The Role of the Thioredoxin System in Brain Diseases
Abstract
:1. Introduction
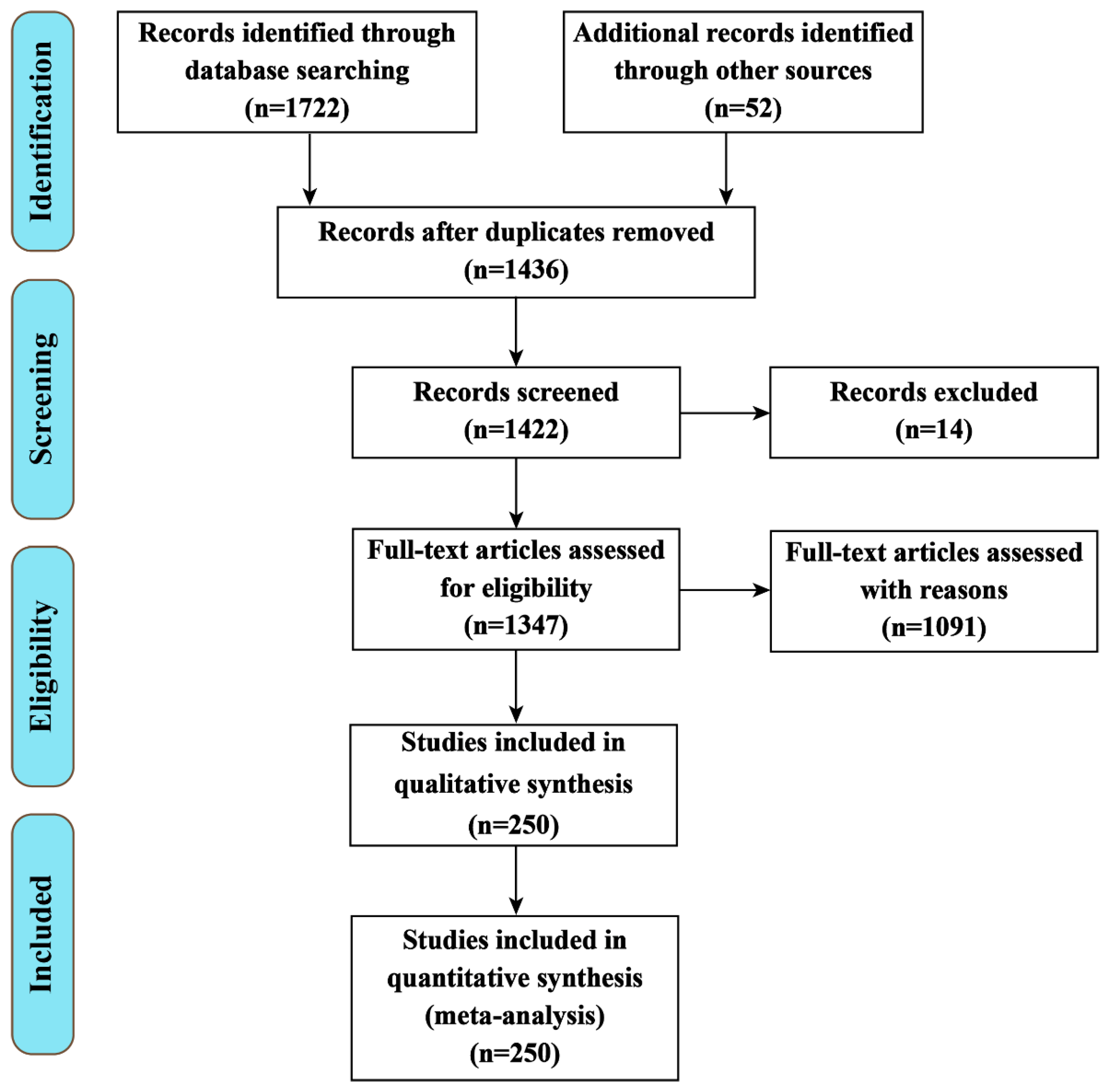
2. The Methodology of the Review Analysis
3. The Thioredoxin System
4. Synergistic and Antagonistic Interactions between Aberrations in Different Signal Transduction Systems
5. Alzheimer’s Disease
5.1. Pathophysiology of Alzheimer’s Disease
5.2. Trx and TrxR in Alzheimer’s Disease
5.3. Age-Related Decline in Memory Function
6. Parkinson’s Disease
6.1. Pathophysiology of Parkinson’s Disease
6.2. Trx and TrxR in Parkinson’s Disease
7. Huntington’s Disease
7.1. Pathophysiology of Huntington’s Disease
7.2. Trx and TrxR in Huntington’s Disease
8. Brain Stroke
8.1. Pathophysiology of Brain Stroke
8.2. Trx and TrxR in Brain Stroke
9. Multiple Sclerosis
9.1. Pathophysiology of Multiple Sclerosis
9.2. Trx and TrxR in Multiple Sclerosis
9.3. Effects of Metals on the Trx System
10. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nasoohi, S.; Ismael, S.; Ishrat, T. Thioredoxin-Interacting Protein (TXNIP) in Cerebrovascular and Neurodegenerative Diseases: Regulation and Implication. Mol. Neurobiol. 2018, 55, 7900–7920. [Google Scholar] [CrossRef] [PubMed]
- Gitler, A.D.; Dhillon, P.; Shorter, J. Neurodegenerative disease: Models, mechanisms, and a new hope. Dis. Model. Mech. 2017, 10, 499–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, B.; Sharma, D. Recent Patent Advances for Neurodegenerative Disorders and its Treatment. Recent Pat. Drug Deliv. Formul. 2017, 11, 158–172. [Google Scholar] [CrossRef] [PubMed]
- Ruszkiewicz, J.; Albrecht, J. Changes in the mitochondrial antioxidant systems in neurodegenerative diseases and acute brain disorders. Neurochem. Int. 2015, 88, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Hattori, F.; Oikawa, S. Peroxiredoxins in the central nervous system. Subcell Biochem. 2007, 44, 357–374. [Google Scholar] [CrossRef] [PubMed]
- Collin, F. Chemical Basis of Reactive Oxygen Species Reactivity and Involvement in Neurodegenerative Diseases. Int. J. Mol. Sci 2019, 20, 2407. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative Stress: A Key Modulator in Neurodegenerative Diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.L.; Wilhelmus, M.M.; de Vries, H.E.; Drukarch, B.; Hoozemans, J.J.; van Horssen, J. Antioxidative defense mechanisms controlled by Nrf2: State-of-the-art and clinical perspectives in neurodegenerative diseases. Arch. Toxicol. 2014, 88, 1773–1786. [Google Scholar] [CrossRef]
- Mahmood, D.F.; Abderrazak, A.; El Hadri, K.; Simmet, T.; Rouis, M. The thioredoxin system as a therapeutic target in human health and disease. Antioxid. Redox Signal. 2013, 19, 1266–1303. [Google Scholar] [CrossRef]
- Bharti, V.; Tan, H.; Chow, D.; Wang, Y.; Nagakannan, P.; Eftekharpour, E.; Wang, J.F. Glucocorticoid Upregulates Thioredoxin-interacting Protein in Cultured Neuronal Cells. Neuroscience 2018, 384, 375–383. [Google Scholar] [CrossRef]
- Holmgren, A.; Lu, J. Thioredoxin and thioredoxin reductase: Current research with special reference to human disease. Biochem. Biophys. Res. Commun. 2010, 396, 120–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wadhwa, R.; Gupta, R.; Maurya, P.K. Oxidative Stress and Accelerated Aging in Neurodegenerative and Neuropsychiatric Disorder. Curr. Pharm. Des. 2018, 24, 4711–4725. [Google Scholar] [CrossRef] [PubMed]
- Hohn, A.; Weber, D.; Jung, T.; Ott, C.; Hugo, M.; Kochlik, B.; Kehm, R.; Konig, J.; Grune, T.; Castro, J.P. Happily (n)ever after: Aging in the context of oxidative stress, proteostasis loss and cellular senescence. Redox Biol. 2017, 11, 482–501. [Google Scholar] [CrossRef]
- Zhang, Y.; Unnikrishnan, A.; Deepa, S.S.; Liu, Y.; Li, Y.; Ikeno, Y.; Sosnowska, D.; van Remmen, H.; Richardson, A. A new role for oxidative stress in aging: The accelerated aging phenotype in Sod1(-/)(-) mice is correlated to increased cellular senescence. Redox Biol. 2017, 11, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Nocella, C.; Cammisotto, V.; Pigozzi, F.; Borrione, P.; Fossati, C.; D’Amico, A.; Cangemi, R.; Peruzzi, M.; Gobbi, G.; Ettorre, E.; et al. Impairment between Oxidant and Antioxidant Systems: Short- and Long-term Implications for Athletes’ Health. Nutrients 2019, 11, 1353. [Google Scholar] [CrossRef] [Green Version]
- Chasapis, C.T.; Makridakis, M.; Damdimopoulos, A.E.; Zoidakis, J.; Lygirou, V.; Mavroidis, M.; Vlahou, A.; Miranda-Vizuete, A.; Spyrou, G.; Vlamis-Gardikas, A. Implications of the mitochondrial interactome of mammalian thioredoxin 2 for normal cellular function and disease. Free Radic. Biol. Med. 2019, 137, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Kahya, M.; Sood, P.; Devos, H.; Krishnan, S.; Hirsch, M.A.; Heyn, P. Falls Risk and Alzheimer Disease: A Patient Guide. Arch. Phys. Med. Rehabil. 2020, 101, 931–935. [Google Scholar] [CrossRef]
- Wang, C.Y.; Xu, Y.; Wang, X.; Guo, C.; Wang, T.; Wang, Z.Y. Dl-3-n-Butylphthalide Inhibits NLRP3 Inflammasome and Mitigates Alzheimer’s-Like Pathology via Nrf2-TXNIP-TrX Axis. Antioxid. Redox Signal. 2019, 30, 1411–1431. [Google Scholar] [CrossRef]
- Armon-Omer, A.; Waldman, C.; Simaan, N.; Neuman, H.; Tamir, S.; Shahien, R. New Insights on the Nutrition Status and Antioxidant Capacity in Multiple Sclerosis Patients. Nutrients 2019, 11, 427. [Google Scholar] [CrossRef] [Green Version]
- Bjørklund, G.; Zou, L.; Wang, J.; Chasapis, C.T.; Peana, M. Thioredoxin reductase as a pharmacological target. Pharmacol. Res. 2021, 174, 105854. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [Green Version]
- Bazinet, R.P.; Laye, S. Polyunsaturated fatty acids and their metabolites in brain function and disease. Nat. Rev. Neurosci. 2014, 15, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.Z.; He, L. The role of polyunsaturated fatty acids and GPR40 receptor in brain. Neuropharmacology 2017, 113, 639–651. [Google Scholar] [CrossRef] [PubMed]
- McNamara, R.K.; Asch, R.H.; Lindquist, D.M.; Krikorian, R. Role of polyunsaturated fatty acids in human brain structure and function across the lifespan: An update on neuroimaging findings. Prostaglandins Leukot. Essent. Fatty Acids 2018, 136, 23–34. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Niedzielska, E.; Smaga, I.; Gawlik, M.; Moniczewski, A.; Stankowicz, P.; Pera, J.; Filip, M. Oxidative Stress in Neurodegenerative Diseases. Mol. Neurobiol. 2016, 53, 4094–4125. [Google Scholar] [CrossRef] [Green Version]
- Pennisi, G.; Cornelius, C.; Cavallaro, M.M.; Salinaro, A.T.; Cambria, M.T.; Pennisi, M.; Bella, R.; Milone, P.; Ventimiglia, B.; Migliore, M.R.; et al. Redox regulation of cellular stress response in multiple sclerosis. Biochem. Pharmacol. 2011, 82, 1490–1499. [Google Scholar] [CrossRef] [Green Version]
- Elmann, A.; Wang, C.K.; Vauzour, D. Polyphenols Targeting Brain Cells Longevity, Brain’s Redox Status, and Neurodegenerative Diseases. Oxid. Med. Cell Longev. 2018, 2018, 7402795. [Google Scholar] [CrossRef]
- Arner, E.S.; Holmgren, A. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 2000, 267, 6102–6109. [Google Scholar] [CrossRef]
- Ouyang, Y.; Peng, Y.; Li, J.; Holmgren, A.; Lu, J. Modulation of thiol-dependent redox system by metal ions via thioredoxin and glutaredoxin systems. Metallomics 2018, 10, 218–228. [Google Scholar] [CrossRef]
- Ren, X.; Zou, L.; Zhang, X.; Branco, V.; Wang, J.; Carvalho, C.; Holmgren, A.; Lu, J. Redox Signaling Mediated by Thioredoxin and Glutathione Systems in the Central Nervous System. Antioxid. Redox Signal. 2017, 27, 989–1010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holmgren, A. Antioxidant function of thioredoxin and glutaredoxin systems. Antioxid. Redox Signal. 2000, 2, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; Martin, S.G. The thioredoxin system: A key target in tumour and endothelial cells. Br. J. Radiol. 2008, 81, S57–S68. [Google Scholar] [CrossRef] [PubMed]
- Bas, A.; Gultekin, G.; Incir, S.; Bas, T.O.; Emul, M.; Duran, A. Level of serum thioredoxin and correlation with neurocognitive functions in patients with schizophrenia using clozapine and other atypical antipsychotics. Psychiatry Res. 2017, 247, 84–89. [Google Scholar] [CrossRef]
- Pekkari, K.; Holmgren, A. Truncated thioredoxin: Physiological functions and mechanism. Antioxid. Redox Signal. 2004, 6, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Holmgren, A. The thioredoxin antioxidant system. Free Radic. Biol. Med. 2014, 66, 75–87. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Holmgren, A. Selenoproteins. J. Biol. Chem. 2009, 284, 723–727. [Google Scholar] [CrossRef] [Green Version]
- Nishiyama, A.; Matsui, M.; Iwata, S.; Hirota, K.; Masutani, H.; Nakamura, H.; Takagi, Y.; Sono, H.; Gon, Y.; Yodoi, J. Identification of thioredoxin-binding protein-2/vitamin D(3) up-regulated protein 1 as a negative regulator of thioredoxin function and expression. J. Biol. Chem. 1999, 274, 21645–21650. [Google Scholar] [CrossRef] [Green Version]
- Patwari, P.; Higgins, L.J.; Chutkow, W.A.; Yoshioka, J.; Lee, R.T. The interaction of thioredoxin with Txnip. Evidence for formation of a mixed disulfide by disulfide exchange. J. Biol. Chem. 2006, 281, 21884–21891. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Holmgren, A. Thioredoxin system in cell death progression. Antioxid. Redox Signal. 2012, 17, 1738–1747. [Google Scholar] [CrossRef]
- Yu, Y.; Xing, K.; Badamas, R.; Kuszynski, C.A.; Wu, H.; Lou, M.F. Overexpression of thioredoxin-binding protein 2 increases oxidation sensitivity and apoptosis in human lens epithelial cells. Free Radic. Biol. Med. 2013, 57, 92–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.G.; Upton, J.P.; Praveen, P.V.; Ghosh, R.; Nakagawa, Y.; Igbaria, A.; Shen, S.; Nguyen, V.; Backes, B.J.; Heiman, M.; et al. IRE1alpha induces thioredoxin-interacting protein to activate the NLRP3 inflammasome and promote programmed cell death under irremediable ER stress. Cell Metab. 2012, 16, 250–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oslowski, C.M.; Hara, T.; O’Sullivan-Murphy, B.; Kanekura, K.; Lu, S.; Hara, M.; Ishigaki, S.; Zhu, L.J.; Hayashi, E.; Hui, S.T.; et al. Thioredoxin-interacting protein mediates ER stress-induced beta cell death through initiation of the inflammasome. Cell Metab. 2012, 16, 265–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, X.; Shih, A.Y.; Johannssen, H.C.; Erb, H.; Li, P.; Murphy, T.H. Two-photon imaging of glutathione levels in intact brain indicates enhanced redox buffering in developing neurons and cells at the cerebrospinal fluid and blood-brain interface. J. Biol. Chem. 2006, 281, 17420–17431. [Google Scholar] [CrossRef] [Green Version]
- Ignowski, E.; Winter, A.N.; Duval, N.; Fleming, H.; Wallace, T.; Manning, E.; Koza, L.; Huber, K.; Serkova, N.J.; Linseman, D.A. The cysteine-rich whey protein supplement, Immunocal(R), preserves brain glutathione and improves cognitive, motor, and histopathological indices of traumatic brain injury in a mouse model of controlled cortical impact. Free Radic. Biol. Med. 2018, 124, 328–341. [Google Scholar] [CrossRef]
- Garcia-Ruiz, C.; Morales, A.; Ballesta, A.; Rodes, J.; Kaplowitz, N.; Fernandez-Checa, J.C. Effect of chronic ethanol feeding on glutathione and functional integrity of mitochondria in periportal and perivenous rat hepatocytes. J. Clin. Investig. 1994, 94, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Castagna, A.; Le Grazie, C.; Accordini, A.; Giulidori, P.; Cavalli, G.; Bottiglieri, T.; Lazzarin, A. Cerebrospinal fluid S-adenosylmethionine (SAMe) and glutathione concentrations in HIV infection: Effect of parenteral treatment with SAMe. Neurology 1995, 45, 1678–1683. [Google Scholar] [CrossRef]
- Zachara, B.A.; Pawluk, H.; Bloch-Boguslawska, E.; Sliwka, K.M.; Korenkiewicz, J.; Skok, Z.; Ryc, K. Tissue level, distribution, and total body selenium content in healthy and diseased humans in Poland. Arch. Environ. Health 2001, 56, 461–466. [Google Scholar] [CrossRef]
- Varikasuvu, S.R.; Prasad, V.S.; Kothapalli, J.; Manne, M. Brain Selenium in Alzheimer’s Disease (BRAIN SEAD Study): A Systematic Review and Meta-Analysis. Biol. Trace Elem. Res. 2019, 189, 361–369. [Google Scholar] [CrossRef]
- Hodgson, N.; Trivedi, M.; Muratore, C.; Li, S.; Deth, R. Soluble oligomers of amyloid-beta cause changes in redox state, DNA methylation, and gene transcription by inhibiting EAAT3 mediated cysteine uptake. J. Alzheimers Dis. 2013, 36, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Branco, V.; Pimentel, J.; Brito, M.A.; Carvalho, C. Thioredoxin, glutathione and related molecules in tumors of the nervous system. Curr. Med. Chem. 2019, 27, 1878–1900. [Google Scholar] [CrossRef] [PubMed]
- Satrustegui, J.; Richter, C. The role of hydroperoxides as calcium release agents in rat brain mitochondria. Arch. Biochem. Biophys. 1984, 233, 736–740. [Google Scholar] [CrossRef]
- Rowe, J.M. The evolving paradigm of prognostic factors in AML: Introduction to the Acute Leukemia Forum 2010. Best Pract. Res. Clin. Haematol. 2010, 23, 453–456. [Google Scholar] [CrossRef] [PubMed]
- Ahn, J.H.; Choi, J.H.; Song, J.M.; Lee, C.H.; Yoo, K.Y.; Hwang, I.K.; Kim, J.S.; Shin, H.C.; Won, M.H. Increase in Trx2/Prx3 redox system immunoreactivity in the spinal cord and hippocampus of aged dogs. Exp. Gerontol. 2011, 46, 946–952. [Google Scholar] [CrossRef]
- Angeles, D.C.; Gan, B.H.; Onstead, L.; Zhao, Y.; Lim, K.L.; Dachsel, J.; Melrose, H.; Farrer, M.; Wszolek, Z.K.; Dickson, D.W.; et al. Mutations in LRRK2 increase phosphorylation of peroxiredoxin 3 exacerbating oxidative stress-induced neuronal death. Hum. Mutat. 2011, 32, 1390–1397. [Google Scholar] [CrossRef]
- Goemaere, J.; Knoops, B. Peroxiredoxin distribution in the mouse brain with emphasis on neuronal populations affected in neurodegenerative disorders. J. Comp. Neurol. 2012, 520, 258–280. [Google Scholar] [CrossRef]
- Irwin, R.W.; Yao, J.; To, J.; Hamilton, R.T.; Cadenas, E.; Brinton, R.D. Selective oestrogen receptor modulators differentially potentiate brain mitochondrial function. J. Neuroendocrinol. 2012, 24, 236–248. [Google Scholar] [CrossRef]
- Pitts, A.; Dailey, K.; Newington, J.T.; Chien, A.; Arseneault, R.; Cann, T.; Thompson, L.M.; Cumming, R.C. Dithiol-based compounds maintain expression of antioxidant protein peroxiredoxin 1 that counteracts toxicity of mutant huntingtin. J. Biol. Chem. 2012, 287, 22717–22729. [Google Scholar] [CrossRef] [Green Version]
- Shim, S.Y.; Kim, H.S.; Kim, E.K.; Choi, J.H. Expression of peroxiredoxin 1, 2, and 6 in the rat brain during perinatal development and in response to dexamethasone. Free Radic. Res. 2012, 46, 231–239. [Google Scholar] [CrossRef]
- Zhao, L.; Morgan, T.E.; Mao, Z.; Lin, S.; Cadenas, E.; Finch, C.E.; Pike, C.J.; Mack, W.J.; Brinton, R.D. Continuous versus cyclic progesterone exposure differentially regulates hippocampal gene expression and functional profiles. PLoS ONE 2012, 7, e31267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Y.; Zhang, X.; Ji, H.; Liu, H.; Li, S.; Li, L. Probucol and atorvastatin in combination protect rat brains in MCAO model: Upregulating Peroxiredoxin2, Foxo3a and Nrf2 expression. Neurosci. Lett. 2012, 509, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.J.; Lin, W.P. Quantitative analysis of multiple exocyclic DNA adducts in human salivary DNA by stable isotope dilution nanoflow liquid chromatography-nanospray ionization tandem mass spectrometry. Anal. Chem. 2011, 83, 8543–8551. [Google Scholar] [CrossRef] [PubMed]
- Voulgaridou, G.P.; Anestopoulos, I.; Franco, R.; Panayiotidis, M.I.; Pappa, A. DNA damage induced by endogenous aldehydes: Current state of knowledge. Mutat. Res. 2011, 711, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.E.; Park, Y.S. Role of lipid peroxidation-derived alpha, beta-unsaturated aldehydes in vascular dysfunction. Oxid Med. Cell Longev. 2013, 2013, 629028. [Google Scholar] [CrossRef] [Green Version]
- Yata, K.; Oikawa, S.; Sasaki, R.; Shindo, A.; Yang, R.; Murata, M.; Kanamaru, K.; Tomimoto, H. Astrocytic neuroprotection through induction of cytoprotective molecules; a proteomic analysis of mutant P301S tau-transgenic mouse. Brain Res. 2011, 1410, 12–23. [Google Scholar] [CrossRef]
- Power, J.H.; Asad, S.; Chataway, T.K.; Chegini, F.; Manavis, J.; Temlett, J.A.; Jensen, P.H.; Blumbergs, P.C.; Gai, W.P. Peroxiredoxin 6 in human brain: Molecular forms, cellular distribution and association with Alzheimer’s disease pathology. Acta Neuropathol. 2008, 115, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Drechsel, D.A.; Patel, M. Respiration-dependent H2O2 removal in brain mitochondria via the thioredoxin/peroxiredoxin system. J. Biol. Chem. 2010, 285, 27850–27858. [Google Scholar] [CrossRef] [Green Version]
- Kudin, A.P.; Augustynek, B.; Lehmann, A.K.; Kovacs, R.; Kunz, W.S. The contribution of thioredoxin-2 reductase and glutathione peroxidase to H2O2 detoxification of rat brain mitochondria. Biochim. Biophys. Acta 2012, 1817, 1901–1906. [Google Scholar] [CrossRef] [Green Version]
- Yuan, S.; Schuster, A.; Tang, C.; Yu, T.; Ortogero, N.; Bao, J.; Zheng, H.; Yan, W. Sperm-borne miRNAs and endo-siRNAs are important for fertilization and preimplantation embryonic development. Development 2016, 143, 635–647. [Google Scholar] [CrossRef]
- Lokken, A.A.; Ralston, A. The Genetic Regulation of Cell Fate during Preimplantation Mouse Development. Curr. Top. Dev. Biol. 2016, 120, 173–202. [Google Scholar] [CrossRef] [PubMed]
- Fessel, J. Alzheimer’s disease combination treatment. Neurobiol. Aging 2018, 63, 165. [Google Scholar] [CrossRef] [PubMed]
- Plassman, B.L.; Langa, K.M.; Fisher, G.G.; Heeringa, S.G.; Weir, D.R.; Ofstedal, M.B.; Burke, J.R.; Hurd, M.D.; Potter, G.G.; Rodgers, W.L.; et al. Prevalence of dementia in the United States: The aging, demographics, and memory study. Neuroepidemiology 2007, 29, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Abolhassani, N.; Leon, J.; Sheng, Z.; Oka, S.; Hamasaki, H.; Iwaki, T.; Nakabeppu, Y. Molecular pathophysiology of impaired glucose metabolism, mitochondrial dysfunction, and oxidative DNA damage in Alzheimer’s disease brain. Mech. Ageing Dev. 2017, 161, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Hebert, L.E.; Beckett, L.A.; Scherr, P.A.; Evans, D.A. Annual incidence of Alzheimer disease in the United States projected to the years 2000 through 2050. Alzheimer Dis. Assoc. Disord 2001, 15, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Prince, M.; Bryce, R.; Albanese, E.; Wimo, A.; Ribeiro, W.; Ferri, C.P. The global prevalence of dementia: A systematic review and metaanalysis. Alzheimers Dement. 2013, 9, 63–75.e62. [Google Scholar] [CrossRef]
- Alzheimer’s, A. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015, 11, 332–384. [Google Scholar] [CrossRef]
- Dye, R.V.; Miller, K.J.; Singer, E.J.; Levine, A.J. Hormone replacement therapy and risk for neurodegenerative diseases. Int. J. Alzheimers Dis. 2012, 2012, 258454. [Google Scholar] [CrossRef] [Green Version]
- Robinson, R.A.; Amin, B.; Guest, P.C. Multiplexing Biomarker Methods, Proteomics and Considerations for Alzheimer’s Disease. Adv. Exp. Med. Biol. 2017, 974, 21–48. [Google Scholar] [CrossRef]
- Gotz, J.; Ittner, A.; Ittner, L.M. Tau-targeted treatment strategies in Alzheimer’s disease. Br. J. Pharmacol. 2012, 165, 1246–1259. [Google Scholar] [CrossRef]
- Aaseth, J.; Skaug, M.A.; Cao, Y.; Andersen, O. Chelation in metal intoxication—Principles and paradigms. J. Trace Elem. Med. Biol. 2015, 31, 260–266. [Google Scholar] [CrossRef] [PubMed]
- Cieslik, M.; Czapski, G.A.; Wojtowicz, S.; Wieczorek, I.; Wencel, P.L.; Strosznajder, R.P.; Jaber, V.; Lukiw, W.J.; Strosznajder, J.B. Alterations of Transcription of Genes Coding Anti-oxidative and Mitochondria-Related Proteins in Amyloid beta Toxicity: Relevance to Alzheimer’s Disease. Mol. Neurobiol. 2020, 57, 1374–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dickerson, B.C.; Brickhouse, M.; McGinnis, S.; Wolk, D.A. Alzheimer’s disease: The influence of age on clinical heterogeneity through the human brain connectome. Alzheimers Dement. 2017, 6, 122–135. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R., Jr.; Wiste, H.J.; Weigand, S.D.; Therneau, T.M.; Lowe, V.J.; Knopman, D.S.; Gunter, J.L.; Senjem, M.L.; Jones, D.T.; Kantarci, K.; et al. Defining imaging biomarker cut points for brain aging and Alzheimer’s disease. Alzheimers Dement. 2017, 13, 205–216. [Google Scholar] [CrossRef] [Green Version]
- Feger, B.J.; Thompson, J.W.; Dubois, L.G.; Kommaddi, R.P.; Foster, M.W.; Mishra, R.; Shenoy, S.K.; Shibata, Y.; Kidane, Y.H.; Moseley, M.A.; et al. Microgravity induces proteomics changes involved in endoplasmic reticulum stress and mitochondrial protection. Sci. Rep. 2016, 6, 34091. [Google Scholar] [CrossRef] [Green Version]
- Scheff, S.W.; Ansari, M.A.; Mufson, E.J. Oxidative stress and hippocampal synaptic protein levels in elderly cognitively intact individuals with Alzheimer’s disease pathology. Neurobiol. Aging 2016, 42, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, N.; Ghosh, R.; Mandal, S.C. Antioxidant protection: A promising therapeutic intervention in neurodegenerative disease. Free Radic. Res. 2011, 45, 888–905. [Google Scholar] [CrossRef]
- Cardoso, B.R.; Ong, T.P.; Jacob-Filho, W.; Jaluul, O.; Freitas, M.I.; Cominetti, C.; Cozzolino, S.M. Glutathione peroxidase 1 Pro198Leu polymorphism in Brazilian Alzheimer’s disease patients: Relations to the enzyme activity and to selenium status. J. Nutrigenet. Nutr. 2012, 5, 72–80. [Google Scholar] [CrossRef]
- Bateman, R.J.; Siemers, E.R.; Mawuenyega, K.G.; Wen, G.; Browning, K.R.; Sigurdson, W.C.; Yarasheski, K.E.; Friedrich, S.W.; Demattos, R.B.; May, P.C.; et al. A gamma-secretase inhibitor decreases amyloid-beta production in the central nervous system. Ann. Neurol. 2009, 66, 48–54. [Google Scholar] [CrossRef] [Green Version]
- Chouraki, V.; Reitz, C.; Maury, F.; Bis, J.C.; Bellenguez, C.; Yu, L.; Jakobsdottir, J.; Mukherjee, S.; Adams, H.H.; Choi, S.H.; et al. Evaluation of a Genetic Risk Score to Improve Risk Prediction for Alzheimer’s Disease. J. Alzheimers Dis. 2016, 53, 921–932. [Google Scholar] [CrossRef]
- Corder, E.H.; Saunders, A.M.; Strittmatter, W.J.; Schmechel, D.E.; Gaskell, P.C.; Small, G.W.; Roses, A.D.; Haines, J.L.; Pericak-Vance, M.A. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer’s disease in late onset families. Science 1993, 261, 921–923. [Google Scholar] [CrossRef] [PubMed]
- Bertram, L.; McQueen, M.B.; Mullin, K.; Blacker, D.; Tanzi, R.E. Systematic meta-analyses of Alzheimer disease genetic association studies: The AlzGene database. Nat. Genet. 2007, 39, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Tran, L.; Emilsson, V.; Zhu, J. Characterization of Genetic Networks Associated with Alzheimer’s Disease. Methods Mol. Biol. 2016, 1303, 459–477. [Google Scholar] [CrossRef]
- Chouliaras, L.; Sierksma, A.S.; Kenis, G.; Prickaerts, J.; Lemmens, M.A.; Brasnjevic, I.; van Donkelaar, E.L.; Martinez-Martinez, P.; Losen, M.; de Baets, M.H.; et al. Gene-environment interaction research and transgenic mouse models of Alzheimer’s disease. Int. J. Alzheimers Dis. 2010, 2010, 859101. [Google Scholar] [CrossRef] [Green Version]
- Huse, J.T.; Doms, R.W. Neurotoxic traffic: Uncovering the mechanics of amyloid production in Alzheimer’s disease. Traffic 2001, 2, 75–81. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A.; Perluigi, M.; Sultana, R. Oxidative stress in Alzheimer’s disease brain: New insights from redox proteomics. Eur. J. Pharmacol. 2006, 545, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Spires-Jones, T.L.; Stoothoff, W.H.; de Calignon, A.; Jones, P.B.; Hyman, B.T. Tau pathophysiology in neurodegeneration: A tangled issue. Trends Neurosci. 2009, 32, 150–159. [Google Scholar] [CrossRef]
- Selenius, M.; Rundlof, A.K.; Olm, E.; Fernandes, A.P.; Bjornstedt, M. Selenium and the selenoprotein thioredoxin reductase in the prevention, treatment and diagnostics of cancer. Antioxid. Redox Signal. 2010, 12, 867–880. [Google Scholar] [CrossRef]
- Lovell, M.A.; Xie, C.; Gabbita, S.P.; Markesbery, W.R. Decreased thioredoxin and increased thioredoxin reductase levels in Alzheimer’s disease brain. Free Radic. Biol. Med. 2000, 28, 418–427. [Google Scholar] [CrossRef]
- Takagi, Y.; Mitsui, A.; Nishiyama, A.; Nozaki, K.; Sono, H.; Gon, Y.; Hashimoto, N.; Yodoi, J. Overexpression of thioredoxin in transgenic mice attenuates focal ischemic brain damage. Proc. Natl. Acad. Sci. USA 1999, 96, 4131–4136. [Google Scholar] [CrossRef]
- Akterin, S.; Cowburn, R.F.; Miranda-Vizuete, A.; Jimenez, A.; Bogdanovic, N.; Winblad, B.; Cedazo-Minguez, A. Involvement of glutaredoxin-1 and thioredoxin-1 in beta-amyloid toxicity and Alzheimer’s disease. Cell Death Differ. 2006, 13, 1454–1465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuzawa, A. Thioredoxin and redox signaling: Roles of the thioredoxin system in control of cell fate. Arch. Biochem. Biophys. 2017, 617, 101–105. [Google Scholar] [CrossRef]
- Bobba, A.; Casalino, E.; Petragallo, V.A.; Atlante, A. Thioredoxin/thioredoxin reductase system involvement in cerebellar granule cell apoptosis. Apoptosis 2014, 19, 1497–1508. [Google Scholar] [CrossRef]
- Gil-Bea, F.; Akterin, S.; Persson, T.; Mateos, L.; Sandebring, A.; Avila-Carino, J.; Gutierrez-Rodriguez, A.; Sundstrom, E.; Holmgren, A.; Winblad, B.; et al. Thioredoxin-80 is a product of alpha-secretase cleavage that inhibits amyloid-beta aggregation and is decreased in Alzheimer’s disease brain. EMBO Mol. Med. 2012, 4, 1097–1111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Ma, H.; Zhang, L.; Cui, Y.; Liu, X.; Fang, J. A small molecule probe reveals declined mitochondrial thioredoxin reductase activity in a Parkinson’s disease model. Chem. Commun. 2016, 52, 2296–2299. [Google Scholar] [CrossRef] [PubMed]
- Lamoke, F.; Ripandelli, G.; Webster, S.; Montemari, A.; Maraschi, A.; Martin, P.; Marcus, D.M.; Liou, G.I.; Bartoli, M. Loss of thioredoxin function in retinas of mice overexpressing amyloid beta. Free Radic. Biol. Med. 2012, 53, 577–588. [Google Scholar] [CrossRef] [PubMed]
- Masutani, H.; Bai, J.; Kim, Y.C.; Yodoi, J. Thioredoxin as a neurotrophic cofactor and an important regulator of neuroprotection. Mol. Neurobiol. 2004, 29, 229–242. [Google Scholar] [CrossRef]
- Seyfried, J.; Wullner, U. Inhibition of thioredoxin reductase induces apoptosis in neuronal cell lines: Role of glutathione and the MKK4/JNK pathway. Biochem. Biophys. Res. Commun. 2007, 359, 759–764. [Google Scholar] [CrossRef]
- Arner, E.S. Focus on mammalian thioredoxin reductases—Important selenoproteins with versatile functions. Biochim. Biophys. Acta 2009, 1790, 495–526. [Google Scholar] [CrossRef]
- Cardoso, B.R.; Hare, D.J.; Bush, A.I.; Li, Q.X.; Fowler, C.J.; Masters, C.L.; Martins, R.N.; Ganio, K.; Lothian, A.; Mukherjee, S.; et al. Selenium Levels in Serum, Red Blood Cells, and Cerebrospinal Fluid of Alzheimer’s Disease Patients: A Report from the Australian Imaging, Biomarker & Lifestyle Flagship Study of Ageing (AIBL). J. Alzheimers Dis. 2017, 57, 183–193. [Google Scholar] [CrossRef]
- Du, X.; Wang, C.; Liu, Q. Potential Roles of Selenium and Selenoproteins in the Prevention of Alzheimer’s Disease. Curr. Top. Med. Chem. 2016, 16, 835–848. [Google Scholar] [CrossRef] [PubMed]
- Bjørklund, G.; Shanaida, M.; Lysiuk, R.; Antonyak, H.; Klishch, I.; Shanaida, V.; Peana, M. Selenium: An Antioxidant with a Critical Role in Anti-Aging. Molecules 2022, 27, 6613. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Markesbery, W.R.; Shao, C.; Lovell, M.A. Seleno-L-methionine protects against beta-amyloid and iron/hydrogen peroxide-mediated neuron death. Antioxid. Redox Signal. 2007, 9, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Pillai, R.; Uyehara-Lock, J.H.; Bellinger, F.P. Selenium and selenoprotein function in brain disorders. IUBMB Life 2014, 66, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Lovell, M.A.; Xiong, S.; Lyubartseva, G.; Markesbery, W.R. Organoselenium (Sel-Plex diet) decreases amyloid burden and RNA and DNA oxidative damage in APP/PS1 mice. Free Radic. Biol. Med. 2009, 46, 1527–1533. [Google Scholar] [CrossRef] [Green Version]
- Ansari, M.A.; Joshi, G.; Huang, Q.; Opii, W.O.; Abdul, H.M.; Sultana, R.; Butterfield, D.A. In vivo administration of D609 leads to protection of subsequently isolated gerbil brain mitochondria subjected to in vitro oxidative stress induced by amyloid beta-peptide and other oxidative stressors: Relevance to Alzheimer’s disease and other oxidative stress-related neurodegenerative disorders. Free Radic. Biol. Med. 2006, 41, 1694–1703. [Google Scholar] [CrossRef] [Green Version]
- Song, J.; Park, K.A.; Lee, W.T.; Lee, J.E. Apoptosis signal regulating kinase 1 (ASK1): Potential as a therapeutic target for Alzheimer’s disease. Int. J. Mol. Sci. 2014, 15, 2119–2129. [Google Scholar] [CrossRef] [Green Version]
- Nishitoh, H.; Matsuzawa, A.; Tobiume, K.; Saegusa, K.; Takeda, K.; Inoue, K.; Hori, S.; Kakizuka, A.; Ichijo, H. ASK1 is essential for endoplasmic reticulum stress-induced neuronal cell death triggered by expanded polyglutamine repeats. Genes Dev. 2002, 16, 1345–1355. [Google Scholar] [CrossRef] [Green Version]
- Moran, C.; Kirk, C.; Powell, E. Spoken persuasive discourse abilities of adolescents with acquired brain injury. Lang. Speech Hear. Serv. Sch. 2012, 43, 264–275. [Google Scholar] [CrossRef]
- Santos, R.X.; Correia, S.C.; Zhu, X.; Smith, M.A.; Moreira, P.I.; Castellani, R.J.; Nunomura, A.; Perry, G. Mitochondrial DNA oxidative damage and repair in aging and Alzheimer’s disease. Antioxid. Redox Signal. 2013, 18, 2444–2457. [Google Scholar] [CrossRef]
- Bradley-Whitman, M.A.; Timmons, M.D.; Beckett, T.L.; Murphy, M.P.; Lynn, B.C.; Lovell, M.A. Nucleic acid oxidation: An early feature of Alzheimer’s disease. J. Neurochem. 2014, 128, 294–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maciejczyk, M.; Zalewska, A.; Gerreth, A.K. Salivary Redox Biomarkers in Selected Neurodegenerative Diseases. J. Clin. Med. 2020, 9, 497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.J.; Song, D.S.; Yoo, J.S.; Hyung, K.E.; Lee, M.J.; Moon, Y.H.; Lee, I.H.; Go, B.S.; Park, S.Y.; Hwang, K.W. Protective functions of peroxiredoxin-1 against cytokine-induced MIN6 pancreatic beta-cell line death. Can. J. Physiol. Pharmacol. 2013, 91, 1037–1043. [Google Scholar] [CrossRef]
- Ahn, S.; Kearbey, J.D.; Li, C.M.; Duke, C.B., 3rd; Miller, D.D.; Dalton, J.T. Biotransformation of a novel antimitotic agent, I-387, by mouse, rat, dog, monkey, and human liver microsomes and in vivo pharmacokinetics in mice. Drug Metab. Dispos. 2011, 39, 636–643. [Google Scholar] [CrossRef] [Green Version]
- Barja, I.; Silvan, G.; Martinez-Fernandez, L.; Illera, J.C. Physiological stress responses, fecal marking behavior, and reproduction in wild European pine martens (Martes martes). J. Chem. Ecol. 2011, 37, 253–259. [Google Scholar] [CrossRef]
- Kuhn, D.M.; Arthur, R.E., Jr. Inactivation of tryptophan hydroxylase by nitric oxide: Enhancement by tetrahydrobiopterin. J. Neurochem. 1997, 68, 1495–1502. [Google Scholar] [CrossRef]
- Stone, D.M.; Johnson, M.; Hanson, G.R.; Gibb, J.W. Acute inactivation of tryptophan hydroxylase by amphetamine analogs involves the oxidation of sulfhydryl sites. Eur. J. Pharmacol. 1989, 172, 93–97. [Google Scholar] [CrossRef]
- Kuhn, D.M.; Ruskin, B.; Lovenberg, W. Tryptophan hydroxylase. The role of oxygen, iron, and sulfhydryl groups as determinants of stability and catalytic activity. J. Biol. Chem. 1980, 255, 4137–4143. [Google Scholar] [CrossRef]
- Kuhn, D.M.; Arthur, R.E., Jr.; Thomas, D.M.; Elferink, L.A. Tyrosine hydroxylase is inactivated by catechol-quinones and converted to a redox-cycling quinoprotein: Possible relevance to Parkinson’s disease. J. Neurochem. 1999, 73, 1309–1317. [Google Scholar] [CrossRef]
- Blanchard-Fillion, B.; Souza, J.M.; Friel, T.; Jiang, G.C.; Vrana, K.; Sharov, V.; Barron, L.; Schoneich, C.; Quijano, C.; Alvarez, B.; et al. Nitration and inactivation of tyrosine hydroxylase by peroxynitrite. J. Biol. Chem. 2001, 276, 46017–46023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadidi, M.; Geddes, T.J.; Kuhn, D.M. S-thiolation of tyrosine hydroxylase by reactive nitrogen species in the presence of cysteine or glutathione. Antioxid. Redox Signal. 2005, 7, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Haugaard, N.; Lee, N.H.; Kostrzewa, R.; Horn, R.S.; Haugaard, E.S. The role of sulfhydryl groups in oxidative phosphorylation and ion transport by rat liver mitochrondia. Biochim. Biophys. Acta 1969, 172, 198–204. [Google Scholar] [CrossRef]
- Wood, J.D. The role of gamma-aminobutyric acid in the mechanism of seizures. Prog. Neurobiol. 1975, 5, 77–95. [Google Scholar] [CrossRef]
- Bondarenko, T.I.; Makletsova, M.G.; Uskova, N.I. Intensity of label incorporation from [1,4-14C]putrescine into homocarnosine in the brain of rats of various ages. Ukr. Biokhim. Zh. 1986, 58, 75–77. [Google Scholar]
- Hori, S. Study on hyperbaric oxygen-induced convulsion with particular reference to gamma-aminobutyric acid in synaptosomes. J. Biochem. 1982, 91, 443–448. [Google Scholar] [CrossRef] [PubMed]
- Krichevskaia, A.A.; Bondarenko, T.I.; Makletsova, M.G.; Mikhaleva, I.I. Protective effect of the neuropeptide homocarnosine in hyperbaric oxygenation. Vopr. Med. Khim. 1985, 31, 75–79. [Google Scholar]
- Li, X.; Gardner, E.L.; Xi, Z.X. The metabotropic glutamate receptor 7 (mGluR7) allosteric agonist AMN082 modulates nucleus accumbens GABA and glutamate, but not dopamine, in rats. Neuropharmacology 2008, 54, 542–551. [Google Scholar] [CrossRef] [Green Version]
- De Rijk, M.C.; Launer, L.J.; Berger, K.; Breteler, M.M.; Dartigues, J.F.; Baldereschi, M.; Fratiglioni, L.; Lobo, A.; Martinez-Lage, J.; Trenkwalder, C.; et al. Prevalence of Parkinson’s disease in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology 2000, 54, S21–S23. [Google Scholar]
- Cook, C.; Stetler, C.; Petrucelli, L. Disruption of protein quality control in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2012, 2, a009423. [Google Scholar] [CrossRef] [Green Version]
- Pringsheim, T.; Jette, N.; Frolkis, A.; Steeves, T.D. The prevalence of Parkinson’s disease: A systematic review and meta-analysis. Mov. Disord. Off. J. Mov. Disord. Soc. 2014, 29, 1583–1590. [Google Scholar] [CrossRef] [PubMed]
- Engelender, S.; Isacson, O. The Threshold Theory for Parkinson’s Disease. Trends Neurosci. 2017, 40, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Fahn, S. Description of Parkinson’s disease as a clinical syndrome. Ann. N. Y. Acad. Sci. 2003, 991, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Forno, L.S.; DeLanney, L.E.; Irwin, I.; Langston, J.W. Electron microscopy of Lewy bodies in the amygdala-parahippocampal region. Comparison with inclusion bodies in the MPTP-treated squirrel monkey. Adv. Neurol. 1996, 69, 217–228. [Google Scholar]
- Chinta, S.J.; Andersen, J.K. Dopaminergic neurons. Int. J. Biochem. Cell Biol. 2005, 37, 942–946. [Google Scholar] [CrossRef]
- Galvin, K.A.; Oorschot, D.E. Postinjury magnesium sulfate treatment is not markedly neuroprotective for striatal medium spiny neurons after perinatal hypoxia/ischemia in the rat. Pediatr. Res. 1998, 44, 740–745. [Google Scholar] [CrossRef] [Green Version]
- Alladi, P.A.; Mahadevan, A.; Vijayalakshmi, K.; Muthane, U.; Shankar, S.K.; Raju, T.R. Ageing enhances alpha-synuclein, ubiquitin and endoplasmic reticular stress protein expression in the nigral neurons of Asian Indians. Neurochem. Int. 2010, 57, 530–539. [Google Scholar] [CrossRef]
- Imai, Y.; Venderova, K.; Lim, K.L. Animal models of Parkinson’s disease 2012. Parkinsons Dis. 2012, 2012, 729428. [Google Scholar] [CrossRef] [Green Version]
- Greenamyre, J.T.; Hastings, T.G. Biomedicine. Parkinson’s—Divergent causes, convergent mechanisms. Science 2004, 304, 1120–1122. [Google Scholar] [CrossRef]
- Gandhi, S.; Wood, N.W. Molecular pathogenesis of Parkinson’s disease. Hum. Mol. Genet. 2005, 14, 2749–2755. [Google Scholar] [CrossRef]
- Terzioglu, M.; Galter, D. Parkinson’s disease: Genetic versus toxin-induced rodent models. FEBS J. 2008, 275, 1384–1391. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.E.; Mouradian, M.M. Cytoprotective mechanisms of DJ-1 against oxidative stress through modulating ERK1/2 and ASK1 signal transduction. Redox Biol. 2018, 14, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.J.; Sailor, K.A.; Mahmood, Q.A.; Chavali, N.; Christian, K.M.; Song, H.; Ming, G.L. Seamless reconstruction of intact adult-born neurons by serial end-block imaging reveals complex axonal guidance and development in the adult hippocampus. J. Neurosci. 2013, 33, 11400–11411. [Google Scholar] [CrossRef] [Green Version]
- Brezova, V.; Dvoranova, D.; Zubor, V.; Breza, M.; Mazur, M.; Valko, M. Photochemical properties of camptothecin in the presence of copper(II) ions: The role of radicals as prospective species in photodynamic therapy. Mol. Biotechnol. 2007, 37, 48–51. [Google Scholar] [CrossRef]
- Bjørklund, G.; Peana, M.; Maes, M.; Dadar, M.; Severin, B. The glutathione system in Parkinson’s disease and its progression. Neurosci. Biobehav. Rev. 2021, 120, 470–478. [Google Scholar] [CrossRef] [PubMed]
- Naoi, M.; Maruyama, W. Monoamine oxidase inhibitors as neuroprotective agents in age-dependent neurodegenerative disorders. Curr. Pharm. Des. 2010, 16, 2799–2817. [Google Scholar] [CrossRef] [PubMed]
- Naoi, M.; Maruyama, W.; Inaba-Hasegawa, K.; Akao, Y. Type A monoamine oxidase regulates life and death of neurons in neurodegeneration and neuroprotection. Int. Rev. Neurobiol. 2011, 100, 85–106. [Google Scholar] [CrossRef]
- Xia, L.; Nordman, T.; Olsson, J.M.; Damdimopoulos, A.; Bjorkhem-Bergman, L.; Nalvarte, I.; Eriksson, L.C.; Arner, E.S.; Spyrou, G.; Bjornstedt, M. The mammalian cytosolic selenoenzyme thioredoxin reductase reduces ubiquinone. A novel mechanism for defense against oxidative stress. J. Biol. Chem. 2003, 278, 2141–2146. [Google Scholar] [CrossRef] [Green Version]
- Blackinton, J.; Kumaran, R.; van der Brug, M.P.; Ahmad, R.; Olson, L.; Galter, D.; Lees, A.; Bandopadhyay, R.; Cookson, M.R. Post-transcriptional regulation of mRNA associated with DJ-1 in sporadic Parkinson disease. Neurosci. Lett. 2009, 452, 8–11. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.S.; Jia, J.J.; Kwon, Y.; Wang, S.D.; Bai, J. The role of thioredoxin-1 in suppression of endoplasmic reticulum stress in Parkinson disease. Free Radic. Biol. Med. 2014, 67, 10–18. [Google Scholar] [CrossRef]
- Hu, X.; Weng, Z.; Chu, C.T.; Zhang, L.; Cao, G.; Gao, Y.; Signore, A.; Zhu, J.; Hastings, T.; Greenamyre, J.T.; et al. Peroxiredoxin-2 protects against 6-hydroxydopamine-induced dopaminergic neurodegeneration via attenuation of the apoptosis signal-regulating kinase (ASK1) signaling cascade. J. Neurosci. 2011, 31, 247–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonifati, V.; Rizzu, P.; van Baren, M.J.; Schaap, O.; Breedveld, G.J.; Krieger, E.; Dekker, M.C.; Squitieri, F.; Ibanez, P.; Joosse, M.; et al. Mutations in the DJ-1 gene associated with autosomal recessive early-onset parkinsonism. Science 2003, 299, 256–259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, J.; Sullards, M.C.; Olzmann, J.A.; Rees, H.D.; Weintraub, S.T.; Bostwick, D.E.; Gearing, M.; Levey, A.I.; Chin, L.S.; Li, L. Oxidative damage of DJ-1 is linked to sporadic Parkinson and Alzheimer diseases. J. Biol. Chem. 2006, 281, 10816–10824. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorner, K.; Holtorf, E.; Waak, J.; Pham, T.T.; Vogt-Weisenhorn, D.M.; Wurst, W.; Haass, C.; Kahle, P.J. Structural determinants of the C-terminal helix-kink-helix motif essential for protein stability and survival promoting activity of DJ-1. J. Biol. Chem. 2007, 282, 13680–13691. [Google Scholar] [CrossRef] [PubMed]
- Bahmed, K.; Boukhenouna, S.; Karim, L.; Andrews, T.; Lin, J.; Powers, R.; Wilson, M.A.; Lin, C.R.; Messier, E.; Reisdorph, N.; et al. The effect of cysteine oxidation on DJ-1 cytoprotective function in human alveolar type II cells. Cell Death Dis. 2019, 10, 638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Im, J.Y.; Lee, K.W.; Woo, J.M.; Junn, E.; Mouradian, M.M. DJ-1 induces thioredoxin 1 expression through the Nrf2 pathway. Hum. Mol. Genet. 2012, 21, 3013–3024. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.Y.; Liu, I.C.; Lin, T.Y. Truncated Escherichia coli thioredoxin induces proliferation of human blood mononuclear cells and production of reactive oxygen species as well as proinflammatory cytokines. Cell Biochem. Funct. 2016, 34, 226–232. [Google Scholar] [CrossRef]
- Chen, J.; Berry, M.J. Selenium and selenoproteins in the brain and brain diseases. J. Neurochem. 2003, 86, 1–12. [Google Scholar] [CrossRef]
- Shahar, A.; Patel, K.V.; Semba, R.D.; Bandinelli, S.; Shahar, D.R.; Ferrucci, L.; Guralnik, J.M. Plasma selenium is positively related to performance in neurological tasks assessing coordination and motor speed. Mov. Disord. Off. J. Mov. Disord. Soc. 2010, 25, 1909–1915. [Google Scholar] [CrossRef] [Green Version]
- Lopert, P.; Day, B.J.; Patel, M. Thioredoxin reductase deficiency potentiates oxidative stress, mitochondrial dysfunction and cell death in dopaminergic cells. PLoS ONE 2012, 7, e50683. [Google Scholar] [CrossRef] [Green Version]
- Maes, M.; Moraes, J.B.; Congio, A.; Bonifacio, K.L.; Barbosa, D.S.; Vargas, H.O.; Michelin, A.P.; Carvalho, A.F.; Nunes, S.O.V. Development of a Novel Staging Model for Affective Disorders Using Partial Least Squares Bootstrapping: Effects of Lipid-Associated Antioxidant Defenses and Neuro-Oxidative Stress. Mol. Neurobiol. 2019, 56, 6626–6644. [Google Scholar] [CrossRef] [PubMed]
- Salimpoor, V.N.; Benovoy, M.; Larcher, K.; Dagher, A.; Zatorre, R.J. Anatomically distinct dopamine release during anticipation and experience of peak emotion to music. Nat. Neurosci. 2011, 14, 257–262. [Google Scholar] [CrossRef] [PubMed]
- Baik, J.H. Dopamine signaling in reward-related behaviors. Front. Neural Circuits 2013, 7, 152. [Google Scholar] [CrossRef] [Green Version]
- Golden, J.P.; Demaro, J.A., 3rd; Knoten, A.; Hoshi, M.; Pehek, E.; Johnson, E.M., Jr.; Gereau, R.W.t.; Jain, S. Dopamine-dependent compensation maintains motor behavior in mice with developmental ablation of dopaminergic neurons. J. Neurosci. 2013, 33, 17095–17107. [Google Scholar] [CrossRef]
- Toates, F. Application of a multilevel model of behavioural control to understanding emotion. Behav. Process. 2002, 60, 99–114. [Google Scholar] [CrossRef]
- Brebner, K.; Wong, T.P.; Liu, L.; Liu, Y.; Campsall, P.; Gray, S.; Phelps, L.; Phillips, A.G.; Wang, Y.T. Nucleus accumbens long-term depression and the expression of behavioral sensitization. Science 2005, 310, 1340–1343. [Google Scholar] [CrossRef] [PubMed]
- Kutlu, A.; Karabacak, E.; Aydin, E.; Ozturk, S.; Bozkurt, B. A patient with steroids and antihistaminic drug allergy and newly occurred chronic urticaria angioedema: What about omalizumab? Hum. Exp. Toxicol. 2014, 33, 882–885. [Google Scholar] [CrossRef]
- Scuvee-Moreau, J. Neurobiology of addiction. Rev. Med. Liege 2013, 68, 211–217. [Google Scholar]
- Eisenstein, M. CRISPR takes on Huntington’s disease. Nature 2018, 557, S42–S43. [Google Scholar] [CrossRef] [Green Version]
- Nowogrodzki, A. Huntington’s disease: 4 big questions. Nature 2018, 557, S48. [Google Scholar] [CrossRef] [Green Version]
- Raymond, L.A.; Andre, V.M.; Cepeda, C.; Gladding, C.M.; Milnerwood, A.J.; Levine, M.S. Pathophysiology of Huntington’s disease: Time-dependent alterations in synaptic and receptor function. Neuroscience 2011, 198, 252–273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fox, J.; Lu, Z.; Barrows, L. Thiol-disulfide Oxidoreductases TRX1 and TMX3 Decrease Neuronal Atrophy in a Lentiviral Mouse Model of Huntington’s Disease. PLoS Curr. 2015, 7. [Google Scholar] [CrossRef] [PubMed]
- Galvan, A.; Wichmann, T. GABAergic circuits in the basal ganglia and movement disorders. Prog. Brain Res. 2007, 160, 287–312. [Google Scholar] [CrossRef] [PubMed]
- Hickey, M.A.; Chesselet, M.F. Apoptosis in Huntington’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2003, 27, 255–265. [Google Scholar] [CrossRef]
- Levine, M.S.; Cepeda, C.; Hickey, M.A.; Fleming, S.M.; Chesselet, M.F. Genetic mouse models of Huntington’s and Parkinson’s diseases: Illuminating but imperfect. Trends Neurosci. 2004, 27, 691–697. [Google Scholar] [CrossRef]
- Gafni, J.; Hermel, E.; Young, J.E.; Wellington, C.L.; Hayden, M.R.; Ellerby, L.M. Inhibition of calpain cleavage of huntingtin reduces toxicity: Accumulation of calpain/caspase fragments in the nucleus. J. Biol. Chem. 2004, 279, 20211–20220. [Google Scholar] [CrossRef] [Green Version]
- Fox, J.H.; Connor, T.; Stiles, M.; Kama, J.; Lu, Z.; Dorsey, K.; Lieberman, G.; Sapp, E.; Cherny, R.A.; Banks, M.; et al. Cysteine oxidation within N-terminal mutant huntingtin promotes oligomerization and delays clearance of soluble protein. J. Biol. Chem. 2011, 286, 18320–18330. [Google Scholar] [CrossRef] [Green Version]
- Arrasate, M.; Mitra, S.; Schweitzer, E.S.; Segal, M.R.; Finkbeiner, S. Inclusion body formation reduces levels of mutant huntingtin and the risk of neuronal death. Nature 2004, 431, 805–810. [Google Scholar] [CrossRef] [Green Version]
- Dunah, A.W.; Jeong, H.; Griffin, A.; Kim, Y.M.; Standaert, D.G.; Hersch, S.M.; Mouradian, M.M.; Young, A.B.; Tanese, N.; Krainc, D. Sp1 and TAFII130 transcriptional activity disrupted in early Huntington’s disease. Science 2002, 296, 2238–2243. [Google Scholar] [CrossRef]
- Vonsattel, J.P.; Myers, R.H.; Stevens, T.J.; Ferrante, R.J.; Bird, E.D.; Richardson, E.P., Jr. Neuropathological classification of Huntington’s disease. J. Neuropathol. Exp. Neurol. 1985, 44, 559–577. [Google Scholar] [CrossRef]
- Zhou, F.; Gomi, M.; Fujimoto, M.; Hayase, M.; Marumo, T.; Masutani, H.; Yodoi, J.; Hashimoto, N.; Nozaki, K.; Takagi, Y. Attenuation of neuronal degeneration in thioredoxin-1 overexpressing mice after mild focal ischemia. Brain Res. 2009, 1272, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Sorolla, M.A.; Reverter-Branchat, G.; Tamarit, J.; Ferrer, I.; Ros, J.; Cabiscol, E. Proteomic and oxidative stress analysis in human brain samples of Huntington disease. Free Radic. Biol. Med. 2008, 45, 667–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sreekala, S.; Indira, M. Impact of co administration of selenium and quinolinic acid in the rat’s brain. Brain Res. 2009, 1281, 101–107. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Rosenstock, T.R.; Cunha-Oliveira, T.; Ferreira, I.L.; Oliveira, C.R.; Rego, A.C. Glutathione redox cycle dysregulation in Huntington’s disease knock-in striatal cells. Free Radic. Biol. Med. 2012, 53, 1857–1867. [Google Scholar] [CrossRef] [PubMed]
- Kannan, R.; Kuhlenkamp, J.F.; Ookhtens, M.; Kaplowitz, N. Transport of glutathione at blood-brain barrier of the rat: Inhibition by glutathione analogs and age-dependence. J. Pharmacol. Exp. Ther. 1992, 263, 964–970. [Google Scholar]
- Mackic, J.B.; Jinagouda, S.; McComb, J.G.; Weiss, M.H.; Kannan, R.; Kaplowitz, N.; Zlokovic, B.V. Transport of circulating reduced glutathione at the basolateral side of the anterior lens epithelium: Physiologic importance and manipulations. Exp. Eye Res. 1996, 62, 29–37. [Google Scholar] [CrossRef]
- Kannan, R.; Mackic, J.B.; Zlokovic, B.V. Corneal transport of circulating glutathione in normal and galactosemic guinea pigs. Cornea 1999, 18, 321–327. [Google Scholar] [CrossRef]
- Kannan, R.; Chakrabarti, R.; Tang, D.; Kim, K.J.; Kaplowitz, N. GSH transport in human cerebrovascular endothelial cells and human astrocytes: Evidence for luminal localization of Na+-dependent GSH transport in HCEC. Brain Res. 2000, 852, 374–382. [Google Scholar] [CrossRef]
- Zlokovic, B.V.; Mackic, J.B.; McComb, J.G.; Weiss, M.H.; Kaplowitz, N.; Kannan, R. Evidence for transcapillary transport of reduced glutathione in vascular perfused guinea-pig brain. Biochem. Biophys. Res. Commun. 1994, 201, 402–408. [Google Scholar] [CrossRef]
- Kannan, R.; Yi, J.R.; Tang, D.; Zlokovic, B.V.; Kaplowitz, N. Identification of a novel, sodium-dependent, reduced glutathione transporter in the rat lens epithelium. Investig. Ophthalmol. Vis. Sci. 1996, 37, 2269–2275. [Google Scholar]
- De Almeida, L.P.; Ross, C.A.; Zala, D.; Aebischer, P.; Deglon, N. Lentiviral-mediated delivery of mutant huntingtin in the striatum of rats induces a selective neuropathology modulated by polyglutamine repeat size, huntingtin expression levels, and protein length. J. Neurosci. 2002, 22, 3473–3483. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, M.; Nishitoh, H.; Fujii, M.; Takeda, K.; Tobiume, K.; Sawada, Y.; Kawabata, M.; Miyazono, K.; Ichijo, H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998, 17, 2596–2606. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peterson, D.A. Umbilical cord blood cells and brain stroke injury: Bringing in fresh blood to address an old problem. J. Clin. Investig. 2004, 114, 312–314. [Google Scholar] [CrossRef] [PubMed]
- Nih, L.R.; Gojgini, S.; Carmichael, S.T.; Segura, T. Dual-function injectable angiogenic biomaterial for the repair of brain tissue following stroke. Nat. Mater. 2018, 17, 642–651. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; Fernandez-Gajardo, R.; Gutierrez, R.; Matamala, J.M.; Carrasco, R.; Miranda-Merchak, A.; Feuerhake, W. Oxidative stress and pathophysiology of ischemic stroke: Novel therapeutic opportunities. CNS Neurol. Disord. Drug Targets 2013, 12, 698–714. [Google Scholar] [CrossRef]
- Norrving, B.; Kissela, B. The global burden of stroke and need for a continuum of care. Neurology 2013, 80, S5–S12. [Google Scholar] [CrossRef]
- Kalladka, D.; Muir, K.W. Brain repair: Cell therapy in stroke. Stem Cells Cloning 2014, 7, 31–44. [Google Scholar] [CrossRef]
- Tsuda, K. Letter by Tsuda Regarding Article, “Oxidative Stress Biomarkers of Brain Damage: Hyperacute Plasma F2-Isoprostane Predicts Infarct Growth in Stroke”. Stroke 2018, 49, e263. [Google Scholar] [CrossRef]
- Lorenzano, S.; Rost, N.S.; Khan, M.; Li, H.; Lima, F.O.; Maas, M.B.; Green, R.E.; Thankachan, T.K.; Dipietro, A.J.; Arai, K.; et al. Oxidative Stress Biomarkers of Brain Damage: Hyperacute Plasma F2-Isoprostane Predicts Infarct Growth in Stroke. Stroke 2018, 49, 630–637. [Google Scholar] [CrossRef]
- Watson, W.H.; Yang, X.; Choi, Y.E.; Jones, D.P.; Kehrer, J.P. Thioredoxin and its role in toxicology. Toxicol. Sci. 2004, 78, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Q.; Che, X.; Zhang, H.; Tan, G.; Liu, L.; Jiang, D.; Zhao, J.; Xiang, X.; Sun, X.; He, Z. Thioredoxin-Interacting Protein Mediates Apoptosis in Early Brain Injury after Subarachnoid Haemorrhage. Int. J. Mol. Sci. 2017, 18, 854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, G.S.; Jung, J.E.; Narasimhan, P.; Sakata, H.; Chan, P.H. Induction of thioredoxin-interacting protein is mediated by oxidative stress, calcium, and glucose after brain injury in mice. Neurobiol. Dis. 2012, 46, 440–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, W.H.; Lin, H.S.; Chen, C.I.; Chou, M.S.; Liou, C.W.; Chen, S.S.; Stroke Center, C.K. The medicolegal issue of tissue plasminogen activator in ischemic stroke: A review of judiciary decrees in Taiwan. Acta Neurol. Taiwan 2011, 20, 163–171. [Google Scholar] [PubMed]
- Hermann, D.M.; Matter, C.M. Tissue plasminogen activator-induced reperfusion injury after stroke revisited. Circulation 2007, 116, 363–365. [Google Scholar] [CrossRef] [Green Version]
- Kelly, P.J.; Morrow, J.D.; Ning, M.; Koroshetz, W.; Lo, E.H.; Terry, E.; Milne, G.L.; Hubbard, J.; Lee, H.; Stevenson, E.; et al. Oxidative stress and matrix metalloproteinase-9 in acute ischemic stroke: The Biomarker Evaluation for Antioxidant Therapies in Stroke (BEAT-Stroke) study. Stroke 2008, 39, 100–104. [Google Scholar] [CrossRef] [Green Version]
- Ishrat, T.; Mohamed, I.N.; Pillai, B.; Soliman, S.; Fouda, A.Y.; Ergul, A.; El-Remessy, A.B.; Fagan, S.C. Erratum to: Thioredoxin-interacting protein: A novel target for neuroprotection in experimental thromboembolic stroke in mice. Mol. Neurobiol. 2015, 51, 779–780. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Ismael, S.; Nasoohi, S.; Sakata, K.; Liao, F.F.; McDonald, M.P.; Ishrat, T. Thioredoxin-Interacting Protein (TXNIP) Associated NLRP3 Inflammasome Activation in Human Alzheimer’s Disease Brain. J. Alzheimers Dis. 2019, 68, 255–265. [Google Scholar] [CrossRef]
- Tian, L.; Nie, H.; Zhang, Y.; Chen, Y.; Peng, Z.; Cai, M.; Wei, H.; Qin, P.; Dong, H.; Xiong, L. Recombinant human thioredoxin-1 promotes neurogenesis and facilitates cognitive recovery following cerebral ischemia in mice. Neuropharmacology 2014, 77, 453–464. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Tian, S.; Wang, J.; Han, F.; Zhao, L.; Wang, R.; Ning, W.; Chen, W.; Qu, Y. Intraperitoneal administration of thioredoxin decreases brain damage from ischemic stroke. Brain Res. 2015, 1615, 89–97. [Google Scholar] [CrossRef]
- Wu, X.; Li, L.; Zhang, L.; Wu, J.; Zhou, Y.; Zhou, Y.; Zhao, Y.; Zhao, J. Inhibition of thioredoxin-1 with siRNA exacerbates apoptosis by activating the ASK1-JNK/p38 pathway in brain of a stroke model rats. Brain Res. 2015, 1599, 20–31. [Google Scholar] [CrossRef]
- Li, L.; Zhu, K.; Liu, Y.; Wu, X.; Wu, J.; Zhao, Y.; Zhao, J. Targeting thioredoxin-1 with siRNA exacerbates oxidative stress injury after cerebral ischemia/reperfusion in rats. Neuroscience 2015, 284, 815–823. [Google Scholar] [CrossRef] [PubMed]
- Qi, A.Q.; Li, Y.; Liu, Q.; Si, J.Z.; Tang, X.M.; Zhang, Z.Q.; Qi, Q.D.; Chen, W.B. Thioredoxin is a novel diagnostic and prognostic marker in patients with ischemic stroke. Free Radic. Biol. Med. 2015, 80, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Baratz-Goldstein, R.; Deselms, H.; Heim, L.R.; Khomski, L.; Hoffer, B.J.; Atlas, D.; Pick, C.G. Thioredoxin-Mimetic-Peptides Protect Cognitive Function after Mild Traumatic Brain Injury (mTBI). PLoS ONE 2016, 11, e0157064. [Google Scholar] [CrossRef] [PubMed]
- Voigt, D.; Scheidt, U.; Derfuss, T.; Bruck, W.; Junker, A. Expression of the Antioxidative Enzyme Peroxiredoxin 2 in Multiple Sclerosis Lesions in Relation to Inflammation. Int. J. Mol. Sci. 2017, 18, 760. [Google Scholar] [CrossRef] [Green Version]
- De Bock, L.; Somers, K.; Fraussen, J.; Hendriks, J.J.; van Horssen, J.; Rouwette, M.; Hellings, N.; Villar, L.M.; Alvarez-Cermeno, J.C.; Espino, M.; et al. Sperm-associated antigen 16 is a novel target of the humoral autoimmune response in multiple sclerosis. J. Immunol. 2014, 193, 2147–2156. [Google Scholar] [CrossRef] [Green Version]
- Edan, G. Multiple Sclerosis over the last 25 years: An introduction. Rev. Neurol. 2018, 174, 353–354. [Google Scholar] [CrossRef]
- Wendebourg, M.J.; Heesen, C.; Finlayson, M.; Meyer, B.; Pottgen, J.; Kopke, S. Patient education for people with multiple sclerosis-associated fatigue: A systematic review. PLoS ONE 2017, 12, e0173025. [Google Scholar] [CrossRef]
- Bar-Or, A. Multiple sclerosis and related disorders: Evolving pathophysiologic insights. Lancet Neurol. 2016, 15, 9–11. [Google Scholar] [CrossRef]
- Ascherio, A.; Munger, K.L. Environmental risk factors for multiple sclerosis. Part II: Noninfectious factors. Ann. Neurol. 2007, 61, 504–513. [Google Scholar] [CrossRef]
- Perron, H.; Bernard, C.; Bertrand, J.B.; Lang, A.B.; Popa, I.; Sanhadji, K.; Portoukalian, J. Endogenous retroviral genes, Herpesviruses and gender in Multiple Sclerosis. J. Neurol. Sci. 2009, 286, 65–72. [Google Scholar] [CrossRef]
- Morandi, E.; Tanasescu, R.; Tarlinton, R.E.; Constantinescu, C.S.; Zhang, W.; Tench, C.; Gran, B. The association between human endogenous retroviruses and multiple sclerosis: A systematic review and meta-analysis. PLoS ONE 2017, 12, e0172415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Horssen, J.; Witte, M.E.; Schreibelt, G.; de Vries, H.E. Radical changes in multiple sclerosis pathogenesis. Biochim. Biophys. Acta 2011, 1812, 141–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Draheim, T.; Liessem, A.; Scheld, M.; Wilms, F.; Weissflog, M.; Denecke, B.; Kensler, T.W.; Zendedel, A.; Beyer, C.; Kipp, M.; et al. Activation of the astrocytic Nrf2/ARE system ameliorates the formation of demyelinating lesions in a multiple sclerosis animal model. Glia 2016, 64, 2219–2230. [Google Scholar] [CrossRef] [PubMed]
- Nijland, P.G.; Witte, M.E.; van het Hof, B.; van der Pol, S.; Bauer, J.; Lassmann, H.; van der Valk, P.; de Vries, H.E.; van Horssen, J. Astroglial PGC-1alpha increases mitochondrial antioxidant capacity and suppresses inflammation: Implications for multiple sclerosis. Acta Neuropathol. Commun. 2014, 2, 170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lassmann, H.; van Horssen, J. Oxidative stress and its impact on neurons and glia in multiple sclerosis lesions. Biochim. Biophys. Acta 2016, 1862, 506–510. [Google Scholar] [CrossRef]
- Perkins, R.B.; Christiansen, C.L. The human papillomavirus vaccination is not associated with risk of multiple sclerosis or other demyelinating diseases. Evid. Based Med. 2015, 20, 116. [Google Scholar] [CrossRef]
- Pellegrini, G.G.; Cregor, M.; McAndrews, K.; Morales, C.C.; McCabe, L.D.; McCabe, G.P.; Peacock, M.; Burr, D.; Weaver, C.; Bellido, T. Nrf2 regulates mass accrual and the antioxidant endogenous response in bone differently depending on the sex and age. PLoS ONE 2017, 12, e0171161. [Google Scholar] [CrossRef]
- Choi, I.Y.; Lee, P.; Adany, P.; Hughes, A.J.; Belliston, S.; Denney, D.R.; Lynch, S.G. In vivo evidence of oxidative stress in brains of patients with progressive multiple sclerosis. Mult. Scler. 2018, 24, 1029–1038. [Google Scholar] [CrossRef]
- Ohl, K.; Tenbrock, K.; Kipp, M. Oxidative stress in multiple sclerosis: Central and peripheral mode of action. Exp. Neurol. 2016, 277, 58–67. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Nurchi, V.M.; Lachowicz, J.I.; Crisponi, G.; Zoroddu, M.A. Noble metals in medicine: Latest advances. Coord. Chem. Rev. 2015, 284, 329–350. [Google Scholar] [CrossRef]
- Zhong, L.; Arner, E.S.; Ljung, J.; Aslund, F.; Holmgren, A. Rat and calf thioredoxin reductase are homologous to glutathione reductase with a carboxyl-terminal elongation containing a conserved catalytically active penultimate selenocysteine residue. J. Biol. Chem. 1998, 273, 8581–8591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zou, L.; Lu, J.; Wang, J.; Ren, X.; Zhang, L.; Gao, Y.; Rottenberg, M.E.; Holmgren, A. Synergistic antibacterial effect of silver and ebselen against multidrug-resistant Gram-negative bacterial infections. EMBO Mol. Med. 2017, 9, 1165–1178. [Google Scholar] [CrossRef] [PubMed]
- Dong, C.; Zhou, J.; Wang, P.; Li, T.; Zhao, Y.; Ren, X.; Lu, J.; Wang, J.; Holmgren, A.; Zou, L. Topical Therapeutic Efficacy of Ebselen Against Multidrug-Resistant Staphylococcus aureus LT-1 Targeting Thioredoxin Reductase. Front. Microbiol. 2019, 10, 3016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunigan-Russell, K.; Lin, V.; Silverberg, M.; Wall, S.B.; Li, R.; Gotham, J.; Nicola, T.; Sridharan, A.; Snowball, J.; Delaney, C.; et al. Aurothioglucose Enhances Pro-Angiogenic Pathway Activation in Lungs from Room Air and Hyperoxia-exposed Newborn Mice. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 318, L1165–L1171. [Google Scholar] [CrossRef] [PubMed]
- Onodera, T.; Momose, I.; Kawada, M. Potential Anticancer Activity of Auranofin. Chem. Pharm. Bull. 2019, 67, 186–191. [Google Scholar] [CrossRef] [Green Version]
- Yumnamcha, T.; Devi, T.S.; Singh, L.P. Auranofin Mediates Mitochondrial Dysregulation and Inflammatory Cell Death in Human Retinal Pigment Epithelial Cells: Implications of Retinal Neurodegenerative Diseases. Front. Neurosci. 2019, 13, 1065. [Google Scholar] [CrossRef]
- Prast-Nielsen, S.; Cebula, M.; Pader, I.; Arner, E.S. Noble metal targeting of thioredoxin reductase—Covalent complexes with thioredoxin and thioredoxin-related protein of 14 kDa triggered by cisplatin. Free Radic. Biol. Med. 2010, 49, 1765–1778. [Google Scholar] [CrossRef]
- Rigobello, M.P.; Messori, L.; Marcon, G.; Agostina Cinellu, M.; Bragadin, M.; Folda, A.; Scutari, G.; Bindoli, A. Gold complexes inhibit mitochondrial thioredoxin reductase: Consequences on mitochondrial functions. J. Inorg. Biochem. 2004, 98, 1634–1641. [Google Scholar] [CrossRef]
- Branco, V.; Canario, J.; Lu, J.; Holmgren, A.; Carvalho, C. Mercury and selenium interaction in vivo: Effects on thioredoxin reductase and glutathione peroxidase. Free Radic. Biol. Med. 2012, 52, 781–793. [Google Scholar] [CrossRef]
- Spiller, H.A. Rethinking mercury: The role of selenium in the pathophysiology of mercury toxicity. Clin. Toxicol. 2018, 56, 313–326. [Google Scholar] [CrossRef]
- Farina, M.; Aschner, M. Glutathione antioxidant system and methylmercury-induced neurotoxicity: An intriguing interplay. Biochim. Biophys. Acta Gen. Subj. 2019, 1863, 129285. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, C.M.; Chew, E.H.; Hashemy, S.I.; Lu, J.; Holmgren, A. Inhibition of the human thioredoxin system. A molecular mechanism of mercury toxicity. J. Biol. Chem. 2008, 283, 11913–11923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, C.L.; Jang, T.H.; Wang, L.H. Effects of mercury on serotonin concentration in the brain of tilapia, Oreochromis mossambicus. Neurosci. Lett. 1995, 184, 208–211. [Google Scholar] [CrossRef]
- Zhou, T.; Rademacher, D.J.; Steinpreis, R.E.; Weis, J.S. Neurotransmitter levels in two populations of larval Fundulus heteroclitus after methylmercury exposure. Comp Biochem. Physiol. C Pharmacol. Toxicol. Endocrinol. 1999, 124, 287–294. [Google Scholar] [CrossRef]
- Bjørklund, G.; Antonyak, H.; Polishchuk, A.; Semenova, Y.; Lesiv, M.; Lysiuk, R.; Peana, M. Effect of methylmercury on fetal neurobehavioral development: An overview of the possible mechanisms of toxicity and the neuroprotective effect of phytochemicals. Arch. Toxicol. 2022, 96, 3175–3199. [Google Scholar] [CrossRef] [PubMed]
- Hattori, K.; Sukenobu, N.; Sasaki, T.; Takasuga, S.; Hayashi, T.; Kasai, R.; Yamasaki, K.; Hazeki, O. Activation of insulin receptors by lagerstroemin. J. Pharmacol. Sci. 2003, 93, 69–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bessems, J.G.; Vermeulen, N.P. Paracetamol (acetaminophen)-induced toxicity: Molecular and biochemical mechanisms, analogues and protective approaches. Crit. Rev. Toxicol. 2001, 31, 55–138. [Google Scholar] [CrossRef]
- Hansen, J.M.; Zhang, H.; Jones, D.P. Differential oxidation of thioredoxin-1, thioredoxin-2, and glutathione by metal ions. Free Radic. Biol. Med. 2006, 40, 138–145. [Google Scholar] [CrossRef]
- Tanaka, K.I.; Shimoda, M.; Chuang, V.T.G.; Nishida, K.; Kawahara, M.; Ishida, T.; Otagiri, M.; Maruyama, T.; Ishima, Y. Thioredoxin-albumin fusion protein prevents copper enhanced zinc-induced neurotoxicity via its antioxidative activity. Int. J. Pharm. 2018, 535, 140–147. [Google Scholar] [CrossRef]
- Maher, P. Potentiation of glutathione loss and nerve cell death by the transition metals iron and copper: Implications for age-related neurodegenerative diseases. Free Radic. Biol. Med. 2018, 115, 92–104. [Google Scholar] [CrossRef]
- Zou, L.; Wang, J.; Gao, Y.; Ren, X.; Rottenberg, M.E.; Lu, J.; Holmgren, A. Synergistic antibacterial activity of silver with antibiotics correlating with the upregulation of the ROS production. Sci. Rep. 2018, 8, 11131. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bjørklund, G.; Zou, L.; Peana, M.; Chasapis, C.T.; Hangan, T.; Lu, J.; Maes, M. The Role of the Thioredoxin System in Brain Diseases. Antioxidants 2022, 11, 2161. https://doi.org/10.3390/antiox11112161
Bjørklund G, Zou L, Peana M, Chasapis CT, Hangan T, Lu J, Maes M. The Role of the Thioredoxin System in Brain Diseases. Antioxidants. 2022; 11(11):2161. https://doi.org/10.3390/antiox11112161
Chicago/Turabian StyleBjørklund, Geir, Lili Zou, Massimiliano Peana, Christos T. Chasapis, Tony Hangan, Jun Lu, and Michael Maes. 2022. "The Role of the Thioredoxin System in Brain Diseases" Antioxidants 11, no. 11: 2161. https://doi.org/10.3390/antiox11112161







