P.J.; Xiao, W.H. Mechanism of increased risk of insulin resistance in aging skeletal muscle. Diabetol. Metab. Syndr. 2020, 12, 14. [Google Scholar] [CrossRef] [Green Version]
- Camporez, J.P.; Petersen, M.C.; Abudukadier, A.; Moreira, G.V.; Jurczak, M.J.; Friedman, G.; Haqq, C.M.; Petersen, K.F.; Shulman, G.I. Anti-myostatin antibody increases muscle mass and strength and improves insulin sensitivity in old mice. Proc. Natl. Acad. Sci. USA 2016, 113, 2212–2217. [Google Scholar] [CrossRef] [Green Version]
- Chang, A.Y.; Skirbekk, V.F.; Tyrovolas, S.; Kassebaum, N.J.; Dieleman, J.L. Measuring population ageing: An analysis of the global burden of disease study 2017. Lancet Public Health 2019, 4, e159–e167. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, C.K. Functional foods, herbs and nutraceuticals: Towards biochemical mechanisms of healthy aging. Biogerontology 2004, 5, 275–289. [Google Scholar] [CrossRef]
- Perez-Lopez, F.R.; Chedraui, P.; Haya, J.; Cuadros, J.L. Effects of the Mediterranean diet on longevity and age-related morbid conditions. Maturitas 2009, 64, 67–79. [Google Scholar] [CrossRef]
- Tapsell, L.C. Foods and food components in the Mediterranean diet: Supporting overall effects. BMC Med. 2014, 12, 100. [Google Scholar] [CrossRef] [Green Version]
- Alkhatib, A.; Tsang, C.; Tuomilehto, J. Olive oil nutraceuticals in the prevention and management of diabetes: From molecules to lifestyle. Int. J. Mol. Sci. 2018, 19, 2024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, P.; Kasper Machado, I.; Garavaglia, J.; Zani, V.T.; de Souza, D.; Morelo Dal Bosco, S. Polyphenols benefits of olive leaf (Olea europaea L.) to human health. Nutr. Hosp. 2014, 31, 1427–1433. [Google Scholar] [CrossRef] [PubMed]
- González-Hedström, D.; García-Villalón, A.; Amor, S.; de la Fuente-Fernández, M.; Almodóvar, P.; Prodanov, M.; Priego, T.; Martín, A.I.; Inarejos-García, A.M.; Granado, M. Supplementation with an olive leaf (Olea europaea L.) extract improves the vascular and metabolic alterations associated with metabolic syndrome in old Wistar rats. Sci. Rep. 2021, 14, 8188. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Chomczynski, P. A reagent for the single-step simultaneous isolation of RNA, DNA and proteins from cell and tissue samples. Biotechniques 1993, 15, 532–534, 536–537. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Coban, J.; Oztezcan, S.; Dogru-Abbasoglu, S.; Bingul, I.; Yesil-Mizrak, K.; Uysal, M. Olive leaf extract decreases age-induced oxidative stress in major organs of aged rats. Geriatr. Gerontol. Int. 2014, 14, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Ramos, M.F.P.; Oliveira, O.B.; de Barros, A.; Razvickas, C.V.; Pessoa, E.A.; da Silva, R.F.; Pereira, A.M.S.; Convento, M.B.; Borges, F.T.; Schor, N. Comparison of olive leaf, olive oil, palm oil, and omega-3 oil in acute kidney injury induced by sepsis in rats. Peer J. 2019, 7, e7219. [Google Scholar] [CrossRef]
- Hong, Y.H.; Song, C.; Shin, K.K.; Choi, E.; Hwang, S.H.; Jang, Y.J.; Taamalli, A.; Yum, J.; Kim, J.H.; Kim, E.; et al. Tunisian Olea europaea L. leaf extract suppresses Freund’s complete adjuvant-induced rheumatoid arthritis and lipopolysaccharide-induced inflammatory responses. J. Ethnopharmacol. 2021, 268, 113602. [Google Scholar] [CrossRef]
- Al-Quraishy, S.; Othman, M.S.; Dkhil, M.A.; Abdel Moneim, A.E. Olive (Olea europaea) leaf methanolic extract prevents HCl/ethanol-induced gastritis in rats by attenuating inflammation and augmenting antioxidant enzyme activities. Biomed. Pharmacother. 2017, 91, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Mohagheghi, F.; Bigdeli, M.R.; Rasoulian, B.; Hashemi, P.; Pour, M.R. The neuroprotective effect of olive leaf extract is related to improved blood-brain barrier permeability and brain edema in rat with experimental focal cerebral ischemia. Phytomedicine 2011, 18, 170–175. [Google Scholar] [CrossRef]
- Dekanski, D.; Selakovic, V.; Piperski, V.; Radulovic, Z.; Korenic, A.; Radenovic, L. Protective effect of olive leaf extract on hippocampal injury induced by transient global cerebral ischemia and reperfusion in Mongolian gerbils. Phytomedicine 2011, 18, 1137–1143. [Google Scholar] [CrossRef] [PubMed]
- Motlagh, S.; Vessal, M.; Arabsolghar, R. The protective effects of Olive leaf extract on Type 2 diabetes, the expression of liver superoxide dismutase and total antioxidant capacity of plasma in rats. Trends Pharm. Sci. 2020, 6, 43–52. [Google Scholar]
- Fki, I.; Sayadi, S.; Mahmoudi, A.; Daoued, I.; Marrekchi, R.; Ghorbel, H. Comparative study on beneficial effects of hydroxytyrosol- and oleuropein-rich olive leaf extracts on high-fat diet-induced lipid metabolism disturbance and liver injury in rats. Biomed. Res. Int. 2020, 2020, 1315202. [Google Scholar] [CrossRef]
- Mahmoudi, A.; Hadrich, F.; Feki, I.; Ghorbel, H.; Bouallagui, Z.; Marrekchi, R.; Fourati, H.; Sayadi, S. Oleuropein and hydroxytyrosol rich extracts from olive leaves attenuate liver injury and lipid metabolism disturbance in bisphenol A-treated rats. Food Funct. 2018, 9, 3220–3234. [Google Scholar] [CrossRef]
- Shen, Y.; Song, S.J.; Keum, N.; Park, T. Olive leaf extract attenuates obesity in high-fat diet-fed mice by modulating the expression of molecules involved in adipogenesis and thermogenesis. Evid. Based Complement. Altern. Med. 2014, 2014, 971890. [Google Scholar] [CrossRef] [Green Version]
- Vezza, T.; Rodriguez-Nogales, A.; Algieri, F.; Garrido-Mesa, J.; Romero, M.; Sanchez, M.; Toral, M.; Martin-Garcia, B.; Gomez-Caravaca, A.M.; Arraez-Roman, D.; et al. The metabolic and vascular protective effects of olive (Olea europaea L.) leaf extract in diet-induced obesity in mice are related to the amelioration of gut microbiota dysbiosis and to its immunomodulatory properties. Pharmacol. Res. 2019, 150, 104487. [Google Scholar] [CrossRef]
- Palmeri, R.; Monteleone, J.I.; Spagna, G.; Restuccia, C.; Raffaele, M.; Vanella, L.; Li Volti, G.; Barbagallo, I. Olive leaf extract from sicilian cultivar reduced lipid accumulation by inducing thermogenic pathway during adipogenesis. Front. Pharmacol. 2016, 7, 143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahima, R.S. Connecting obesity, aging and diabetes. Nat. Med. 2009, 15, 996–997. [Google Scholar] [CrossRef] [PubMed]
- Perez, L.M.; Pareja-Galeano, H.; Sanchis-Gomar, F.; Emanuele, E.; Lucia, A.; Galvez, B.G. ‘Adipaging’: Ageing and obesity share biological hallmarks related to a dysfunctional adipose tissue. J. Physiol. 2016, 594, 3187–3207. [Google Scholar] [CrossRef]
- Jung, Y.C.; Kim, H.W.; Min, B.K.; Cho, J.Y.; Son, H.J.; Lee, J.Y.; Kim, J.Y.; Kwon, S.B.; Li, Q.; Lee, H.W. Inhibitory effect of olive leaf extract on obesity in high-fat diet-induced mice. In Vivo 2019, 33, 707–715. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheurfa, M.; Abdallah, H.H.; Allem, R.; Noui, A.; Picot-Allain, C.M.N.; Mahomoodally, F. Hypocholesterolaemic and antioxidant properties of Olea europaea L. leaves from Chlef province, Algeria using in vitro, in vivo and in silico approaches. Food Chem. Toxicol. 2019, 123, 98–105. [Google Scholar] [CrossRef]
- Olmez, E.; Vural, K.; Gok, S.; Ozturk, Z.; Kayalar, H.; Ayhan, S.; Var, A. Olive leaf extract improves the atherogenic lipid profile in rats fed a high cholesterol diet. Phytother. Res. 2015, 29, 1652–1657. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, H.; Campbell, F.; Brown, L. Olive leaf extract attenuates cardiac, hepatic, and metabolic changes in high carbohydrate-, high fat-fed rats. J. Nutr. 2010, 140, 946–953. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Geng, C.; Jiang, L.; Gong, D.; Liu, D.; Yoshimura, H.; Zhong, L. The anti-atherosclerotic effect of olive leaf extract is related to suppressed inflammatory response in rabbits with experimental atherosclerosis. Eur. J. Nutr. 2008, 47, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Yoon, L.; Liu, Y.N.; Park, H.; Kim, H.S. Olive leaf extract elevates hepatic PPAR alpha mRNA expression and improves serum lipid profiles in ovariectomized rats. J. Med. Food 2015, 18, 738–744. [Google Scholar] [CrossRef] [Green Version]
- Araki, R.; Fujie, K.; Yuine, N.; Watabe, Y.; Nakata, Y.; Suzuki, H.; Isoda, H.; Hashimoto, K. Olive leaf tea is beneficial for lipid metabolism in adults with prediabetes: An exploratory randomized controlled trial. Nutr. Res. 2019, 67, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Lockyer, S.; Rowland, I.; Spencer, J.P.E.; Yaqoob, P.; Stonehouse, W. Impact of phenolic-rich olive leaf extract on blood pressure, plasma lipids and inflammatory markers: A randomised controlled trial. Eur. J. Nutr. 2017, 56, 1421–1432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darcy, J.; McFadden, S.; Fang, Y.; Huber, J.A.; Zhang, C.; Sun, L.Y.; Bartke, A. Brown adipose tissue function is enhanced in long-lived, male ames dwarf mice. Endocrinology 2016, 157, 4744–4753. [Google Scholar] [CrossRef] [PubMed]
- Vatner, D.E.; Zhang, J.; Oydanich, M.; Guers, J.; Katsyuba, E.; Yan, L.; Sinclair, D.; Auwerx, J.; Vatner, S.F. Enhanced longevity and metabolism by brown adipose tissue with disruption of the regulator of G protein signaling 14. Aging Cell 2018, 17, e12751. [Google Scholar] [CrossRef]
- Santanasto, A.J.; Goodpaster, B.H.; Kritchevsky, S.B.; Miljkovic, I.; Satterfield, S.; Schwartz, A.V.; Cummings, S.R.; Boudreau, R.M.; Harris, T.B.; Newman, A.B. Body composition remodeling and mortality: The health aging and body composition study. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 513–519. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szychlinska, M.A.; Castrogiovanni, P.; Trovato, F.M.; Nsir, H.; Zarrouk, M.; Lo Furno, D.; Di Rosa, M.; Imbesi, R.; Musumeci, G. Physical activity and Mediterranean diet based on olive tree phenolic compounds from two different geographical areas have protective effects on early osteoarthritis, muscle atrophy and hepatic steatosis. Eur. J. Nutr. 2019, 58, 565–581. [Google Scholar] [CrossRef]
- Arsyad, M.A.; Akazawa, T.; Nozaki, C.; Yoshida, M.; Oyama, K.; Mukai, T.; Ogawa, M. Effects of olive leaf powder supplemented to fish feed on muscle protein of red sea bream. Fish. Physiol. Biochem. 2018, 44, 1299–1308. [Google Scholar] [CrossRef]
- Rong, Y.D.; Bian, A.L.; Hu, H.Y.; Ma, Y.; Zhou, X.Z. Study on relationship between elderly sarcopenia and inflammatory cytokine IL-6, anti-inflammatory cytokine IL-10. BMC Geriatr. 2018, 18, 308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, H.; Macpherson, P.; Marvin, M.; Meadows, E.; Klein, W.H.; Yang, X.J.; Goldman, D. A histone deacetylase 4/myogenin positive feedback loop coordinates denervation-dependent gene induction and suppression. Mol. Biol. Cell 2009, 20, 1120–1131. [Google Scholar] [CrossRef] [Green Version]
- Moresi, V.; Williams, A.H.; Meadows, E.; Flynn, J.M.; Potthoff, M.J.; McAnally, J.; Shelton, J.M.; Backs, J.; Klein, W.H.; Richardson, J.A.; et al. Myogenin and class II HDACs control neurogenic muscle atrophy by inducing E3 ubiquitin ligases. Cell 2010, 143, 35–45. [Google Scholar] [CrossRef] [Green Version]
- Siriett, V.; Platt, L.; Salerno, M.S.; Ling, N.; Kambadur, R.; Sharma, M. Prolonged absence of myostatin reduces sarcopenia. J. Cell Physiol. 2006, 209, 866–873. [Google Scholar] [CrossRef]
- Chang, W.T.; Chen, C.S.; Cheng, M.C.; Wu, M.F.; Cheng, F.T.; Hsu, C.L. Effects of resveratrol, epigallocatechin gallate, and epicatechin on mitochondrial functions in C2C12 myotubes. J. Funct. Foods 2017, 35, 507–512. [Google Scholar] [CrossRef]
- Gauze-Gnagne, C.; Raynaud, F.; Djohan, Y.F.; Lauret, C.; Feillet-Coudray, C.; Coudray, C.; Monde, A.; Koffi, G.; Morena, M.; Camara-Cisse, M.; et al. Impact of diets rich in olive oil, palm oil or lard on myokine expression in rats. Food Funct. 2020, 11, 9114–9128. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Hwang, J.K. Flavonoids: Nutraceutical potential for counteracting muscle atrophy. Food Sci. Biotechnol. 2020, 29, 1619–1640. [Google Scholar] [CrossRef]
- Castillero, E.; Martin, A.I.; Lopez-Menduina, M.; Granado, M.; Villanua, M.A.; Lopez-Calderon, A. IGF-I system, atrogenes and myogenic regulatory factors in arthritis induced muscle wasting. Mol. Cell Endocrinol. 2009, 309, 8–16. [Google Scholar] [CrossRef]
- Hyatt, J.P.; Roy, R.R.; Baldwin, K.M.; Edgerton, V.R. Nerve activity-independent regulation of skeletal muscle atrophy: Role of MyoD and myogenin in satellite cells and myonuclei. Am. J. Physiol. Cell Physiol. 2003, 285, C1161–C1173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.N.; Jung, J.H.; Park, H.; Kim, H. Olive leaf extract suppresses messenger RNA expression of proinflammatory cytokines and enhances insulin receptor substrate 1 expression in the rats with streptozotocin and high-fat diet-induced diabetes. Nutr. Res. 2014, 34, 450–457. [Google Scholar] [CrossRef]
- Giacometti, J.; Muhvic, D.; Grubic-Kezele, T.; Nikolic, M.; Soic-Vranic, T.; Bajek, S. Olive leaf polyphenols (OLPs) stimulate GLUT4 expression and translocation in the skeletal muscle of diabetic rats. Int. J. Mol. Sci. 2020, 21, 8981. [Google Scholar] [CrossRef] [PubMed]




| Young | Old | Old + OLE | |
|---|---|---|---|
| Heart (mg/100 g body weight) | 314.6 ± 10.8 | 291.0 ± 14.4 | 264.6 ± 13.7 * |
| Epidydimal visceral adipose tissue (mg/100 g body weight) | 2107.1 ± 183.7 | 3201.8 ± 139.6 *** | 2970.7 ± 265.1 ** |
| Lumbar subcutaneous adipose tissue (mg/100 g body weight) | 1063.1 ± 139.6 | 3974.2 ± 661.4 *** | 4249.9 ± 396.9 *** |
| Interscapular brown adipose tissue (mg/100 g body weight) | 105.6 ± 9.8 | 111.1 ± 12.9 | 140.4 ± 14.4 *# |
| Periaortic adipose tissue (mg/100 g body weight) | 39.3 ± 5.9 | 47.8 ± 7.5 | 55.2 ± 5.9 * |
| Liver (mg/100 g body weight) | 2932.8 ± 103.6 | 2204.2 ± 138.6 *** | 2343.0 ± 92.6 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Hedström, D.; Priego, T.; Amor, S.; de la Fuente-Fernández, M.; Martín, A.I.; López-Calderón, A.; Inarejos-García, A.M.; García-Villalón, Á.L.; Granado, M. Olive Leaf Extract Supplementation to Old Wistar Rats Attenuates Aging-Induced Sarcopenia and Increases Insulin Sensitivity in Adipose Tissue and Skeletal Muscle. Antioxidants 2021, 10, 737. https://doi.org/10.3390/antiox10050737
González-Hedström D, Priego T, Amor S, de la Fuente-Fernández M, Martín AI, López-Calderón A, Inarejos-García AM, García-Villalón ÁL, Granado M. Olive Leaf Extract Supplementation to Old Wistar Rats Attenuates Aging-Induced Sarcopenia and Increases Insulin Sensitivity in Adipose Tissue and Skeletal Muscle. Antioxidants. 2021; 10(5):737. https://doi.org/10.3390/antiox10050737
Chicago/Turabian StyleGonzález-Hedström, Daniel, Teresa Priego, Sara Amor, María de la Fuente-Fernández, Ana Isabel Martín, Asunción López-Calderón, Antonio Manuel Inarejos-García, Ángel Luís García-Villalón, and Miriam Granado. 2021. "Olive Leaf Extract Supplementation to Old Wistar Rats Attenuates Aging-Induced Sarcopenia and Increases Insulin Sensitivity in Adipose Tissue and Skeletal Muscle" Antioxidants 10, no. 5: 737. https://doi.org/10.3390/antiox10050737






