Genetic Polymorphisms in Oxidative Stress and Inflammatory Pathways as Potential Biomarkers in Alzheimer’s Disease and Dementia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. Assesment
2.3. Cerebrospinal Fluid Analysis
2.4. Genotyping
2.5. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Association of Investigated SNPs with Dementia and AD Susceptibility
3.3. Association of Investigated SNPs with CSF Biomarker Levels and MMSE
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kawas, C.; Gray, S.; Brookmeyer, R.; Fozard, J.; Zonderman, A. Age-specific incidence rates of Alzheimer’s disease: The Baltimore longitudinal study of aging. Neurology 2000, 54, 2072–2077. [Google Scholar] [CrossRef] [PubMed]
- 2020 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2020, 16, 391–460. [CrossRef] [PubMed]
- Masters, C.L.; Bateman, R.; Blennow, K.; Rowe, C.C.; Sperling, R.A.; Cummings, J.L. Alzheimer’s disease. Nat. Rev. Dis. Prim. 2015, 1, 15056. [Google Scholar] [CrossRef] [PubMed]
- Förstl, H.; Kurz, A. Clinical features of Alzheimer’s disease. Eur. Arch. Psychiatry Clin. Neurosci. 1999, 249, 288–290. [Google Scholar] [CrossRef]
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 57, 1105–1121. [Google Scholar] [CrossRef] [Green Version]
- Lovell, M.A.; Markesbery, W.R. Oxidative DNA damage in mild cognitive impairment and late-stage Alzheimer’s disease. Nucleic Acids Res. 2007, 35, 7497–7504. [Google Scholar] [CrossRef] [Green Version]
- Schippling, S.; Kontush, A.; Arlt, S.; Buhmann, C.; Stürenburg, H.-J.; Mann, U.; Müller-Thomsen, T.; Beisiegel, U. Increased lipoprotein oxidation in alzheimer’s disease. Free Radic. Biol. Med. 2000, 28, 351–360. [Google Scholar] [CrossRef]
- Forster, M.J.; Dubey, A.; Dawson, K.M.; Stutts, W.A.; Lal, H.; Sohal, R.S. Age-related losses of cognitive function and motor skills in mice are associated with oxidative protein damage in the brain. Proc. Natl. Acad. Sci. USA 1996, 93, 4765–4769. [Google Scholar] [CrossRef] [Green Version]
- Shelat, P.B.; Chalimoniuk, M.; Wang, J.-H.; Strosznajder, J.B.; Lee, J.C.; Sun, A.Y.; Simonyi, A.; Sun, G.Y. Amyloid beta peptide and NMDA induce ROS from NADPH oxidase and AA release from cytosolic phospholipase A 2 in cortical neurons. J. Neurochem. 2008, 106, 45–55. [Google Scholar] [CrossRef]
- Ill-Raga, G.; Ramos-Fernández, E.; Guix, F.X.; Tajes, M.; Bosch-Morató, M.; Palomer, E.; Godoy, J.; Belmar, S.; Cerpa, W.; Simpkins, J.W.; et al. Amyloid-β Peptide Fibrils Induce Nitro-Oxidative Stress in Neuronal Cells. J. Alzheimer’s Dis. 2010, 22, 641–652. [Google Scholar] [CrossRef] [Green Version]
- Bai, R.; Guo, J.; Ye, X.-Y.; Xie, Y.; Xie, T. Oxidative stress: The core pathogenesis and mechanism of Alzheimer’s disease. Ageing Res. Rev. 2022, 77, 101619. [Google Scholar] [CrossRef] [PubMed]
- Pappolla, M.A.; Omar, R.A.; Kim, K.S.; Robakis, N.K. Immunohistochemical evidence of oxidative [corrected] stress in Alzheimer’s disease. Am. J. Pathol. 1992, 140, 621–628. [Google Scholar] [PubMed]
- Saha, S.; Buttari, B.; Profumo, E.; Tucci, P.; Saso, L. A Perspective on Nrf2 Signaling Pathway for Neuroinflammation: A Potential Therapeutic Target in Alzheimer’s and Parkinson’s Diseases. Front. Cell. Neurosci. 2022, 15, 551. [Google Scholar] [CrossRef] [PubMed]
- Weisman, D.; Hakimian, E.; Ho, G.J. Interleukins, Inflammation, and Mechanisms of Alzheimer’s Disease. Vitam. Horm. 2006, 74, 505–530. [Google Scholar] [CrossRef]
- Disabato, D.J.; Quan, N.; Godbout, J.P. Neuroinflammation: The devil is in the details. J. Neurochem. 2016, 139, 136–153. [Google Scholar] [CrossRef] [Green Version]
- Heneka, M.T.; Golenbock, D.T.; Latz, E. Innate immunity in Alzheimer’s disease. Nat. Immunol. 2015, 16, 229–236. [Google Scholar] [CrossRef]
- Frost, G.R.; Jonas, L.A.; Li, Y.M. Friend, Foe or Both? Immune Activity in Alzheimer’s Disease. Front. Aging Neurosci. 2019, 11, 337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leng, F.; Edison, P. Neuroinflammation and microglial activation in Alzheimer disease: Where do we go from here? Nat. Rev. Neurol. 2021, 17, 157–172. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef] [Green Version]
- Liddelow, S.A.; Barres, B.A. Reactive Astrocytes: Production, Function, and Therapeutic Potential. Immunity 2017, 46, 957–967. [Google Scholar] [CrossRef] [Green Version]
- Olah, M.; Biber, K.; Vinet, J.; Boddeke, H.W.G.M. Microglia Phenotype Diversity. CNS Neurol. Disord. Drug Targets 2011, 10, 108–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Liu, Y.; Hao, W.; Wolf, L.; Kiliaan, A.J.; Penke, B.; Rübe, C.E.; Walter, J.; Heneka, M.T.; Hartmann, T.; et al. TLR2 Is a Primary Receptor for Alzheimer’s Amyloid β Peptide To Trigger Neuroinflammatory Activation. J. Immunol. 2012, 188, 1098–1107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murgas, P.; Godoy, B.; von Bernhardi, R. Aβ Potentiates Inflammatory Activation of Glial Cells Induced by Scavenger Receptor Ligands and Inflammatory Mediators in Culture. Neurotox. Res. 2012, 22, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-H.; Zhou, W.; Liu, S.; Deng, Y.; Cai, F.; Tone, M.; Tone, Y.; Tong, Y.; Song, W. Increased NF-κB signalling up-regulates BACE1 expression and its therapeutic potential in Alzheimer’s disease. Int. J. Neuropsychopharmacol. 2012, 15, 77–90. [Google Scholar] [CrossRef] [Green Version]
- Petralia, M.C.; Battaglia, G.; Bruno, V.; Pennisi, M.; Mangano, K.; Lombardo, S.D.; Fagone, P.; Cavalli, E.; Saraceno, A.; Nicoletti, F.; et al. The role of macrophage migration inhibitory factor in Alzheimer′s disease: Conventionally pathogenetic or unconventionally protective? Molecules 2020, 25, 291. [Google Scholar] [CrossRef] [Green Version]
- Basile, M.S.; Battaglia, G.; Bruno, V.; Mangano, K.; Fagone, P.; Petralia, M.C.; Nicoletti, F.; Cavalli, E. The dichotomic role of macrophage migration inhibitory factor in neurodegeneration. Int. J. Mol. Sci. 2020, 21, 3023. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, A.; Xia, C.; Lin, Q.; Chen, C. A single nucleotide polymorphism in primary-microRNA-146a reduces the expression of mature microRNA-146a in patients with Alzheimer’s disease and is associated with the pathogenesis of Alzheimer’s disease. Mol. Med. Rep. 2015, 12, 4037–4042. [Google Scholar] [CrossRef] [Green Version]
- Morales, I.; Jiménez, J.M.; Mancilla, M.; Maccioni, R.B. Tau Oligomers and Fibrils Induce Activation of Microglial Cells. J. Alzheimer’s Dis. 2013, 37, 849–856. [Google Scholar] [CrossRef]
- Bolós, M.; Llorens-Martín, M.; Jurado-Arjona, J.; Hernández, F.; Rábano, A.; Avila, J. Direct Evidence of Internalization of Tau by Microglia In Vitro and In Vivo. J. Alzheimer’s Dis. 2015, 50, 77–87. [Google Scholar] [CrossRef] [Green Version]
- Wes, P.D.; Easton, A.; Corradi, J.; Barten, D.M.; Devidze, N.; DeCarr, L.B.; Truong, A.; He, A.; Barrezueta, N.X.; Polson, C.; et al. Tau Overexpression Impacts a Neuroinflammation Gene Expression Network Perturbed in Alzheimer’s Disease. PLoS ONE 2014, 9, e106050. [Google Scholar] [CrossRef]
- Asai, H.; Ikezu, S.; Tsunoda, S.; Medalla, M.; Luebke, J.; Haydar, T.; Wolozin, B.; Butovsky, O.; Kügler, S.; Ikezu, T. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat. Neurosci. 2015, 18, 1584–1593. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.C.; et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ising, C.; Venegas, C.; Zhang, S.; Scheiblich, H.; Schmidt, S.V.; Vieira-Saecker, A.; Schwartz, S.; Albasset, S.; McManus, R.M.; Tejera, D.; et al. NLRP3 inflammasome activation drives tau pathology. Nature 2019, 575, 669–673. [Google Scholar] [CrossRef]
- Hamelin, L.; Lagarde, J.; Dorothée, G.; Leroy, C.; Labit, M.; Comley, R.A.; de Souza, L.C.; Corne, H.; Dauphinot, L.; Bertoux, M.; et al. Early and protective microglial activation in Alzheimer’s disease: A prospective study using 18 F-DPA-714 PET imaging. Brain 2016, 139, 1252–1264. [Google Scholar] [CrossRef] [Green Version]
- Fan, Z.; Brooks, D.J.; Okello, A.; Edison, P. An early and late peak in microglial activation in Alzheimer’s disease trajectory. Brain 2017, 140, aww349. [Google Scholar] [CrossRef] [PubMed]
- Swardfager, W.; Lanctt, K.; Rothenburg, L.; Wong, A.; Cappell, J.; Herrmann, N. A meta-analysis of cytokines in Alzheimer’s disease. Biol. Psychiatry 2010, 68, 930–941. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.; Tam, W.W.; Zhang, M.W.; Ho, C.S.; Husain, S.F.; McIntyre, R.S.; Ho, R.C. IL-1β, IL-6, TNF- α and CRP in Elderly Patients with Depression or Alzheimer’s disease: Systematic Review and Meta-Analysis. Sci. Rep. 2018, 8, 12050. [Google Scholar] [CrossRef]
- Sartori, A.C.; Vance, D.E.; Slater, L.Z.; Crowe, M. The Impact of Inflammation on Cognitive Function in Older Adults. J. Neurosci. Nurs. 2012, 44, 206–217. [Google Scholar] [CrossRef] [Green Version]
- Coon, K.D.; Myers, A.J.; Craig, D.W.; Webster, J.A.; Pearson, J.V.; Lince, D.H.; Zismann, V.L.; Beach, T.G.; Leung, D.; Bryden, L.; et al. A high-density whole-genome association study reveals that APOE is the major susceptibility gene for sporadic late-onset Alzheimer’s disease. J. Clin. Psychiatry 2007, 68, 613–618. [Google Scholar] [CrossRef]
- Newcombe, E.A.; Camats-Perna, J.; Silva, M.L.; Valmas, N.; Huat, T.J.; Medeiros, R. Inflammation: The link between comorbidities, genetics, and Alzheimer’s disease. J. Neuroinflamm. 2018, 15, 276. [Google Scholar] [CrossRef] [Green Version]
- Vogrinc, D.; Goričar, K.; Dolžan, V. Genetic Variability in Molecular Pathways Implicated in Alzheimer’s Disease: A Comprehensive Review. Front. Aging Neurosci. 2021, 13, 646901. [Google Scholar] [CrossRef] [PubMed]
- Kunkle, B.W.; Grenier-Boley, B.; Sims, R.; Bis, J.C.; Damotte, V.; Naj, A.C.; Boland, A.; Vronskaya, M.; van der Lee, S.J.; Amlie-Wolf, A.; et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Aβ, tau, immunity and lipid processing. Nat. Genet. 2019, 51, 414–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonsson, T.; Stefansson, H.; Steinberg, S.; Jonsdottir, I.; Jonsson, P.V.; Snaedal, J.; Bjornsson, S.; Huttenlocher, J.; Levey, A.I.; Lah, J.J.; et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med. 2013, 368, 107–116. [Google Scholar] [CrossRef] [Green Version]
- Haddick, P.C.G.; Larson, J.L.; Rathore, N.; Bhangale, T.R.; Phung, Q.T.; Srinivasan, K.; Hansen, D.V.; Lill, J.R.; Pericak-Vance, M.A.; Haines, J.; et al. A Common Variant of IL-6R is Associated with Elevated IL-6 Pathway Activity in Alzheimer’s Disease Brains. J. Alzheimer’s Dis. 2017, 56, 1037–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kauwe, J.S.K.; Bailey, M.H.; Ridge, P.G.; Perry, R.; Wadsworth, M.E.; Hoyt, K.L.; Staley, L.A.; Karch, C.M.; Harari, O.; Cruchaga, C.; et al. Genome-Wide Association Study of CSF Levels of 59 Alzheimer’s Disease Candidate Proteins: Significant Associations with Proteins Involved in Amyloid Processing and Inflammation. PLoS Genet. 2014, 10, e1004758. [Google Scholar] [CrossRef]
- Lambert, J.C.; Ibrahim-Verbaas, C.A.; Harold, D.; Naj, A.C.; Sims, R.; Bellenguez, C.; DeStafano, A.L.; Bis, J.C.; Beecham, G.W.; Grenier-Boley, B.; et al. Meta-analysis of 74,046 individuals identifies 11 new susceptibility loci for Alzheimer’s disease. Nat. Genet. 2013, 45, 1452–1458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, K.L.; Marcora, E.; Pimenova, A.A.; Di Narzo, A.F.; Kapoor, M.; Jin, S.C.; Harari, O.; Bertelsen, S.; Fairfax, B.P.; Czajkowski, J.; et al. A common haplotype lowers PU.1 expression in myeloid cells and delays onset of Alzheimer’s disease. Nat. Neurosci. 2017, 20, 1052–1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deming, Y.; Xia, J.; Cai, Y.; Lord, J.; Holmans, P.; Bertelsen, S.; Holtzman, D.; Morris, J.C.; Bales, K.; Pickering, E.H.; et al. A potential endophenotype for Alzheimer’s disease: Cerebrospinal fluid clusterin Alzheimer’s Disease Neuroimaging Initiative (ADNI ) HHS Public Access. Neurobiol. Aging 2016, 37, 208.e1–208.e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramanan, V.K.; Risacher, S.L.; Nho, K.; Kim, S.; Shen, L.; McDonald, B.C.; Yoder, K.K.; Hutchins, G.D.; West, J.D.; Tallman, E.F.; et al. GWAS of longitudinal amyloid accumulation on 18F-florbetapir PET in Alzheimer’s disease implicates microglial activation gene IL1RAP. Brain 2015, 138, 3076–3088. [Google Scholar] [CrossRef] [Green Version]
- Beecham, G.W.; Hamilton, K.; Naj, A.C.; Martin, E.R.; Huentelman, M.; Myers, A.J.; Corneveaux, J.J.; Hardy, J.; Vonsattel, J.P.; Younkin, S.G.; et al. Genome-Wide Association Meta-analysis of Neuropathologic Features of Alzheimer’s Disease and Related Dementias. PLoS Genet. 2014, 10, e1004606. [Google Scholar] [CrossRef] [Green Version]
- Reisberg, B.; Ferris, S.; De Leon, M.; Crook, T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am. J. Psychiatry 1982, 139, 1136–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; American Psychiatric Association: Arlington, VA, USA, 2013; ISBN 0-89042-555-8. [Google Scholar]
- Winblad, B.; Palmer, K.; Kivipelto, M.; Jelic, V.; Fratiglioni, L.; Wahlund, L.-O.; Nordberg, A.; Backman, L.; Albert, M.; Almkvist, O.; et al. Mild cognitive impairment—Beyond controversies, towards a consensus: Report of the International Working Group on Mild Cognitive Impairment. J. Intern. Med. 2004, 256, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Ashford, J.W. APOE Genotype Effects on Alzheimer’s Disease Definition of AD Epidemiology of AD. J. Mol. Neurosci. 2004, 23, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.; Borchelt, D.R.; Chakrabarty, P. Therapeutic approaches targeting Apolipoprotein e function in Alzheimer’s disease. Mol. Neurodegener. 2020, 15, 8. [Google Scholar] [CrossRef] [Green Version]
- Dupont, W.D.; Plummer, W.D.J. Power and sample size calculations: A review and computer program. Control. Clin. Trials 1990, 11, 116–128. [Google Scholar] [CrossRef]
- Yang, R.; Duan, J.; Luo, F.; Tao, P.; Hu, C. IL-6, IL-8 and IL-10 polymorphisms may impact predisposition of Alzheimer’s disease: A meta-analysis. Acta Neurol. Belg. 2021, 121, 1505–1512. [Google Scholar] [CrossRef]
- Wang, M.; Li, Y.; Lin, L.; Song, G.; Deng, T. GSTM1 Null Genotype and GSTP1 Ile105Val Polymorphism Are Associated with Alzheimer’s Disease: A Meta-Analysis. Mol. Neurobiol. 2016, 53, 1355–1364. [Google Scholar] [CrossRef]
- Mun, M.-J.; Kim, J.-H.; Choi, J.-Y.; Jang, W.-C. Genetic polymorphisms of interleukin genes and the risk of Alzheimer’s disease: An update meta-analysis. Meta Gene 2016, 8, 1–10. [Google Scholar] [CrossRef]
- Laws, S.M.; Perneczky, R.; Wagenpfeil, S.; Müller, U.; Förstl, H.; Martins, R.N.; Kurz, A.; Riemenschneider, M. TNF polymorphisms in Alzheimer disease and functional implications on CSF beta-amyloid levels. Hum. Mutat. 2005, 26, 29–35. [Google Scholar] [CrossRef]
- Sarajärvi, T.; Helisalmi, S.; Antikainen, L.; Mäkinen, P.; Koivisto, A.M.; Herukka, S.K.; Haapasalo, A.; Soininen, H.; Hiltunen, M. An association study of 21 potential Alzheimer’s disease risk genes in a Finnish population. J. Alzheimer’s Dis. 2010, 21, 763–767. [Google Scholar] [CrossRef]
- Babić Leko, M.; Nikolac Perković, M.; Klepac, N.; Štrac, D.Š.; Borovečki, F.; Pivac, N.; Hof, P.R.; Šimić, G. IL-1β, IL-6, IL-10, and TNFα Single Nucleotide Polymorphisms in Human Influence the Susceptibility to Alzheimer’s Disease Pathology. J. Alzheimer’s Dis. 2020, 75, 1029–1047. [Google Scholar] [CrossRef] [PubMed]
- Capurso, C.; Solfrizzi, V.; D’Introno, A.; Colacicco, A.M.; Capurso, S.A.; Bifaro, L.; Menga, R.; Santamato, A.; Seripa, D.; Pilotto, A.; et al. Short arm of chromosome 11 and sporadic Alzheimer’s disease: Catalase and cathepsin D gene polymorphisms. Neurosci. Lett. 2008, 432, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Habib, L.K.; Lee, M.T.C.; Yang, J. Inhibitors of Catalase-Amyloid Interactions Protect Cells from β-Amyloid-Induced Oxidative Stress and Toxicity. J. Biol. Chem. 2010, 285, 38933–38943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behl, C. Hydrogen peroxide mediates amyloid β protein toxicity. Cell 1994, 77, 817–827. [Google Scholar] [CrossRef]
- Nandi, A.; Yan, L.J.; Jana, C.K.; Das, N. Role of catalase in oxidative stress-and age-associated degenerative diseases. Oxidative medicine and cellular longevity, 2019. Oxid. Med. Cell. Longev. 2019, 2019, 9613090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forsberg, L.; Lyrenäs, L.; Morgenstern, R.; de Faire, U. A common functional C-T substitution polymorphism in the promoter region of the human catalase gene influences transcription factor binding, reporter gene transcription and is correlated to blood catalase levels. Free Radic. Biol. Med. 2001, 30, 500–505. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; O’Donnell, R.; Srivastava, S.K.; Xia, H.; Zimniak, P.; Nanduri, B.; Bleicher, R.J.; Awasthi, S.; Awasthi, Y.C.; Ji, X.; et al. Active site architecture of polymorphic forms of human glutathione S-transferase P1-1 accounts for their enantioselectivity and disparate activity in the glutathione conjugation of 7β,8α-dihydroxy-9α,10α-oxy-7,8,9,10-tetrahydrobenzo(a)pyrene. Biochem. Biophys. Res. Commun. 1997, 235, 424–428. [Google Scholar] [CrossRef] [PubMed]
- Ali-Osman, F.; Akande, O.; Antoun, G.; Mao, J.X.; Buolamwini, J. Molecular cloning, characterization, and expression in Escherichia coli of full-length cDNAs of three human glutathione S-transferase Pi gene variants: Evidence for differential catalytic activity of the encoded proteins. J. Biol. Chem. 1997, 272, 10004–10012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayes, J.D.; Flanagan, J.U.; Jowsey, I.R. Glutathione transferases. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 51–88. [Google Scholar] [CrossRef]
- Dasari, S.; Gonuguntla, S.; Ganjayi, M.S.; Bukke, S.; Sreenivasulu, B.; Meriga, B. Genetic polymorphism of glutathione S-transferases: Relevance to neurological disorders. Pathophysiology 2018, 25, 285–292. [Google Scholar] [CrossRef]
- Serrano, J.; Fernández, A.P.; Martínez-Murillo, R.; Martínez, A. High sensitivity to carcinogens in the brain of a mouse model of Alzheimer’s disease. Oncogene 2010, 29, 2165–2171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesa-Herrera, F.; Marín, R.; Torrealba, E.; Díaz, M. Multivariate Assessment of Lipoxidative Metabolites, Trace Biometals, and Antioxidant and Detoxifying Activities in the Cerebrospinal Fluid Define a Fingerprint of Preclinical Stages of Alzheimer’s Disease. J. Alzheimer’s Dis. 2022, 86, 387–402. [Google Scholar] [CrossRef]
- del Campo, M.; Galimberti, D.; Elias, N.; Boonkamp, L.; Pijnenburg, Y.A.; van Swieten, J.C.; Watts, K.; Paciotti, S.; Beccari, T.; Hu, W.; et al. Novel CSF biomarkers to discriminate FTLD and its pathological subtypes. Ann. Clin. Transl. Neurol. 2018, 5, 1163–1175. [Google Scholar] [CrossRef] [PubMed]
- Teunissen, C.E.; Elias, N.; Koel-Simmelink, M.J.A.; Durieux-Lu, S.; Malekzadeh, A.; Pham, T.V.; Piersma, S.R.; Beccari, T.; Meeter, L.H.H.; Dopper, E.G.P.; et al. Novel diagnostic cerebrospinal fluid biomarkers for pathologic subtypes of frontotemporal dementia identified by proteomics. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2016, 2, 86–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torres, L.L.; Quaglio, N.B.; de Souza, G.T.; Garcia, R.T.; Dati, L.M.M.; Moreira, W.L.; de Melo Loureiro, A.P.; de souza-Talarico, J.N.; Smid, J.; Porto, C.S.; et al. Peripheral Oxidative Stress Biomarkers in Mild Cognitive Impairment and Alzheimer’s Disease. J. Alzheimer’s Dis. 2011, 26, 59–68. [Google Scholar] [CrossRef]
- Jafarian, Z.; Saliminejad, K.; Kamali, K.; Ohadi, M.; Kowsari, A.; Nasehi, L.; Khorram Khorshid, H.R. Association of glutathione S-transferases M1, P1 and T1 variations and risk of late-onset Alzheimer’s disease. Neurol. Res. 2018, 40, 41–44. [Google Scholar] [CrossRef]
- Pinhel, M.A.S.; Nakazone, M.A.; Cação, J.C.; Piteri, R.C.O.; Dantas, R.T.; Godoy, M.F.; Godoy, M.R.P.; Tognola, W.A.; Conforti-Froes, N.D.T.; Souza, D.R.S. Glutathione S-transferase variants increase susceptibility for late-onset Alzheimer’s disease: Association study and relationship with apolipoprotein E ε4 allele. Clin. Chem. Lab. Med. 2008, 46, 439–445. [Google Scholar] [CrossRef]
- Spalletta, G.; Bernardini, S.; Bellincampi, L.; Federici, G.; Trequattrini, A.; Ciappi, F.; Bria, P.; Caltagirone, C.; Bossù, P. Glutathione S-Transferase P1 and T1 Gene Polymorphisms Predict Longitudinal Course and Age at Onset of Alzheimer Disease. Am. J. Geriatr. Psychiatry 2007, 15, 879–887. [Google Scholar] [CrossRef]
- Roth, N.J.; Zipperich, S.; Kopf, J.; Deckert, J.; Reif, A. Influence of two functional polymorphisms in NOS1 on baseline cortisol and working memory in healthy subjects. Nitric Oxide 2019, 88, 45–49. [Google Scholar] [CrossRef]
- Yoo, S.D.; Park, J.S.; Yun, D.H.; Kim, H.S.; Kim, S.K.; Kim, D.H.; Chon, J.; Je, G.; Kim, Y.S.; Chung, J.H.; et al. Polymorphism of nitric oxide synthase 1 affects the clinical phenotypes of ischemic stroke in Korean population. Ann. Rehabil. Med. 2016, 40, 102–110. [Google Scholar] [CrossRef] [Green Version]
- Redenšek, S.; Jenko Bizjan, B.; Trošt, M.; Dolžan, V. Clinical-Pharmacogenetic Predictive Models for Time to Occurrence of Levodopa Related Motor Complications in Parkinson’s Disease. Front. Genet. 2019, 10, 461. [Google Scholar] [CrossRef] [PubMed]
- Reif, A.; Herterich, S.; Wichart, I.; Rainer, M.K.; Jungwirth, S.; Danielczyk, W.; Deckert, J.; Tragl, K.H.; Riederer, P.; Fischer, P. Association of a functional NOS1 promoter repeat with alzheimer’s disease in the VITA cohort. J. Alzheimer’s Dis. 2011, 23, 327–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galimberti, D.; Scarpini, E.; Venturelli, E.; Strobel, A.; Herterich, S.; Fenoglio, C.; Guidi, I.; Scalabrini, D.; Cortini, F.; Bresolin, N.; et al. Association of a NOS1 promoter repeat with Alzheimer’s disease. Neurobiol. Aging 2008, 29, 1359–1365. [Google Scholar] [CrossRef]
- von Otter, M.; Landgren, S.; Nilsson, S.; Zetterberg, M.; Celojevic, D.; Bergström, P.; Minthon, L.; Bogdanovic, N.; Andreasen, N.; Gustafson, D.R.; et al. Nrf2-encoding NFE2L2 haplotypes influence disease progression but not risk in Alzheimer’s disease and age-related cataract. Mech. Ageing Dev. 2010, 131, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Davies, K.J.A.; Forman, H.J. Oxidative stress response and Nrf2 signaling in aging. Free Radic. Biol. Med. 2015, 88, 314–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramsey, C.P.; Glass, C.A.; Montgomery, M.B.; Lindl, K.A.; Ritson, G.P.; Chia, L.A.; Hamilton, R.L.; Chu, C.T.; Jordan-Sciutto, K.L. Expression of Nrf2 in Neurodegenerative Diseases. J. Neuropathol. Exp. Neurol. 2007, 66, 75–85. [Google Scholar] [CrossRef]
- Rojo, A.I.; Pajares, M.; Rada, P.; Nuñez, A.; Nevado-Holgado, A.J.; Killik, R.; Van Leuven, F.; Ribe, E.; Lovestone, S.; Yamamoto, M.; et al. NRF2 deficiency replicates transcriptomic changes in Alzheimer’s patients and worsens APP and TAU pathology. Redox Biol. 2017, 13, 444–451. [Google Scholar] [CrossRef]
- Joshi, G.; Gan, K.A.; Johnson, D.A.; Johnson, J.A. Increased Alzheimer’s disease–like pathology in the APP/ PS1ΔE9 mouse model lacking Nrf2 through modulation of autophagy. Neurobiol. Aging 2015, 36, 664–679. [Google Scholar] [CrossRef] [Green Version]
- Branca, C.; Ferreira, E.; Nguyen, T.-V.; Doyle, K.; Caccamo, A.; Oddo, S. Genetic reduction of Nrf2 exacerbates cognitive deficits in a mouse model of Alzheimer’s disease. Hum. Mol. Genet. 2017, 26, 4823–4835. [Google Scholar] [CrossRef] [PubMed]
- Kerr, F.; Sofola-Adesakin, O.; Ivanov, D.K.; Gatliff, J.; Gomez Perez-Nievas, B.; Bertrand, H.C.; Martinez, P.; Callard, R.; Snoeren, I.; Cochemé, H.M.; et al. Direct Keap1-Nrf2 disruption as a potential therapeutic target for Alzheimer’s disease. PLoS Genet. 2017, 13, e1006593. [Google Scholar] [CrossRef] [Green Version]
- Uruno, A.; Matsumaru, D.; Ryoke, R.; Saito, R.; Kadoguchi, S.; Saigusa, D.; Saito, T.; Saido, T.C.; Kawashima, R.; Yamamoto, M. Nrf2 Suppresses Oxidative Stress and Inflammation in App Knock-In Alzheimer’s Disease Model Mice. Mol. Cell. Biol. 2020, 40, e00467-19. [Google Scholar] [CrossRef] [PubMed]
- Micheau, O.; Tschopp, J. Induction of TNF Receptor I-Mediated Apoptosis via Two Sequential Signaling Complexes. Cell 2003, 114, 181–190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kroeger, K.M.; Carville, K.S.; Abraham, L.J. The −308 tumor necrosis factor-α promoter polymorphism effects transcription. Mol. Immunol. 1997, 34, 391–399. [Google Scholar] [CrossRef]
- Kimura, K.; Takayanagi, R.; Yokoyama, H.; Yamada, Y. Effects of tumor necrosis factor α-857C/T polymorphism on the expression of tumor necrosis factor α. Apmis 2016, 124, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Fricke-Galindo, I.; Buendía-Roldán, I.; Ruiz, A.; Palacios, Y.; Pérez-Rubio, G.; Hernández-Zenteno, R.d.J.; Reyes-Melendres, F.; Zazueta-Márquez, A.; Alarcón-Dionet, A.; Guzmán-Vargas, J.; et al. TNFRSF1B and TNF variants are associated with differences in soluble TNF receptors’ levels in patients with severe COVID-19. J. Infect. Dis. 2022, 226, 778–787. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Xue, M.; Yan, Z.; Song, B.; Hong, H.; Gao, X. Correlation between TNF-α −308 and +489 Gene Polymorphism and Acute Exacerbation of Chronic Obstructive Pulmonary Diseases. BioMed Res. Int. 2021, 2021, 6661281. [Google Scholar] [CrossRef] [PubMed]
- Tarkowski, E.; Liljeroth, A.M.; Nilsson, Å.; Ricksten, A.; Davidsson, P.; Minthon, L.; Blennow, K. TNF gene polymorphism and its relation to intracerebral production of TNFα and TNFβ in AD. Neurology 2000, 54, 2077–2081. [Google Scholar] [CrossRef] [PubMed]
- Culjak, M.; Perkovic, M.N.; Uzun, S.; Strac, D.S.; Erjavec, G.N.; Leko, M.B.; Simic, G.; Tudor, L.; Konjevod, M.; Kozumplik, O.; et al. The Association between TNF-alpha, IL-1 alpha and IL-10 with Alzheimer’s Disease. Curr. Alzheimer Res. 2021, 17, 972–984. [Google Scholar] [CrossRef]
- Brosseron, F.; Krauthausen, M.; Kummer, M.; Heneka, M.T. Body Fluid Cytokine Levels in Mild Cognitive Impairment and Alzheimer’s Disease: A Comparative Overview. Mol. Neurobiol. 2014, 50, 534–544. [Google Scholar] [CrossRef] [Green Version]
- Tarkowski, E.; Andreasen, N.; Tarkowski, A.; Blennow, K. Intrathecal inflammation precedes development of Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2003, 74, 1200–1205. [Google Scholar] [CrossRef]
- Abdi, S.; Alghamdi, A.A.; AlGhunaim, N.N.A.; Almutairi, R.M.; Ataya, F.S.; Ansari, M.G.A.; Hussain, S.D.; Masoud, M.; Alamro, A.; Almutairi, O.T.; et al. Association of Alzheimer’s Disease with Genetic Variants of Apolipoprotein E, Clusterin, TNF-α and IL-6 Among Elderly Saudis. Curr. Pharm. Biotechnol. 2022, 23, 1893–1920. [Google Scholar] [CrossRef]
- Mishra, A.; Kim, H.J.; Shin, A.H.; Thayer, S.A. Synapse loss induced by interleukin-1β requires pre-and post-synaptic mechanisms. J. Neuroimmune Pharmacol. 2012, 7, 571–578. [Google Scholar] [CrossRef] [Green Version]
- Tayel, S.I.; Fouda, E.A.M.; Elshayeb, E.I.; Eldakamawy, A.R.A.; El-kousy, S.M. Biochemical and molecular study on interleukin-1β gene expression and relation of single nucleotide polymorphism in promoter region with Type 2 diabetes mellitus. J. Cell. Biochem. 2018, 119, 5343–5349. [Google Scholar] [CrossRef] [PubMed]
- Iglesias Molli, A.E.; Bergonzi, M.F.; Spalvieri, M.P.; Linari, M.A.; Frechtel, G.D.; Cerrone, G.E. Relationship between the IL-1β serum concentration, mRNA levels and rs16944 genotype in the hyperglycemic normalization of T2D patients. Sci. Rep. 2020, 10, 9985. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Y.; Wang, G.; Dong, S.; Yang, D.; Zuo, X. The association between interleukin-1 polymorphisms and their protein expression in Chinese Han patients with breast cancer. Mol. Genet. Genom. Med. 2019, 7, e804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarabino, D.; Peconi, M.; Broggio, E.; Gambina, G.; Maggi, E.; Armeli, F.; Mantuano, E.; Morello, M.; Corbo, R.M.; Businaro, R. Relationship between proinflammatory cytokines (Il-1beta, Il-18) and leukocyte telomere length in mild cognitive impairment and Alzheimer’s disease. Exp. Gerontol. 2020, 136, 110945. [Google Scholar] [CrossRef]
- Huang, C.W.; Hsu, S.W.; Tsai, S.J.; Chen, N.C.; Liu, M.E.; Lee, C.C.; Huang, S.H.; Chang, W.N.; Chang, Y.T.; Tsai, W.C.; et al. Genetic effect of interleukin-1 beta (C-511T) polymorphism on the structural covariance network and white matter integrity in Alzheimer’s disease. J. Neuroinflamm. 2017, 14, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, X.; Zhang, L.; Meng, Q.; Gao, Q. Association Between Interleukin-1A, Interleukin-1B, and Bridging integrator 1 Polymorphisms and Alzheimer’s Disease: A standard and Cumulative Meta-analysis. Mol. Neurobiol. 2017, 54, 736–747. [Google Scholar] [CrossRef]
- Li, L.; Chen, X.-P.; Li, Y.-J. MicroRNA-146a and Human Disease. Scand. J. Immunol. 2010, 71, 227–231. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-F.; Shu, R.; Jiang, S.-Y.; Liu, D.-L.; Ni, J.; Zhang, X.-L. MicroRNA-146 inhibits pro-inflammatory cytokine secretion through IL-1 receptor-associated kinase 1 in human gingival fibroblasts. J. Inflamm. 2013, 10, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nahid, M.A.; Satoh, M.; Chan, E.K.L. Interleukin 1β-Responsive MicroRNA-146a Is Critical for the Cytokine-Induced Tolerance and Cross-Tolerance to Toll-Like Receptor Ligands. J. Innate Immun. 2015, 7, 428–440. [Google Scholar] [CrossRef]
- Cui, L.; Li, Y.; Ma, G.; Wang, Y.; Cai, Y.; Liu, S.; Chen, Y.; Li, J.; Xie, Y.; Liu, G.; et al. A functional polymorphism in the promoter region of microRNA-146a is associated with the risk of alzheimer disease and the rate of cognitive decline in patients. PLoS ONE 2014, 9, e89019. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Russell, A.E.; Wang, W.; Gemoets, D.E.; Sarkar, S.N.; Simpkins, J.W.; Brown, C.M. miR-146a Dysregulates Energy Metabolism During Neuroinflammation. J. Neuroimmune Pharmacol. 2021, 17, 228–241. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.S.; Cai, D. Integrated miRNA-Seq and mRNA-Seq Study to Identify miRNAs Associated With Alzheimer’s Disease Using Post-mortem Brain Tissue Samples. Front. Neurosci. 2021, 15, 620899. [Google Scholar] [CrossRef] [PubMed]
- Jia, L.; Zhu, M.; Yang, J.; Pang, Y.; Wang, Q.; Li, Y.; Li, T.; Li, F.; Wang, Q.; Li, Y.; et al. Prediction of P-tau/Aβ42 in the cerebrospinal fluid with blood microRNAs in Alzheimer’s disease. BMC Med. 2021, 19, 92. [Google Scholar] [CrossRef]
- Cuadrado, A.; Rojo, A.I.; Wells, G.; Hayes, J.D.; Cousin, S.P.; Rumsey, W.L.; Attucks, O.C.; Franklin, S.; Levonen, A.-L.; Kensler, T.W.; et al. Therapeutic targeting of the NRF2 and KEAP1 partnership in chronic diseases. Nat. Rev. Drug Discov. 2019, 18, 295–317. [Google Scholar] [CrossRef] [Green Version]
- Qu, Z.; Sun, J.; Zhang, W.; Yu, J.; Zhuang, C. Transcription factor NRF2 as a promising therapeutic target for Alzheimer’s disease. Free Radic. Biol. Med. 2020, 159, 87–102. [Google Scholar] [CrossRef]

| Characteristic | Category/Unit | Dementia | AD | MCI (AD) | MCI (NOT AD) | p |
|---|---|---|---|---|---|---|
| Sex | Male, N (%) | 38 (43.2) | 23 (42.6) | 4 (28.6) | 11 (55) | 0.326 a |
| Female, N (%) | 50 (56.8) | 31 (57.4) | 10 (71.4) | 9 (45) | ||
| Age | Years, median (25–75%) | 77 (72.25–80) | 78 (74.75–81) | 76.5 (72.25–79.25) | 71.5 (67–77.5) | 0.010 b |
| Education | Years, median (25–75%) | 12 (8–12.25) [2] | 11 (8–12) [1] | 12 (11.5–15.5) [1] | 11.5 (8–14.5) | 0.156 b |
| Height | cm, median (25–75%) | 168 (160.50–172) [11] | 168 (160–172) [8] | 162.5 (160.3–168.8) [2] | 172 (163–175) [1] | 0.333 b |
| Weight | kg, median (25–75%) | 68 (57–79) [11] | 63 (55–77) [7] | 70 (54–79) [3] | 70 (62–85) [1] | 0.153 b |
| BMI | kg/m2, median (25–75%) | 25 (21–28) [13] | 24.5 (21–27) [9] | 27 (20.6–29.2) [3] | 26.3 (22.6–28.6) [1] | 0.151 b |
| APOE status | APOE4 carriers, N (%) | 39 (44.3) | 29 (53.7) | 5 (35.7) | 5 (25) | 0.138 a |
| MMSE | Score, median (25–75%) | 24 (17.8–26) [18] | 20 (15–24) [12] | 26 (25–27) [3] | 27 (24.5–27.5) [3] | <0.001 b |
| Aβ42 | pg/mL, median (25–75%) | 745 (570–964.5) [2] | 688 (538.5–772) [1] | 740.5 (556–894.8) | 1297 (1091–1540) [1] | <0.001 b |
| Aβ42/40 ratio | Median (25–75%) | 0.06 (0.05–0.08) [8] | 0.06 (0.04–0.06) [3] | 0.06 (0.03–0.07) [2] | 0.11 (0.09–0.13) [3] | <0.001 b |
| Total tau | pg/mL, median (25–75%) | 605 (466.3–884.3) [2] | 778 (573.5–991) [1] | 557 (467.8–902.3) | 320 (243–404) [1] | <0.001 b |
| p-tau | pg/mL, median (25–75%) | 88.5 (72.8–122.3) [2] | 98 (82.5–128) [1] | 86.5 (73–127.3) | 53 (43–72) [1] | <0.001 b |
| Gene | SNP | Genotype | Controls N (%) | Dementia N (%) | OR (95% CI) | p | ORadj (95% CI) | Padj |
|---|---|---|---|---|---|---|---|---|
| SOD2 | rs4880 | CC | 15 (24.2) | 20 (22.7) | Reference | Reference | ||
| CT | 29 (46.8) | 46 (52.3) | 1.19 (0.527–2.688) | 0.676 | 1.538 (0.604–3.917) | 0.366 | ||
| TT | 18 (29) | 22 (25) | 0.917 (0.367–2.287) | 0.852 | 1.037 (0.366–2.937) | 0.945 | ||
| CT + TT | 47 (75.8) | 68 (77.3) | 1.085 (0.505–2.334) | 0.834 | 1.336 (0.558–3.201) | 0.558 | ||
| CAT | rs1001179 | CC | 38 (61.3) | 61 (69.3) | Reference | Reference | ||
| CT | 19 (30.6) | 21 (23.9) | 0.689 (0.328–1.445) | 0.324 | 1.245 (0.312–4.966) | 0.756 | ||
| TT | 5 (8.1) | 6 (6.8) | 0.748 (0.213–2.62) | 0.649 | 0.789 (0.181–3.433) | 0.753 | ||
| CT + TT | 24 (38.7) | 27 (30.7) | 0.701 (0.354–1.387) | 0.308 | 1.498 (0.691–3.244) | 0.306 | ||
| GPX1 | rs1050450 | CC | 29 (46.8) | 42 (47.7) | Reference | Reference | ||
| CT | 27 (43.5) | 37 (42) | 0.946 (0.477–1.878) | 0.874 | 0.966 (0.445–2.099) | 0.931 | ||
| TT | 6 (9.7) | 9 (10.2) | 1.036 (0.332–3.226) | 0.952 | 1.212 (0.330–4.457) | 0.772 | ||
| CT + TT | 33 (53.2) | 46 (52.2) | 0.962 (0.502–1.846) | 0.962 | 1.009 (0.485–2.101) | 0.980 | ||
| IL1B | rs1143623 | GG | 36 (58.1) | 49 (55.7) | Reference | Reference | ||
| GC | 21 (33.9) | 25 (28.4) | 0.875 (0.425–1.801) | 0.716 | 0.789 (0.353–1.767) | 0.565 | ||
| CC | 5 (8.1) | 14 (15.9) | 2.057 (0.679–6.23) | 0.202 | 1.610 (0.443–5.852) | 0.470 | ||
| GC + CC | 26 (42) | 39 (43.3) | 1.102 (0.571–2.125) | 0.772 | 0.935 (0.444–1.968) | 0.860 | ||
| rs16944 | TT | 6 (9.7) | 17 (19.3) | Reference | Reference | |||
| TC | 27 (43.5) | 31 (35.2) | 0.405 (0.14–1.174) | 0.096 | 0.462 (0.140–1.524) | 0.205 | ||
| CC | 29 (46.8) | 40 (45.5) | 0.487 (0.171–1.386) | 0.177 | 0.631 (0.194–2.057) | 0.445 | ||
| TC + CC | 56 (90.3) | 71 (80.7) | 0.447 (0.166–1.21) | 0.113 | 0.545 (0.178–1.665) | 0.287 | ||
| rs1071676 | CC | 37 (59.7) | 50 (56.8) | Reference | Reference | |||
| CG | 18 (29) | 31 (35.2) | 1.274 (0.621–2.618) | 0.509 | 1.176 (0.533–2.597) | 0.687 | ||
| GG | 7 (11.3) | 7 (8) | 0.74 (0.239–2.292) | 0.602 | 0.638 (0.171–2.379) | 0.503 | ||
| CG + GG | 25 (40.3) | 38 (43.2) | 1.125 (0.581–2.176) | 0.727 | 1.037 (0.497–2.164) | 0.922 | ||
| MIR146A | rs2910164 | GG | 38 (61.3) | 51 (58) | Reference | Reference | ||
| GC | 21 (33.9) | 30 (34.1) | 1.064 (0.53–2.139) | 0.861 | 1.058 (0.485–2.307) | 0.887 | ||
| CC | 3 (4.8) | 7 (8) | 1.739 (0.422–7.166) | 0.444 | 2.544 (0.466–13.88) | 0.281 | ||
| GC + CC | 24 (38.7) | 37 (42) | 1.149 (0.592–2.23) | 0.682 | 1.191 (0.565–2.512) | 0.646 | ||
| IL6 | rs1800795 | GG | 21 (33.9) | 32 (36.4) | Reference | Reference | ||
| GC | 27 (43.5) | 43 (48.9) | 1.045 (0.503–2.171) | 0.906 | 1.16 (0.503–2.672) | 0.728 | ||
| CC | 14 (22.6) | 13 (14.8) | 0.609 (0.239–1.551) | 0.299 | 0.627 (0.215–1.830) | 0.393 | ||
| GC + CC | 41 (66.1) | 56 (63.6) | 0.896 (0.453–1.773) | 0.753 | 0.973 (0.447–2.117) | 0.945 | ||
| TNF | rs1800629 | GG | 42 (67.7) | 72 (81.8) | Reference | Reference | ||
| GA | 19 (30.6) | 12 (13.6) | 0.368 (0.163–0.834) | 0.017 | 0.274 (0.101–0.741) | 0.011 | ||
| AA | 1 (1.6) | 4 (4.5) | 2.333 (0.252–21.57) | 0.455 | 5.445 (0.451–65.75) | 0.182 | ||
| GA + AA | 20 (32.2) | 16 (18.1) | 0.467 (0.218–0.997) | 0.049 | 0.398 (0.159–0.996) | 0.049 | ||
| CARD8 | rs2043211 | AA | 32 (51.6) | 37 (42) | Reference | Reference | ||
| AT | 26 (41.9) | 40 (45.5) | 1.331 (0.671–2.637) | 0.413 | 1.074 (0.490–2.354) | 0.858 | ||
| TT | 4 (6.5) | 11 (12.5) | 2.378 (0.689–8.205) | 0.170 | 1.977 (0.515–7.585) | 0.321 | ||
| AT + TT | 30 (48.4) | 51 (57.7) | 1.47 (0.765–2.827) | 0.248 | 1.202 (0.569–2.536) | 0.630 | ||
| NLRP3 | rs35829419 | CC | 58 (93.5) | 85 (96.6) | Reference | Reference | ||
| CA | 4 (6.5) | 3 (3.4) | 0.512 (0.11–2.372) | 0.392 | 0.560 (0.098–3.208) | 0.515 | ||
| GSTP1 | rs1695 | AA | 28 (45.2) | 36 (40.9) | Reference | Reference | ||
| AG | 28 (45.2) | 38 (43.2) | 1.056 (0.527–2.114) | 0.879 | 1.279 (0.583–2.806) | 0.540 | ||
| GG | 6 (9.7) | 14 (15.9) | 1.815 (0.619–5.325) | 0.278 | 1.731 (0.523–5.729) | 0.369 | ||
| AG + GG | 34 (54.9) | 52 (59.1) | 1.19 (0.617–2.293) | 0.604 | 1.366 (0.648–2.877) | 0.412 | ||
| rs1138272 | CC | 51 (82.3) | 72 (81.8) | Reference | Reference | |||
| CT + TT | 11 (17.7) | 16 (18.2) | 1.03 (0.442–2.404) | 0.945 | 0.869 (0.338–2.236) | 0.772 | ||
| NOS1 | rs2293054 | AA | 37 (59.7) | 44 (50) | Reference | Reference | ||
| AG | 25 (40.3) | 36 (40.9) | 1.211 (0.618–2.371) | 0.577 | 1.104 (0.515–2.364) | 0.800 | ||
| GG | 0 | 8 (9.1) | / | 0.019 * | / | / | ||
| AG + GG | 25 (40.3) | 44 (50) | 1.48 (0.767–2.856) | 0.242 | 1.409 (0.674–2.944) | 0.362 | ||
| rs2682826 | GG | 32 (51.6) | 40 (45.5) | Reference | Reference | |||
| GA | 26 (41.9) | 39 (44.3) | 1.2 (0.608–2.369) | 0.599 | 0.985 (0.456–2.128) | 0.969 | ||
| AA | 4 (6.5) | 9 (10.2) | 1.8 (0.507–6.385) | 0.363 | 0.932 (0.230–3.782) | 0.922 | ||
| GA + AA | 30 (48.4) | 48 (54.5) | 1.28 (0.667–2.455) | 0.458 | 0.976 (0.466–2.044) | 0.950 | ||
| KEAP1 | rs1048290 | GG | 21 (33.9) | 29 (33) | Reference | Reference | ||
| GC | 36 (58.1) | 41 (46.6) | 0.825 (0.402–1.691) | 0.599 | 0.582 (0.253–1.340) | 0.203 | ||
| CC | 5 (8) | 18 (20.5) | 2.607 (0.835–8.142) | 0.099 | 1.947 (0.534–7.105) | 0.313 | ||
| GC + CC | 41 (66.1) | 59 (67) | 1.042 (0.523–2.075) | 0.907 | 0.732 (0.328–1.631) | 0.445 | ||
| rs9676881 | GG | 20 (32.2) | 30 (34.1) | Reference | Reference | |||
| GA | 37 (59.7) | 40 (45.5) | 0.721 (0.35–1.482) | 0.373 | 0.540 (0.234–1.243) | 0.147 | ||
| AA | 5 (8) | 18 (20.5) | 2.4 (0.767–7.512) | 0.133 | 1.851 (0.506–6.765) | 0.352 | ||
| GA + AA | 42 (67.7) | 58 (66) | 0.921 (0.461–1.838) | 0.815 | 0.68 (0.305–1.519) | 0.347 | ||
| NFE2L2 | rs6706649 | CC | 50 (80.6) | 68 (77.3) | Reference | Reference | ||
| CT | 9 (14.5) | 18 (20.5) | 1.471 (0.61–3.544) | 0.390 | 1.266 (0.476–3.369) | 0.637 | ||
| TT | 3 (4.8) | 2 (2.3) | 0.49 (0.079–3.044) | 0.444 | 0.336 (0.044–2.557) | 0.292 | ||
| CT + TT | 12 (19.3) | 20 (22.8) | 1.225 (0.549–2.737) | 0.620 | 1.021 (0.415–2.511) | 0.963 | ||
| rs6721961 | GG | 48 (77.4) | 65 (73.9) | Reference | Reference | |||
| GT + TT | 14 (22.6) | 23 (26.1) | 1.213 (0.566–2.599) | 0.619 | 1.312 (0.557–3.09) | 0.535 | ||
| rs35652124 | TT | 23 (37.1) | 37 (42) | Reference | Reference | |||
| TC | 26 (41.9) | 39 (44.3) | 0.932 (0.454–1.914) | 0.849 | 0.689 (0.3–1.584) | 0.381 | ||
| CC | 13 (21) | 12 (13.6) | 0.574 (0.224–1.471) | 0.248 | 0.530 (0.187–1.508) | 0.234 | ||
| TC + CC | 39 (62.9) | 51 (57.9) | 0.813 (0.417–1.584) | 0.543 | 0.633 (0.296–1.357) | 0.240 |
| SNP | Genotype | Aβ42 (pg/mL) | p | Aβ42/40 Ratio | p | Total tau (pg/mL) | p | pTau (pg/mL) | p |
|---|---|---|---|---|---|---|---|---|---|
| SOD2 rs4880 | CC | 764.5 (640.3–1032.5) | 0.104 | 0.06 (0.05–0.08) | 0.059 | 558 (491.5–770.5) | 0.523 | 85.5 (69.8–102.5) | 0.263 |
| CT | 786.5 (600–1023) | 0.06 (0.05–0.09) | 601.5 (403.8–895) | 86.5 (61–123) | |||||
| TT | 624.5 (536–799.8) | 0.05 (0.04–0.07) | 615 (486.5–1064) | 99 (76.3–148) | |||||
| CT + TT | 740.5 (563.3–952.3) | Pdom = 0.758 | 0.06 (0.04–0.08) | Pdom = 0.501 | 611 (461–897) | Pdom = 0.683 | 88.5 (70.5–123.8) | Pdom = 0.530 | |
| CAT rs1001179 | CC | 747 (553.5–917.5) | 0.372 | 0.06 (0.05–0.08) | 0.737 | 601 (462–879.5) | 0.891 | 87 (71–123) | 0.835 |
| CT | 731 (580.5–1030.5) | 0.06 (0.05–0.07) | 594 (465.5–883) | 88 (72–111.5) | |||||
| TT | 913.5 (742.5–1076.5) | 0.06 (0.03–0.11) | 586.5 (279.8–903.8) | 84 (42.3–140.3) | |||||
| CT + TT | 759 (591–1005) | Pdom = 0.486 | 0.06 (0.05–0.08) | Pdom = 0.435 | 594 (461–894) | Pdom = 0.825 | 88 (70–114) | Pdom = 0.562 | |
| GPX1 rs1050450 | CC | 759 (597–957) | 0.793 | 0.06 (0.05–0.08) | 0.401 | 601.5 (482.8–836) | 0.974 | 92.5 (75.8–123.5) | 0.771 |
| CT | 743 (542–1082) | 0.06 (0.05–0.08) | 601 (429.5–904.5) | 87 (59–119.5) | |||||
| TT | 747 (592.5–852) | 0.06 (0.05–0.06) | 567 (473.5–858) | 84 (72–116.5) | |||||
| CT + TT | 745 (564–964.5) | Pdom = 0.581 | 0.06 (0.05–0.08) | Pdom = 0.932 | 584 (459.8–895) | Pdom = 0.854 | 85.5 (66.3–118.3) | Pdom = 0.525 | |
| IL1B rs1143623 | GG | 795 (626–997.5) | 0.154 | 0.06 (0.05–0.08) | 0.684 | 617 (471.5–887.5) | 0.760 | 89 (75.5–116.5) | 0.863 |
| GC | 688 (534.5–1182) | 0.06 (0.05–0.09) | 549 (401.5–871) | 84 (60.5–126.5) | |||||
| CC | 686 (509.3–866.3) | 0.06 (0.05–0.08) | 596.5 (493.5–900.3) | 95.5 (68–118.5) | |||||
| GC + CC | 688 (530–885) | Pdom = 0.070 | 0.06 (0.05–0.08) | Pdom = 0.475 | 567 (404–878) | Pdom = 0.650 | 87 (68–123) | Pdom = 0.644 | |
| IL1B rs16944 | TT | 725 (524.5–900.5) | 0.020 | 0.06 (0.05–0.08) | 0.071 | 547 (435.5–875) | 0.573 | 79 (64.5–115.5) | 0.293 |
| TC | 670 (530–770) | 0.06 (0.04–0.07) | 613 (470–1013) | 98 (72–128) | |||||
| CC | 808.5 (708–1054.5) | 0.06 (0.06–0.09) | 582.5 (457.3–874.5) | 81 (74.3–110.8) | |||||
| TC + CC | 748 (582–990) | Pdom = 0.369 | 0.06 (0.05–0.08) | Pdom = 0.920 | 609 (461–894) | Pdom = 0.627 | 88 (74–123) | Pdom = 0.428 | |
| IL1B rs1071676 | GG | 723 (557.3–917.3) | 0.337 | 0.06 (0.04–0.08) | 0.414 | 611 (473.8–885.3) | 0.672 | 93.5 (73.8–125.8) | 0.398 |
| GC | 788 (570–1077) | 0.06 (0.05–0.08) | 544 (404–894) | 81 (61–114) | |||||
| CC | 799 (711–990) | 0.07 (0.06–0.09) | 709 (400–855) | 95 (60–111) | |||||
| GC + CC | 793.5 (627–1072.5) | Pdom = 0.179 | 0.06 (0.06–0.08) | Pdom = 0.430 | 546.5 (403–864.8) | Pdom = 0.386 | 82.5 (60.8–111.8) | Pdom = 0.200 | |
| MIR146A rs2910164 | GG | 748 (561–1005) | 0.748 | 0.06 (0.05–0.08) | 0.994 | 525 (454–881) | 0.556 | 81 (70–117) | 0.728 |
| GC | 714.5 (570–893) | 0.06 (0.04–0.07) | 617 (509–901.3) | 92 (73–123.5) | |||||
| CC | 795 (638–1297) | 0.05 (0.05–0.09) | 722 (324–872) | 93 (50–123) | |||||
| GC + CC | 718 (576–915) | Pdom = 0.735 | 0.06 (0.05–0.08) | Pdom = 0.943 | 617 (497–886) | Pdom = 0.310 | 93 (72–122.5) | Pdom = 0.465 | |
| IL6 rs1800795 | GG | 745 (579.8–816.3) | 0.407 | 0.06 (0.05–0.07) | 0.402 | 582.5 (471–902.8) | 0.832 | 87.5 (77–120.8) | 0.849 |
| GC | 759 (570–1087) | 0.06 (0.05–0.09) | 601 (404–894) | 87 (61–114) | |||||
| CC | 669 (538.5–1081) | 0.06 (0.04–0.11) | 609 (386–837) | 92 (63.5–126) | |||||
| GC + CC | 758.5 (570–1083.3) | Pdom = 0.225 | 0.06 (0.05–0.09) | Pdom = 0.178 | 605 (418.3–878.8) | Pdom = 0.690 | 88 (62.8–120.8) | Pdom = 0.579 | |
| TNF rs1800629 | GG | 753 (570–952.3) | 0.881 | 0.06 (0.05–0.08) | 0.309 | 615 (462.8–907.8) | 0.246 | 92 (70.5–123.8) | 0.354 |
| GA | 745 (594.5–863.8) | 0.07 (0.05–0.10) | 555.5 (471–704) | 88 (71–96) | |||||
| AA | 889.5 (532–1566.5) | 0.08 (0.06–0.12) | 510.5 (261.3–592.5) | 79.5 (47.5–86) | |||||
| GA + AA | 745 (594.5–1039.5) | Pdom = 0.854 | 0.07 (0.05–0.10) | Pdom = 0.170 | 534.5 (458–641.8) | Pdom = 0.144 | 85 (71–92.8) | Pdom = 0.221 | |
| CARD8 rs2043211 | AA | 731 (565–869) | 0.451 | 0.06 (0.05–0.07) | 0.663 | 650 (469–875) | 0.134 | 90 (76–123.5) | 0.147 |
| AT | 742.5 (563.3–977.8) | 0.06 (0.05–0.08) | 603.5 (478.3–914) | 94.5 (70.5–122.8) | |||||
| TT | 785 (721–1277) | 0.08 (0.04–0.10) | 470 (320–571) | 68 (50–87) | |||||
| AT + TT | 758 (570–1005) | Pdom = 0.437 | 0.06 (0.05–0.08) | Pdom = 0.588 | 567 (404–898) | Pdom = 0.491 | 83 (61–117) | Pdom = 0.291 | |
| NLRP3 rs35829419 | CC | 748 (572.5–948.5) | 0.413 | 0.06 (0.05–0.08) | 0.168 | 576 (461–879.5) | 0.264 | 87 (70–119.5) | 0.130 |
| CA+AA | 591 (516–790.5) | 0.04 (0.04–0.05) | 709 (663–1003.5) | 109 (103–149.5) | |||||
| GSTP1 rs1695 | AA | 759 (576–936) | 0.883 | 0.06 (0.05–0.08) | 0.416 | 562.5 (468.5–890.8) | 0.846 | 87.5 (69.5–123.8) | 0.550 |
| AG | 758.5 (572.3–984.8) | 0.06 (0.04–0.08) | 615 (403.8–980) | 95.5 (67.8–124.3) | |||||
| GG | 688.5 (557.3–1010.5) | 0.06 (0.06–0.08) | 616.5 (467.8–788) | 80 (72.3–98) | |||||
| AG + GG | 742.5 (564.5–981.5) | Pdom = 0.822 | 0.06 (0.05–0.08) | Pdom = 0.583 | 615 (461–876.5) | Pdom = 0.855 | 90 (70.5–120.8) | Pdom = 0.779 | |
| GSTP1 rs1138272 | CC | 745 (552–919.8) | 0.06 (0.05–0.08) | 601.5 (470–880.3) | 88.5 (70.8–120.8) | ||||
| CT + TT | 790 (674.5–1086.3) | Pdom = 0.224 | 0.07 (0.06–0.10) | Pdom = 0.294 | 572.5 (359–887.8) | Pdom = 0.650 | 86.5 (60.8–118.3) | Pdom = 0.944 | |
| NOS1 rs2293054 | AA | 753 (584.3–1042.3) | 0.738 | 0.06 (0.05–0.09) | 0.420 | 569 (403.3–891.5) | 0.670 | 86.5 (63.3–121.5) | 0.914 |
| AG | 765 (549.8–907) | 0.06 (0.05–0.07) | 605 (506.8–880.3) | 90.5 (74.3–120) | |||||
| GG | 642.5 (571.3–981.5) | 0.07 (0.05–0.07) | 665 (312.8–936.3) | 87.5 (55–120.5) | |||||
| AG + GG | 736 (563.3–907) | Pdom = 0.670 | 0.06 (0.05–0.07) | Pdom = 0.299 | 605 (497.5–880.3) | Pdom = 0.450 | 90.5 (73.3–120) | Pdom = 0.950 | |
| NOS1 rs2682826 | GG | 734.5 (546.8–977.8) | 0.371 | 0.06 (0.05–0.08) | 0.731 | 615 (358.8–880.3) | 0.204 | 91.5 (58–121.5) | 0.261 |
| GA | 770 (658–1005) | 0.06 (0.05–0.08) | 567 (470–782) | 83 (70–107) | |||||
| AA | 602 (552–742.5) | 0.06 (0.05–0.07) | 911 (499–1152) | 122 (76.5–144) | |||||
| GA + AA | 753 (611–947.3) | Pdom = 0.728 | 0.06 (0.05–0.08) | Pdom = 0.745 | 573.5 (473.3–894.3) | Pdom = 0.606 | 85.5 (74–120) | Pdom = 0.712 | |
| KEAP1 rs1048290 | GG | 795 (578.5–1031) | 0.222 | 0.06 (0.06–0.09) | 0.547 | 549 (436.5–875) | 0.723 | 93 (64.5–123.5) | 0.957 |
| GC | 711 (534–938.5) | 0.06 (0.05–0.07) | 617 (464.5–902.5) | 88 (73–118) | |||||
| CC | 767 (656.8–975) | 0.06 (0.04–0.08) | 543 (455.5–885.3) | 84 (74.5–119.8) | |||||
| GC + CC | 725 (570–941) | Pdom = 0.250 | 0.06 (0.05–0.08) | Pdom = 0.331 | 601 (461–894) | Pdom = 0.908 | 87 (74–117) | Pdom = 0.986 | |
| KEAP1 rs9676881 | GG | 797.5 (580.3–1010.5) | 0.188 | 0.06 (0.06–0.08) | 0.565 | 581 (453.3–873.5) | 0.735 | 91 (66.3–123.3) | 0.959 |
| GA | 703 (532–947.3) | 0.06 (0.05–0.07) | 609 (462.8–906.8) | 87.5 (72.5–120) | |||||
| AA | 767 (656.8–975) | 0.05 (0.04–0.08) | 543 (455.5–885.3) | 84 (74.5–119.8) | |||||
| GA + AA | 723 (562.3–944.8) | Pdom = 0.218 | 0.06 (0.04–0.08) | Pdom = 0.349 | 597.5 (461–895) | Pdom = 0.947 | 87 (73.5–118.3) | Pdom = 0.996 | |
| NFE2L2 rs6706649 | CC | 765 (576.8–1054.5) | 0.235 | 0.06 (0.05–0.08) | 0.940 | 573.5 (416.5–878.8) | 0.329 | 85.5 (62.8–116.3) | 0.223 |
| CT | 690 (531.3–817.5) | 0.06 (0.04–0.08) | 736 (486–942) | 103.5 (74.8–131) | |||||
| TT | 790 (758–822) | 0.06 (0.06–0.06) | 534.5 (525–544) | 83.5 (81–86) | |||||
| CT + TT | 714.5 (539.3–815.3) | Pdom = 0.143 | 0.06 (0.05–0.07) | Pdom = 0.733 | 669.5 (493–902.8) | Pdom = 0.210 | 97.5 (76–122.8) | Pdom = 0.117 | |
| NFE2L2 rs6721961 | GG | 758 (578.5–915) | 0.06 (0.05–0.08) | 650 (493.5–916) | 94 (74.5–122.5) | ||||
| GT + TT | 738 (539–1091) | Pdom = 0.827 | 0.06 (0.06–0.09) | Pdom = 0.313 | 473 (324–802) | Pdom = 0.020 | 79 (50–114) | Pdom = 0.063 | |
| NFE2L2 rs35652124 | TT | 817 (663–1125.5) | 0.031 | 0.07 (0.06–0.09) | 0.140 | 544 (430–868) | 0.428 | 81 (69–117) | 0.439 |
| TC | 669 (538–795) | 0.06 (0.04–0.07) | 613 (496–894) | 94 (76–123) | |||||
| CC | 794 (599.3–1055.3) | 0.06 (0.05–0.11) | 610.5 (430.8–913.8) | 85 (63.3–111.5) | |||||
| TC + CC | 711 (539–916) | Pdom = 0.053 | 0.06 (0.05–0.07) | Pdom = 0.097 | 613 (496–894) | Pdom = 0.219 | 93 (74–122) | Pdom = 0.304 |
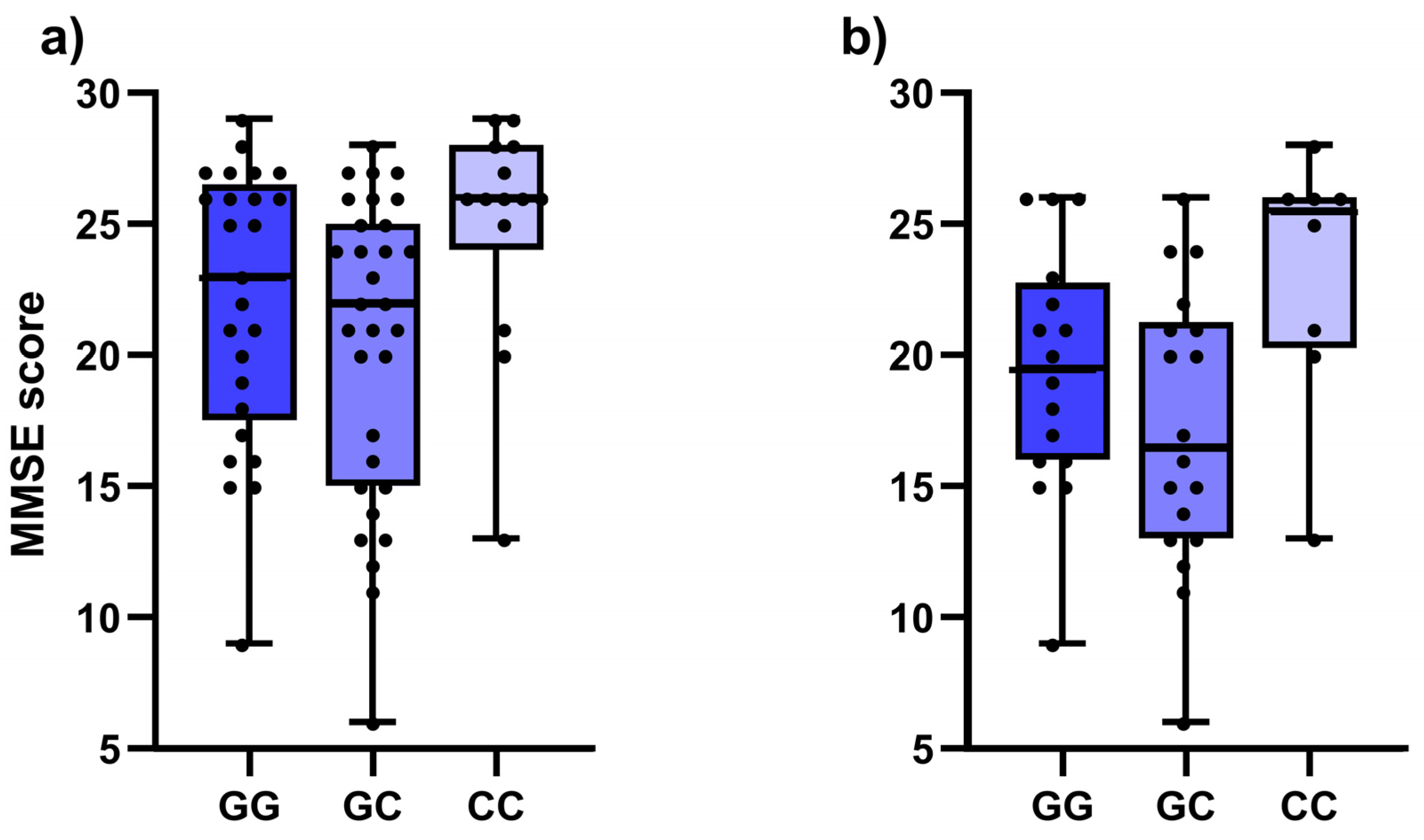
| Dementia | AD | ||||
|---|---|---|---|---|---|
| SNP | Genotype | MMSE | p | MMSE | p |
| SOD2 rs4880 | CC | 21.5 (20–25.3) | 0.703 | 21 (17.8–23.5) | 0.062 |
| CT | 25 (16–27) | 16 (13–21.5) | |||
| TT | 24 (19–26.5) | 21 (17.5–26) | |||
| CT + TT | 24.5 (16.3–26.8) | Pdom = 0.474 | 21 (17.8–23.5) | Pdom = 0.417 | |
| CAT rs1001179 | CC | 24 (19.8–26.3) | 0.022 | 20 (16–24) | 0.114 |
| CT | 22 (13.5–25.5) | 15 (13–23) | |||
| TT | 28 (27–28.5) | / a | |||
| CT + TT | 23 (14.3–26) | Pdom = 0.449 | 15 (13–23) | Pdom = 0.114 | |
| GPX1 rs1050450 | CC | 24.5 (20–26) | 0.849 | 21 (16–26) | 0.411 |
| CT | 23 (17–27) | 20 (15–24) | |||
| TT | 23 (15–26) | 15.5 (13.5–21.3) | |||
| CT + TT | 23 (16–26.3) | Pdom = 0.687 | 21 (16–26) | Pdom = 0.275 | |
| IL1B rs1143623 | GG | 24.5 (20–26) | 0.057 | 21 (16.5–25.5) | 0.231 |
| GC | 25 (17–27) | 16 (13.5–22.8) | |||
| CC | 21 (13–23) | 16 (12–22.5) | |||
| GC + CC | 22.5 (15–26) | Pdom = 0.337 | 21 (16.5–25.5) | Pdom = 0.087 | |
| IL1B rs16944 | TT | 21.5 (14.5–25.3) | 0.213 | 18.5 (12.5–23.8) | 0.879 |
| TC | 26 (19.3–27) | 19.5 (15–25.5) | |||
| CC | 24 (18.5–26) | 20 (16–23.5) | |||
| TC + CC | 24 (19.3–26.8) | Pdom = 0.127 | 20 (15.3–24) | Pdom = 0.631 | |
| IL1B rs1071676 | GG | 25 (20–26) | 0.440 | 20.5 (16.3–24) | 0.004 |
| GC | 21.5 (14.3–26.8) | 14.5 (12.3–17) | |||
| CC | 21.5 (18.8–25.5) | 21 (17.5–26) | |||
| GC + CC | 21.5 (15–26.3) | Pdom = 0.200 | 20 (15–24) | Pdom = 0.820 | |
| MIR146A rs2910164 | GG | 25 (19.3–26.8) | 0.131 | 20 (16–24.8) | 0.328 |
| GC | 23.5 (17.8–26) | 20.5 (14.8–24.5) | |||
| CC | 18.5 (12–23.3) | 14.5 (10–21.3) | |||
| GC + CC | 22.5 (15.8–26) | Pdom = 0.317 | 18.5 (13.8–23.3) | Pdom = 0.338 | |
| IL6 rs1800795 | GG | 21.5 (15–26) | 0.151 | 19.5 (14.5–22.8) | 0.391 |
| GC | 25 (20.5–27) | 21 (17–26) | |||
| CC | 23 (16–25) | 16 (13–23) | |||
| GC + CC | 24 (20–26) | Pdom = 0.291 | 20.5 (16–24) | Pdom = 0.483 | |
| TNF rs1800629 | GG | 23 (17.5–26.5) | 0.459 | 20 (15–23) | 0.652 |
| GA | 22 (16–25.5) | 18 (14.75–24.5) | |||
| AA | 26 (24.5–26) | / b | |||
| GA + AA | 24 (18–26) | Pdom = 0.964 | 20 (16–24) | Pdom = 0.869 | |
| CARD8 rs2043211 | AA | 22.5 (15.8–26) | 0.249 | 20 (13–22) | 0.399 |
| AT | 25 (17.8–26) | 20 (16–26) | |||
| TT | 25.5 (20.8–28.3) | 23 (16.3–26) | |||
| AT + TT | 25 (19.3–26) | Pdom = 0.215 | 20 (16–26) | Pdom = 0.228 | |
| NLRP3 rs35829419 | CC | 23.5 (17.3–26) | 0.365 | 20 (15–23.5) | / |
| CA | 26 (26–26) | / a | |||
| GSTP1 rs1695 | AA | 22 (15–26) | 0.410 | 16.5 (15–21.8) | 0.308 |
| AG | 25 (20–26) | 21 (16–26) | |||
| GG | 21 (19–26.5) | 20 (14.3–24.8) | |||
| AG + GG | 24.5 (20–26) | Pdom = 0.299 | 20.5 (16–26) | Pdom = 0.153 | |
| GSTP1 rs1138272 | CC | 22 (16–26) | 20 (15–22.3) | ||
| CT + TT | 26.5 (24.3–27) | Pdom = 0.005 | 24 (19.5–25.5) | Pdom = 0.144 | |
| NOS1 rs2293054 | AA | 25 (20.3–27) | 0.342 | 21 (15–24) | 0.992 |
| AG | 21 (17–26) | 20 (15.3–23.3) | |||
| GG | 25.5 (16.8–26.3) | 18 (15.5–22) | |||
| AG + GG | 21 (17–26) | Pdom = 0.192 | 20 (15–24) | Pdom = 0.970 | |
| NOS1 rs2682826 | GG | 25 (16.5–26.5) | 0.306 | 20 (14.8–25.3) | 0.754 |
| GA | 24 (19–26) | 21 (15–24) | |||
| AA | 20 (16.3–22.3) | 20 (14.5–20.5) | |||
| GA + AA | 22 (18.5–26) | Pdom = 0.591 | 20 (15.3–23.5) | Pdom = 0.980 | |
| KEAP1 rs1048290 | GG | 23 (17.5–26.5) | 0.019 | 19.5 (16–22.8) | 0.035 |
| GC | 22 (15–25) | 16.5 (13–21.3) | |||
| CC | 26 (24–28) | 25.5 (20.3–26) | |||
| GC + CC | 24 (18.5–26) | Pdom = 0.917 | 20 (13.8–24.3) | Pdom = 0.825 | |
| KEAP1 rs9676881 | GG | 23 (17.5–26.5) | 0.019 | 19.5 (16–22.8) | 0.035 |
| GA | 22 (15–25) | 16.5 (13–21.3) | |||
| AA | 26 (24–28) | 25.5 (20.3–26) | |||
| GA + AA | 24 (18.5–26) | Pdom = 0.917 | 20 (13.8–24.3) | Pdom = 0.825 | |
| NFE2L2 rs6706649 | CC | 24.5 (18.3–26.8) | 20 (15–24.5) | ||
| CT + TT | 22 (16–26) | Pdom = 0.335 | 21 (15.5–23) | Pdom = 0.893 | |
| NFE2L2 rs6721961 | GG | 23.5 (17.3–26) | 20 (16–24) | ||
| GT + TT | 25.5 (18.8–27) | Pdom = 0.391 | 15 (13–22) | Pdom = 0.272 | |
| NFE2L2 rs35652124 | TT | 25 (21–27) | 0.030 | 20 (15–24.5) | 0.456 |
| TC | 21 (15–26) | 20 (14–23) | |||
| CC | 23.5 (20.5–26.3) | 21 (18.3–26) | |||
| TC + CC | 21 (16–26) | Pdom = 0.024 | 20 (15–23.5) | Pdom = 0.893 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vogrinc, D.; Gregorič Kramberger, M.; Emeršič, A.; Čučnik, S.; Goričar, K.; Dolžan, V. Genetic Polymorphisms in Oxidative Stress and Inflammatory Pathways as Potential Biomarkers in Alzheimer’s Disease and Dementia. Antioxidants 2023, 12, 316. https://doi.org/10.3390/antiox12020316
Vogrinc D, Gregorič Kramberger M, Emeršič A, Čučnik S, Goričar K, Dolžan V. Genetic Polymorphisms in Oxidative Stress and Inflammatory Pathways as Potential Biomarkers in Alzheimer’s Disease and Dementia. Antioxidants. 2023; 12(2):316. https://doi.org/10.3390/antiox12020316
Chicago/Turabian StyleVogrinc, David, Milica Gregorič Kramberger, Andreja Emeršič, Saša Čučnik, Katja Goričar, and Vita Dolžan. 2023. "Genetic Polymorphisms in Oxidative Stress and Inflammatory Pathways as Potential Biomarkers in Alzheimer’s Disease and Dementia" Antioxidants 12, no. 2: 316. https://doi.org/10.3390/antiox12020316






