Potential Impacts of Hydralazine as a Novel Antioxidant on Cardiovascular and Renal Disease—Beyond Vasodilation and Blood Pressure Lowering
Abstract
:1. Introduction
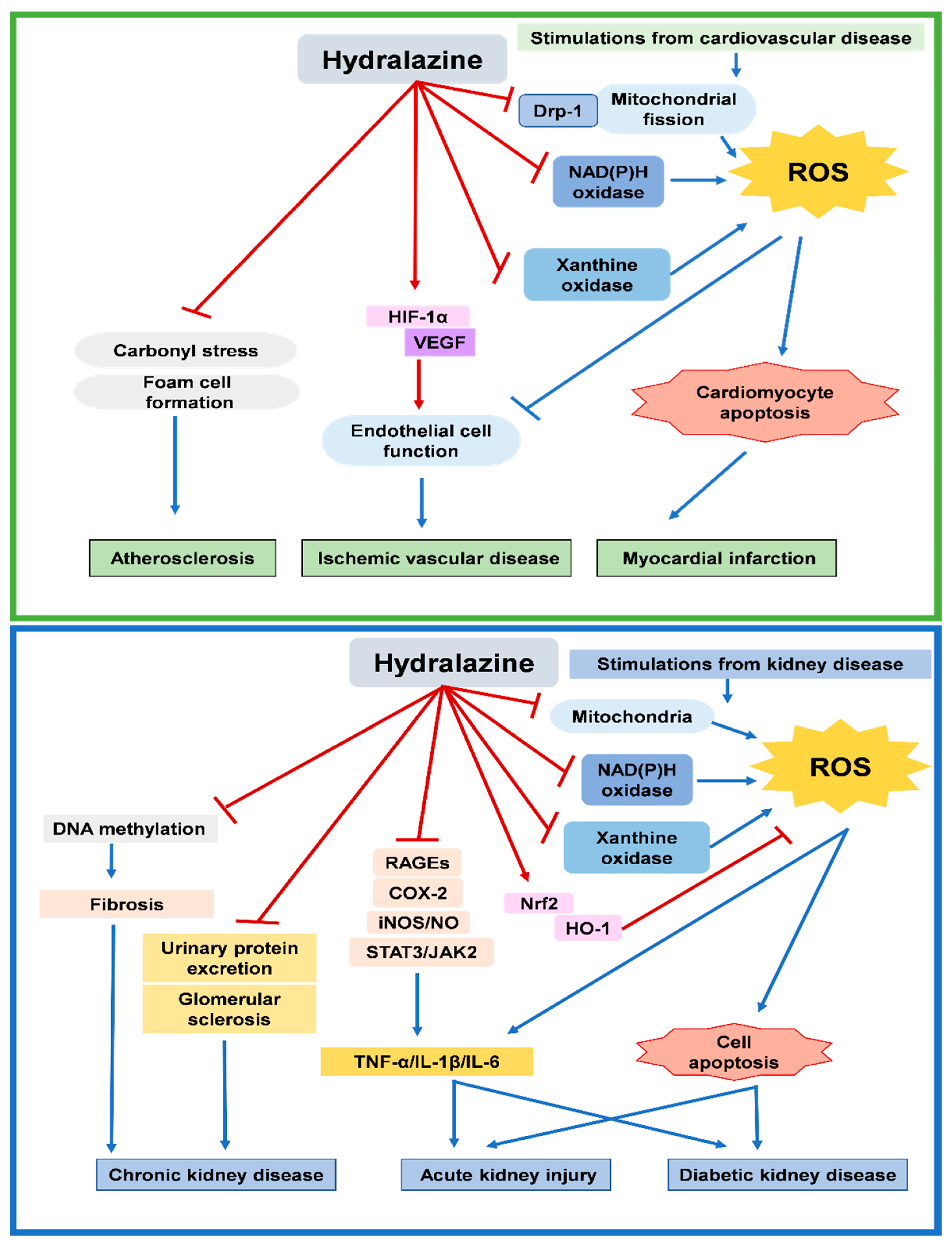
2. Potential Effects of Hydralazine on Cardiovascular Disease
2.1. Hydralazine for Cardiac Protection after Myocardial Infarction
2.2. Hydralazine for Angiogenesis in Ischemic Vascular Disease
2.3. Hydralazine for Plaque Stabilization in Atherosclerosis Disease
3. Potential Effects of Hydralazine on Kidney Disease
3.1. Hydralazine for Chronic Kidney Disease
3.2. Hydralazine for Acute Kidney Injury
3.3. Hydralazine for Diabetic Kidney Disease
4. Potential Mechanisms for Beneficial Effects of Hydralazine
4.1. Potential Mechanisms for Systemic Effects of Hydralazine
4.2. Potential Mechanisms for Intracellular Effects of Hydralazine
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AGE | Advanced glycation end product |
| AKI | Acute kidney injury |
| CKD | Chronic kidney disease |
| DKD | Diabetic kidney disease |
| EPC | Endothelial progenitor cells |
| HIF | Hypoxia-inducible factor |
| iNOS | Inducible nitric oxide synthase |
| IL | Interleukin |
| IRI | Ischemia/reperfusion injury |
| NO | Nitric oxide |
| RAGE | Receptor for advanced glycation end products |
| STZ | Streptozotocin |
| TNF | Tumor necrosis factor |
| XO | Xanthine oxidase |
References
- Arce, C.; Segura-Pacheco, B.; Perez-Cardenas, E.; Taja-Chayeb, L.; Candelaria, M.; Dueñnas-Gonzalez, A. Hydralazine target: From blood vessels to the epigenome. J. Transl. Med. 2006, 4, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ellershaw, D.C.; Gurney, A.M. Mechanisms of hydralazine induced vasodilation in rabbit aorta and pulmonary artery. Br. J. Pharmacol. 2001, 134, 621–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobs, M. Mechanism of action of hydralazine on vascular smooth muscle. Biochem. Pharmacol. 1984, 33, 2915–2919. [Google Scholar] [CrossRef]
- Münzel, T.; Kurz, S.; Rajagopalan, S.; Thoenes, M.; Berrington, W.R.; Thompson, J.A.; Freeman, B.A.; Harrison, D.G. Hydralazine prevents nitroglycerin tolerance by inhibiting activation of a membrane-bound NADH oxidase. A new action for an old drug. J. Clin. Investig. 1996, 98, 1465–1470. [Google Scholar] [CrossRef] [Green Version]
- Kesavan, S.K.; Bhat, S.; Golegaonkar, S.B.; Jagadeeshaprasad, M.G.; Deshmukh, A.B.; Patil, H.S.; Bhosale, S.D.; Shaikh, M.L.; Thulasiram, H.V.; Boppana, R.; et al. Proteome wide reduction in AGE modification in streptozotocin induced diabetic mice by hydralazine mediated transglycation. Sci. Rep. 2013, 3, 2941. [Google Scholar] [CrossRef] [Green Version]
- Dudek, J.; Maack, C. Grandfathe’s moonlighting: Hydralazin’s novel liaison with mitochondria. Cardiovasc. Res. 2022, 118, 13–15. [Google Scholar] [CrossRef]
- Robson, A. Hydralazine reduces myocardial infarct size. Nat. Rev. Cardiol. 2021, 18, 232. [Google Scholar] [CrossRef]
- Kalkhoran, S.B.; Kriston-Vizi, J.; Hernandez-Resendiz, S.; Crespo-Avilan, G.E.; Rosdah, A.A.; Lees, J.G.; Da Costa, J.R.S.; Ling, N.X.Y.; Holien, J.K.; Samangouei, P.; et al. Hydralazine protects the heart against acute ischemia/reperfusion injury by inhibiting Drp1-mediated mitochondrial fission. Cardiovasc. Res. 2022, 118, 282–294. [Google Scholar] [CrossRef]
- Hoenig, M.R.; Bianchi, C.; Sellke, F.W. Hypoxia inducible factor-1 alpha, endothelial progenitor cells, monocytes, cardiovascular risk, wound healing, cobalt and hydralazine: A unifying hypothesis. Curr. Drug Targets 2008, 9, 422–435. [Google Scholar] [CrossRef]
- Knowles, H.J.; Tian, Y.M.; Mole, D.R.; Harris, A.L. Novel mechanism of action for hydralazine: Induction of hypoxia-inducible factor-1alpha, vascular endothelial growth factor, and angiogenesis by inhibition of prolyl hydroxylases. Circ. Res. 2004, 95, 162–169. [Google Scholar] [CrossRef]
- Chang, T.T.; Chen, J.W. Hydralazine improves ischemia-induced neovasculogenesis via xanthine-oxidase inhibition in chronic renal insufficiency. Pharmacol. Res. 2020, 151, 104509. [Google Scholar] [CrossRef] [PubMed]
- Weiss, D.; Taylor, W.R. Deoxycorticosterone acetate salt hypertension in apolipoprotein E−/− mice results in accelerated atherosclerosis: The role of angiotensin II. Hypertension 2008, 51, 218–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galvani, S.; Coatrieux, C.; Elbaz, M.; Grazide, M.H.; Thiers, J.C.; Parini, A.; Uchida, K.; Kamar, N.; Rostaing, L.; Baltas, M.; et al. Carbonyl scavenger and antiatherogenic effects of hydrazine derivatives. Free. Radic. Biol. Med. 2008, 45, 1457–1467. [Google Scholar] [CrossRef] [PubMed]
- Camaré, C.; Vanucci-Bacqué, C.; Augé, N.; Pucelle, M.; Bernis, C.; Swiader, A.; Baltas, M.; Bedos-Belval, F.; Salvayre, R.; Nègre-Salvayre, A. 4-Hydroxynonenal Contributes to Angiogenesis through a Redox-Dependent Sphingolipid Pathway: Prevention by Hydralazine Derivatives. Oxidative Med. Cell. Longev. 2017, 2017, 9172741. [Google Scholar] [CrossRef] [PubMed]
- Finks, S.W.; Finks, A.L.; Self, T.H. Hydralazine-induced lupus: Maintaining vigilance with increased use in patients with heart failure. South. Med. J. 2006, 99, 18–22. [Google Scholar] [CrossRef]
- Panchapakesan, U.; Pollock, C. Drug repurposing in kidney disease. Kidney Int. 2018, 94, 40–48. [Google Scholar] [CrossRef]
- Sugama, I.; Kohagura, K.; Yamazato, M.; Nakamura, T.; Shinzato, T.; Ohya, Y. Superoxide dismutase mimetic, tempol, aggravates renal injury in advanced-stage stroke-prone spontaneously hypertensive rats. J. Hypertens. 2014, 32, 534–541. [Google Scholar] [CrossRef]
- Uramatsu, T.; Nishino, T.; Obata, Y.; Sato, Y.; Furusu, A.; Koji, T.; Miyazaki, T.; Kohno, S. Involvement of apoptosis inhibitor of macrophages in a rat hypertension model with nephrosclerosis: Possible mechanisms of action of olmesartan and azelnidipine. Biol. Pharm. Bull. 2013, 36, 1271–1277. [Google Scholar] [CrossRef] [Green Version]
- Hartono, S.P.; Knudsen, B.E.; Lerman, L.O.; Textor, S.C.; Grande, J.P. Combined effect of hyperfiltration and renin angiotensin system activation on development of chronic kidney disease in diabetic db/db mice. BMC Nephrol. 2014, 15, 58. [Google Scholar] [CrossRef] [Green Version]
- Larkin, B.P.; Nguyen, L.T.; Hou, M.; Glastras, S.J.; Chen, H.; Wang, R.; Pollock, C.A.; Saad, S. Novel Role of Gestational Hydralazine in Limiting Maternal and Dietary Obesity-Related Chronic Kidney Disease. Front. Cell Dev. Biol. 2021, 9, 705263. [Google Scholar] [CrossRef]
- Mizumoto, T.; Kakizoe, Y.; Nakagawa, T.; Iwata, Y.; Miyasato, Y.; Uchimura, K.; Adachi, M.; Deng, Q.; Hayata, M.; Morinaga, J.; et al. A serine protease inhibitor camostat mesilate prevents podocyte apoptosis and attenuates podocyte injury in metabolic syndrome model rats. J. Pharmacol. Sci. 2021, 146, 192–199. [Google Scholar] [CrossRef] [PubMed]
- Larkin, B.P.; Saad, S.; Glastras, S.J.; Nguyen, L.T.; Hou, M.; Chen, H.; Wang, R.; Pollock, C.A. Low-dose hydralazine during gestation reduces renal fibrosis in rodent offspring exposed to maternal high fat diet. PLoS ONE 2021, 16, e0248854. [Google Scholar] [CrossRef] [PubMed]
- Tampe, B.; Steinle, U.; Tampe, D.; Carstens, J.L.; Korsten, P.; Zeisberg, E.M.; Müller, G.A.; Kalluri, R.; Zeisberg, M. Low-dose hydralazine prevents fibrosis in a murine model of acute kidney injury-to-chronic kidney disease progression. Kidney Int. 2017, 91, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Tampe, B.; Tampe, D.; Zeisberg, E.M.; Müller, G.A.; Bechtel-Walz, W.; Koziolek, M.; Kalluri, R.; Zeisberg, M. Induction of Tet3-dependent Epigenetic Remodeling by Low-dose Hydralazine Attenuates Progression of Chronic Kidney Disease. EBioMedicine 2015, 2, 19–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leu, J.G.; Su, W.H.; Chen, Y.C.; Liang, Y.J. Hydralazine attenuates renal inflammation in diabetic rats with ischemia/reperfusion acute kidney injury. Eur. J. Pharmacol. 2021, 910, 174468. [Google Scholar] [CrossRef]
- Li, Y.; Hou, D.; Chen, X.; Zhu, J.; Zhang, R.; Sun, W.; Li, P.; Tian, Y.; Kong, X. Hydralazine protects against renal ischemia-reperfusion injury in rats. Eur. J. Pharmacol. 2019, 843, 199–209. [Google Scholar] [CrossRef]
- Nishiyama, A.; Nakagawa, T.; Kobori, H.; Nagai, Y.; Okada, N.; Konishi, Y.; Morikawa, T.; Okumura, M.; Meda, I.; Kiyomoto, H.; et al. Strict angiotensin blockade prevents the augmentation of intrarenal angiotensin II and podocyte abnormalities in type 2 diabetic rats with microalbuminuria. J. Hypertens. 2008, 26, 1849–1859. [Google Scholar] [CrossRef] [Green Version]
- Habibi, J.; Aroor, A.R.; Das, N.A.; Manrique-Acevedo, C.M.; Johnson, M.S.; Hayden, M.R.; Nistala, R.; Wiedmeyer, C.; Chandrasekar, B.; DeMarco, V.G. The combination of a neprilysin inhibitor (sacubitril) and angiotensin-II receptor blocker (valsartan) attenuates glomerular and tubular injury in the Zucker Obese rat. Cardiovasc. Diabetol. 2019, 18, 40. [Google Scholar] [CrossRef] [Green Version]
- Nangaku, M.; Miyata, T.; Sada, T.; Mizuno, M.; Inagi, R.; Ueda, Y.; Ishikawa, N.; Yuzawa, H.; Koike, H.; van Ypersele de Strihou, C.; et al. Anti-hypertensive agents inhibit in vivo the formation of advanced glycation end products and improve renal damage in a type 2 diabetic nephropathy rat model. J. Am. Soc. Nephrol. JASN 2003, 14, 1212–1222. [Google Scholar] [CrossRef] [Green Version]
- Chang, T.T.; Chiang, C.H.; Chen, C.; Lin, S.C.; Lee, H.J.; Chen, J.W. Antioxidation and Nrf2-mediated heme oxygenase-1 activation contribute to renal protective effects of hydralazine in diabetic nephropathy. Biomed. Pharmacother. 2022, 151, 113139. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, T.-T.; Chen, J.-W. Potential Impacts of Hydralazine as a Novel Antioxidant on Cardiovascular and Renal Disease—Beyond Vasodilation and Blood Pressure Lowering. Antioxidants 2022, 11, 2224. https://doi.org/10.3390/antiox11112224
Chang T-T, Chen J-W. Potential Impacts of Hydralazine as a Novel Antioxidant on Cardiovascular and Renal Disease—Beyond Vasodilation and Blood Pressure Lowering. Antioxidants. 2022; 11(11):2224. https://doi.org/10.3390/antiox11112224
Chicago/Turabian StyleChang, Ting-Ting, and Jaw-Wen Chen. 2022. "Potential Impacts of Hydralazine as a Novel Antioxidant on Cardiovascular and Renal Disease—Beyond Vasodilation and Blood Pressure Lowering" Antioxidants 11, no. 11: 2224. https://doi.org/10.3390/antiox11112224




