Response of Oxidative Stress and Antioxidant System in Pea Plants Exposed to Drought and Boron Nanoparticles
Abstract
:1. Introduction
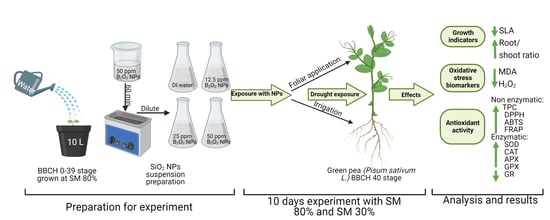
2. Materials and Methods
2.1. Research Conditions
2.2. Aqueous Suspension of Boron Nanoparticles
2.3. Research Object
2.4. Growth Parameters
2.5. Biochemical Analysis
2.5.1. Non-Enzymatic Antioxidant Activity
2.5.2. Enzymatic Antioxidant Activity
2.5.3. Oxidative Stress Biomarkers
- CMDA—concentration of MDA, µM;
- A532, A600—absorbance at wavelength;
- EMDA—MDA extinction coefficient 155 mM−1 cm−1.
2.6. Elemental Composition Analysis
2.7. Statistical Analysis
3. Results
3.1. Boron Nanoparticles’ Impact on Morphological Parameters
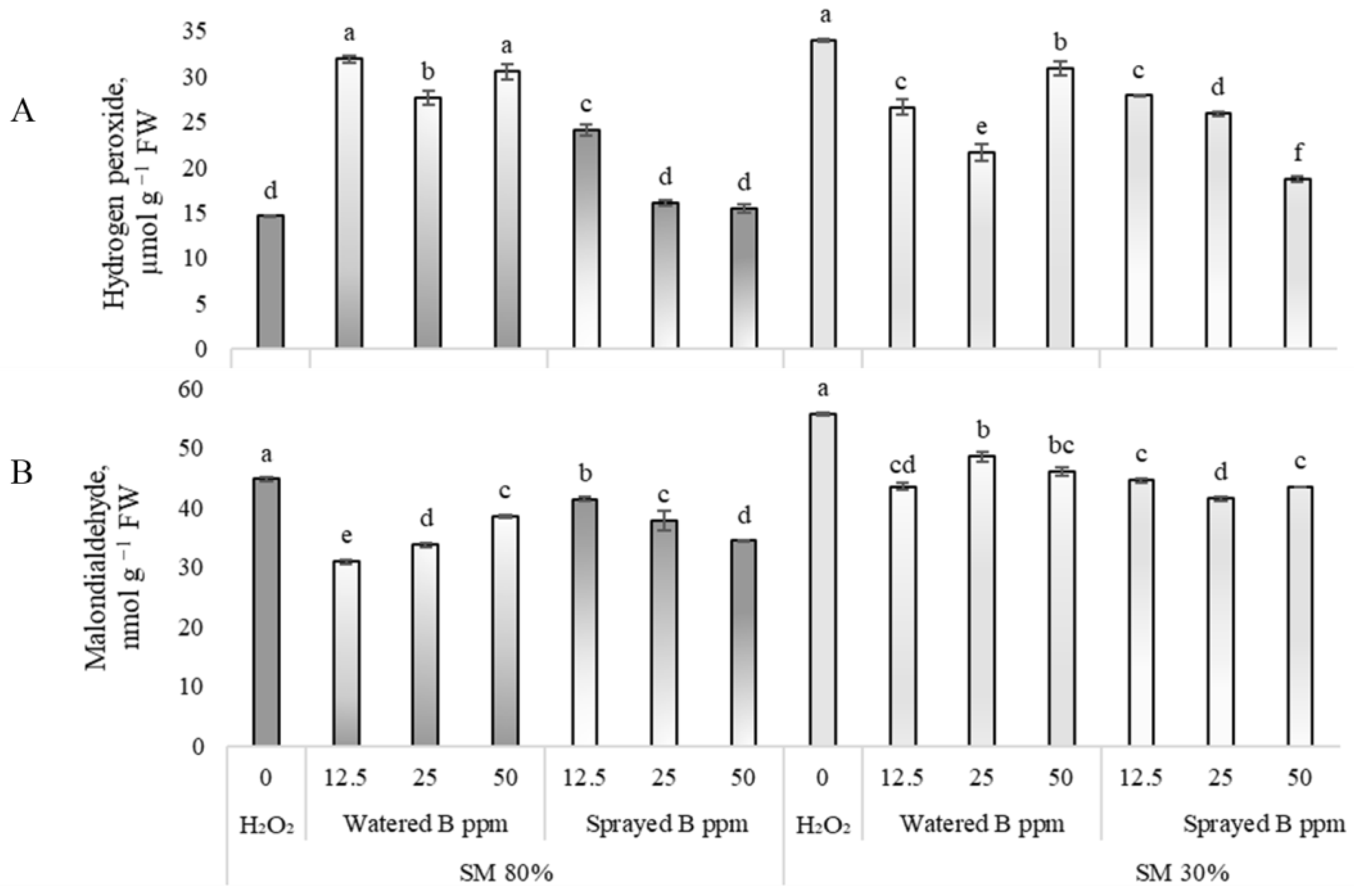
3.2. Effects on Oxidative Stress Biomarkers
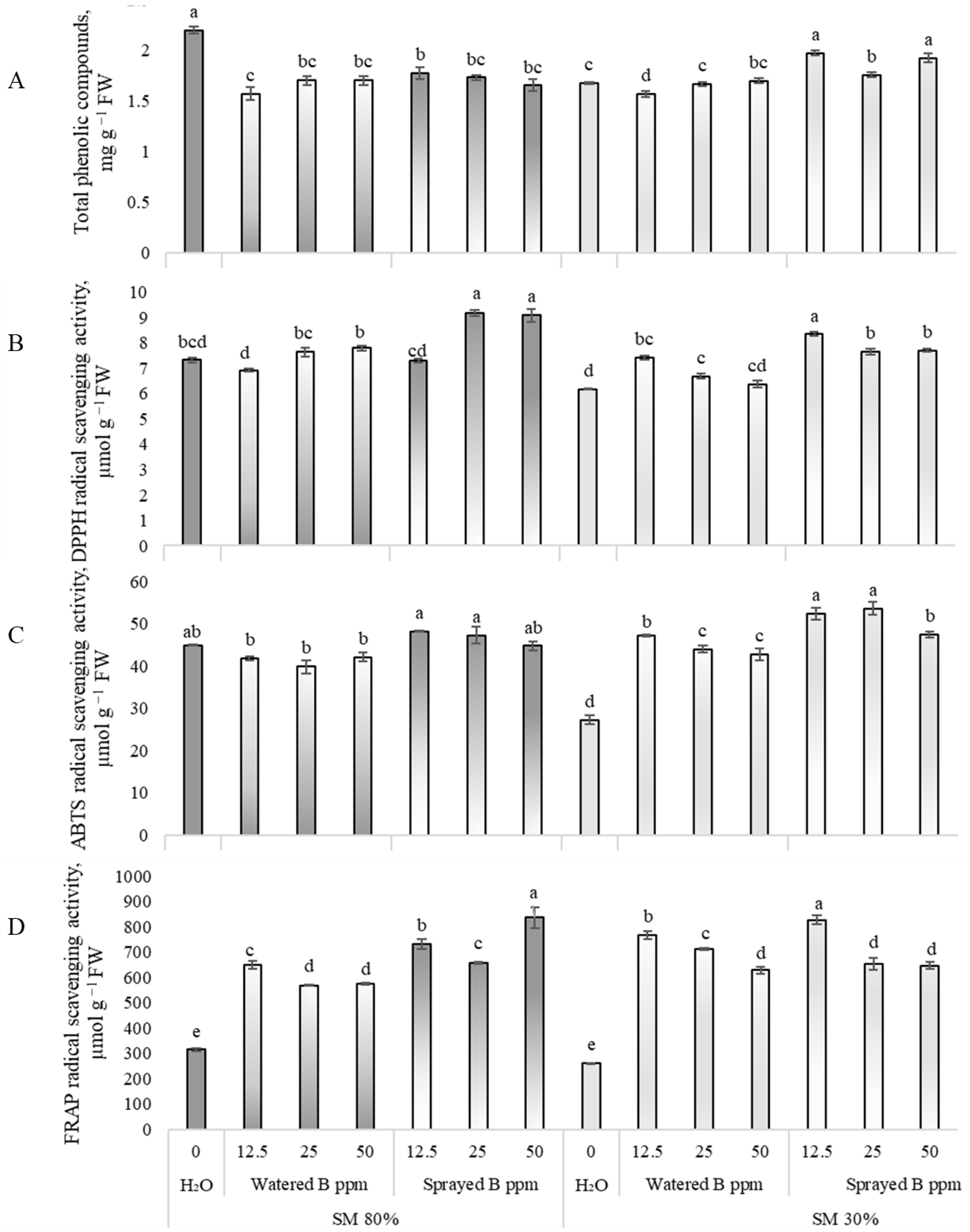
3.3. Effects on Non-Enzymatic Antioxidants
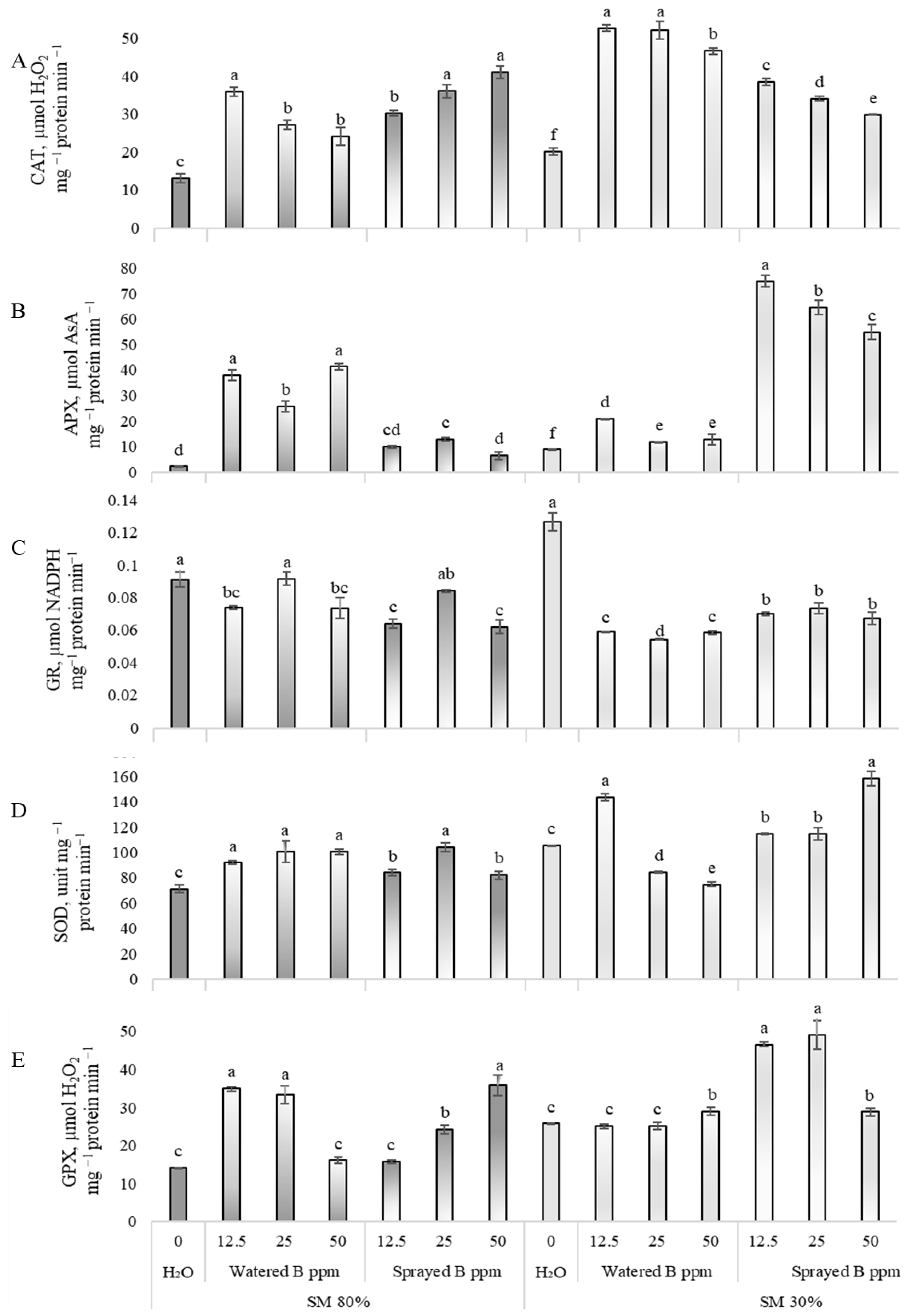
3.4. Effects on Enzymatic Antioxidants
3.5. Comparison and Summary of Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alpaslan, M.; Gunes, A. Interactive effects of boron and salinity stress on the growth, membrane permeability and mineral composition of tomato and cucumber plants. Plant Soil 2001, 236, 123–128. [Google Scholar] [CrossRef]
- Bonilla, I.; El Hamdaoui, A.; Bolaños, L. Boron and calcium increase Pisum sativum seed germination and seedling development under salt stress. Plant Soil 2004, 267, 97–107. [Google Scholar] [CrossRef]
- Bastías, E.I.; González-Moro, M.B.; González-Murua, C. Zea mays L. amylacea from the Lluta Valley (Arica-Chile) tolerates salinity stress when high levels of boron are available. Plant Soil 2004, 267, 73–84. [Google Scholar] [CrossRef]
- Dimkpa, C.O.; Singh, U.; Bindraban, P.S.; Adisa, I.O.; Elmer, W.H.; Gardea-Torresdey, J.L.; White, J.C. Addition-omission of zinc, copper, and boron nano and bulk oxide particles demonstrate element and size-specific response of soybean to micronutrients exposure. Sci. Total Environ. 2019, 665, 606–616. [Google Scholar] [CrossRef]
- Zewail, R.M.; Ali, M.; El-Gamal, I.S.; Al-Maracy, S.H.; Islam, K.R.; Elsadek, M.; Azab, E.; Gobouri, A.A.; ElNahhas, N.; Mohamed, M.H.; et al. Interactive effects of arbuscular mycorrhizal inoculation with nano boron, zinc, and molybdenum fertilization on stevioside contents of stevia (Stevia rebaudiana L.) plants. Horticulturae 2021, 7, 260. [Google Scholar] [CrossRef]
- Mahmoud, A.W.; Abdeldaym, E.A.; Abdelaziz, S.M.; El-Sawy, M.B.; Mottaleb, S.A. Synergetic Effects of Zinc, Boron, Silicon, and Zeolite Nanoparticles on Confer Tolerance in Potato Plants Subjected to Salinity. Agronomy 2020, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Redondo-Nieto, M.; Maunoury, N.; Mergaert, P.; Kondorosi, E.; Bonilla, I.; Bolaños, L. Boron and calcium induce major changes in gene expression during legume nodule organogenesis. Does boron have a role in signalling? New Phytol. 2012, 195, 14–19. [Google Scholar] [CrossRef]
- Cakmak, I.; Römheld, V. Boron deficiency-induced impairments of cellular functions in plants. Plant Soil 1997, 193, 71–83. [Google Scholar] [CrossRef]
- Chen, D.; Chen, D.; Xue, R.; Long, J.; Lin, X.; Lin, Y.; Jia, L.; Zeng, R.; Song, Y. Effects of boron, silicon and their interactions on cadmium accumulation and toxicity in rice plants. J. Hazard. Mater. 2019, 367, 447–455. [Google Scholar] [CrossRef]
- Shireen, F.; Nawaz, M.A.; Lu, J.; Xiong, M.; Kaleem, M.; Huang, Y.; Bie, Z. Application of boron reduces vanadium toxicity by altering the subcellular distribution of vanadium, enhancing boron uptake and enhancing the antioxidant defense system of watermelon. Ecotoxicol. Environ. Saf. 2021, 226, 112828. [Google Scholar] [CrossRef]
- Karim, M.R.; Zhang, Y.Q.; Zhao, R.R.; Chen, X.P.; Zhang, F.S.; Zou, C.Q. Alleviation of drought stress in winter wheat by late foliar application of zinc, boron, and manganese. J. Plant Nutr. Soil Sci. 2012, 175, 142–151. [Google Scholar] [CrossRef]
- Abdel-Motagally, F.; El-Zohri, M. Improvement of wheat yield grown under drought stress by boron foliar application at different growth stages. J. Saudi Soc. Agric. Sci. 2018, 17, 178–185. [Google Scholar] [CrossRef] [Green Version]
- Aydin, M.; Tombuloglu, G.; Sakcali, M.S.; Hakeem, K.R.; Tombuloglu, H. Boron Alleviates Drought Stress by Enhancing Gene Expression and Antioxidant Enzyme Activity. J. Soil Sci. Plant Nutr. 2019, 19, 545–555. [Google Scholar] [CrossRef]
- Lehotai, N.; Pető, A.; Bajkán, S.; Erdei, L.; Tari, I.; Kolbert, Z. In vivo and in situ visualization of early physiological events induced by heavy metals in pea root meristem. Acta Physiol. Plant. 2011, 33, 2199–2207. [Google Scholar] [CrossRef]
- Meier, U. Growth Stages of Mono- and Dicotyledonous Plants: BBCH Monograph; Meier, U., Ed.; Julius Kühn-Institut: Quedlinburg, Germany, 2018. [Google Scholar]
- Barr, H.D.; Weatherley, P.E. A re-examination of the relative turgidity technique for estimating water deficit in leaves. Aust. J. Biol. Sci. 1962, 15, 413–428. [Google Scholar] [CrossRef] [Green Version]
- Koiter, A.; Bill Ashton, M.C.I.P. Rural Development Institute. 2021. Available online: https://www.brandonu.ca/rdi/files/2021/06/2020-2021-Rural-Development-Institute-Annual-Report.pdf (accessed on 7 February 2023).
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an amproved ABTS radical cation decolorization sssay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf Senescence: Correlated with Increased Levels of Membrane Permeability and Lipid Peroxidation, and Decreased Levels of Superoxide Dismutase and Catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Aebi, H. Catalase in vitro. Meth. Enzymol. 1984, 105, 121–126. [Google Scholar]
- Nakano, Y.; Asada, K. Hydrogen Peroxide is Scavenged by Ascorbate-specific Peroxidase in Spinach Chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar] [CrossRef]
- Sofo, A.; Tuzio, A.C.; Dichio, B.; Xiloyannis, C. Influence of water deficit and rewatering on the components of the ascorbate–glutathione cycle in four interspecific Prunus hybrids. Plant Sci. 2005, 169, 403–412. [Google Scholar] [CrossRef]
- Kvaratskhelia, M.; Winkel, C.; Thorneley, R.; Wenzl, P.; Patiño, G.M.; Chaves, A.L.; Mayer, J.E.; Rao, I.M. Purification and Characterization of a Novel Class III Peroxidase Isoenzyme from Tea Leaves. Plant Physiol. 1997, 114, 1237–1245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant system in acid rain treated bean plants: Protective role of exogenous polyammines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in Isolated Chloroplasts I. Kinetics and Stoichiometry of Fatty Acid Peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Araújo, G.C.; Gonzalez, M.H.; Ferreira, A.G.; Nogueira, A.R.A.; Nóbrega, J.A. Effect of acid concentration on closed-vessel microwave-assisted digestion of plant materials. Spectrochim. Acta Part B 2002, 57, 2121–2132. [Google Scholar] [CrossRef]
- Barbosa, J.T.P.; Santos, C.M.; Peralva, V.N.; Flores, E.M.; Korn, M.; Nóbrega, J.A.; Korn, M.G.A. Microwave-assisted diluted acid digestion for trace elements analysis of edible soybean products. Food Chem. 2015, 175, 212–217. [Google Scholar] [CrossRef]
- Barreto, A.; Santos, J.; Amorim, M.; Maria, V. Environmental Hazards of Boron and Vanadium Nanoparticles in the Terrestrial Ecosystem—A Case Study with Enchytraeus crypticus. Nanomaterials 2021, 11, 1937. [Google Scholar] [CrossRef]
- Pereira, G.L.; Siqueira, J.A.; Batista-Silva, W.; Cardoso, F.B.; Nunes-Nesi, A.; Araújo, W.L. Boron: More Than an Essential Element for Land Plants? Front. Plant Sci. 2021, 11, 610307. [Google Scholar] [CrossRef] [PubMed]
- Camacho-Cristóbal, J.J.; Rexach, J.; González-Fontes, A. Boron in Plants: Deficiency and Toxicity. J. Integr. Plant Biol. 2008, 50, 1247–1255. [Google Scholar] [CrossRef] [PubMed]
- Goldbach, H.E.; Wimmer, M.A. Boron in plants and animals: Is there a role beyond cell-wall structure? J. Plant Nutr. Soil Sci. 2007, 170, 39–48. [Google Scholar] [CrossRef]
- Ryden, P.; Sugimoto-Shirasu, K.; Smith, A.C.; Findlay, K.; Reiter, W.-D.; McCann, M.C. Tensile Properties of Arabidopsis Cell Walls Depend on Both a Xyloglucan Cross-Linked Microfibrillar Network and Rhamnogalacturonan II-Borate Complexes. Plant Physiol. 2003, 132, 1033–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolaños, L.; Cebrián, A.; Redondo-Nieto, M.; Rivilla, R.; Bonilla, I. Lectin-Like Glycoprotein PsNLEC-1 Is Not Correctly Glycosylated and Targeted in Boron-Deficient Pea Nodules. MPMI 2001, 14, 663–670. [Google Scholar] [CrossRef] [Green Version]
- Bolaños, L.; Lukaszewski, K.; Bonilla, I.; Blevins, D. Why boron? Plant Physiol. Biochem. 2004, 42, 907–912. [Google Scholar] [CrossRef]
- Dağlioğlu, Y.; Türkiş, S. A Comparative Study on the Effect of Acute Toxicity of Nano and Micro Boron Particles in Lemna minor (Linneaus 1753). J. Boron 2021, 6, 263–273. [Google Scholar] [CrossRef]
| B2O3 NPs, ppm | Leaf Area, cm2 | Plant Height, cm | RWC, % | Root/Shoot Ratio | SLA, m2 kg −1 | Number of Nodules | Yield, t ha−1 | ||
|---|---|---|---|---|---|---|---|---|---|
| SM 80% | 0 | 36.1 c | 28.4 d | 82.5 d | 7.8 c | 5.3 ab | 1.7 d | 3.9 b | |
| Watered | 12.5 | 46.1 a | 32.4 bc | 84.3 c | 9.4 b | 6.1 a | 11.0 a | 3.1 c | |
| 25 | 39.2 bc | 30.3 cd | 86.1 ab | 10.5 b | 5.1 ab | 5.0 c | 4.0 ab | ||
| 50 | 46.8 a | 36.1 a | 86.8 ab | 13.1 a | 4.7 ab | 9.7 ab | 4.7 a | ||
| Sprayed | 12.5 | 49.9 a | 36.3 a | 87.3 a | 13.1 a | 4.5 b | 7.3 bc | 4.4 a | |
| 25 | 44.9 ab | 30.9 bcd | 85.5 bc | 9.2 b | 5.0 ab | 5.7 c | 4.3 a | ||
| 50 | 44.6 ab | 33.8 ab | 85.6 bc | 10.4 b | 5.1 ab | 5.0 c | 3.9 b | ||
| SM 30% | 0 | 33.1 b | 26.0 e | 53.0 c | 9.2 c | 5.0 a | 2.0 c | 2.5 c | |
| Watered | 12.5 | 27.5 b | 28.4 d | 52.6 c | 9.6 b | 4.2 ab | 2.3 c | 2.9 a | |
| 25 | 42.9 a | 32.7 a | 58.3 ab | 10.5 a | 4.8 a | 6.0 b | 2.7 ab | ||
| 50 | 46.4 a | 30.4 bc | 59.1 a | 11.9 a | 4.2 ab | 8.3 b | 2.6 bc | ||
| Sprayed | 12.5 | 31.6 b | 29.3 cd | 51.9 c | 10.2 a | 4.4 ab | 2.0 c | 3.0 a | |
| 25 | 33.4 b | 31.2 ab | 55.2 bc | 10.5 a | 3.9 ab | 3.3 c | 2.9 a | ||
| 50 | 31.2 b | 29.6 cd | 57.8 ab | 9.6 b | 3.5 b | 13.7 a | 2.9 a | ||
| Treatment B2O3 NPs, ppm | SM 80% | SM 30% | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Watered | Sprayed | Watered | Sprayed | |||||||||
| 12.5 | 25 | 50 | 12.5 | 25 | 50 | 12.5 | 25 | 50 | 12.5 | 25 | 50 | |
| Plant height | 14 | 7 | 27 | 28 | 9 | 19 | 9 | 26 | 17 | 13 | 20 | 14 |
| Leaf area | 28 | 9 | 30 | 38 | 25 | 24 | −17 | 30 | 40 | −4 | 1 | −6 |
| Nodules | 560 | 200 | 480 | 340 | 240 | 200 | 17 | 200 | 317 | 0 | 67 | 583 |
| RWC | 2 | 4 | 5 | 6 | 4 | 4 | −1 | 10 | 11 | −2 | 4 | 9 |
| Root/shoot | 21 | 36 | 68 | 69 | 18 | 34 | 4 | 14 | 30 | 11 | 14 | 5 |
| SLA | 14 | −5 | −11 | −15 | −6 | −3 | −17 | −4 | −16 | −13 | −23 | −29 |
| Yield | −21 | 2 | 20 | 12 | 11 | −1 | 16 | 10 | 6 | 19 | 16 | 14 |
| ABTS | −7 | −11 | −6 | 7 | 5 | 0 | 73 | 61 | 56 | 92 | 96 | 74 |
| DPPH | −5 | 4 | 7 | 0 | 25 | 24 | 20 | 8 | 3 | 35 | 24 | 25 |
| TPC | −29 | −22 | −22 | −19 | −21 | −25 | −6 | −1 | 1 | 18 | 5 | 15 |
| FRAP | 106 | 81 | 83 | 132 | 109 | 166 | 194 | 174 | 141 | 217 | 151 | 148 |
| HP | 119 | 89 | 109 | 65 | 10 | 6 | −22 | −37 | −9 | −18 | −24 | −45 |
| MDA | −31 | −25 | −14 | −7 | −15 | −23 | −22 | −13 | −17 | −20 | −25 | −22 |
| GR | −19 | 0 | −19 | −30 | −8 | −32 | −53 | −57 | −54 | −45 | −42 | −47 |
| GPX | 147 | 136 | 14 | 11 | 71 | 153 | −3 | −2 | 13 | 81 | 91 | 12 |
| APX | 1657 | 1100 | 1817 | 363 | 498 | 200 | 136 | 33 | 46 | 750 | 634 | 522 |
| SOD | 29 | 41 | 41 | 18 | 46 | 15 | 36 | −20 | −29 | 9 | 9 | 51 |
| CAT | 173 | 107 | 84 | 131 | 175 | 214 | 161 | 159 | 132 | 91 | 69 | 48 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sutulienė, R.; Brazaitytė, A.; Małek, S.; Jasik, M.; Samuolienė, G. Response of Oxidative Stress and Antioxidant System in Pea Plants Exposed to Drought and Boron Nanoparticles. Antioxidants 2023, 12, 528. https://doi.org/10.3390/antiox12020528
Sutulienė R, Brazaitytė A, Małek S, Jasik M, Samuolienė G. Response of Oxidative Stress and Antioxidant System in Pea Plants Exposed to Drought and Boron Nanoparticles. Antioxidants. 2023; 12(2):528. https://doi.org/10.3390/antiox12020528
Chicago/Turabian StyleSutulienė, Rūta, Aušra Brazaitytė, Stanisław Małek, Michał Jasik, and Giedrė Samuolienė. 2023. "Response of Oxidative Stress and Antioxidant System in Pea Plants Exposed to Drought and Boron Nanoparticles" Antioxidants 12, no. 2: 528. https://doi.org/10.3390/antiox12020528