“Golden” Tomato Consumption Ameliorates Metabolic Syndrome: A Focus on the Redox Balance in the High-Fat-Diet-Fed Rat
Abstract
:1. Introduction
2. Materials and Methods
2.1. Treatment of Tomato Samples
2.1.1. Chemical–Physical Analysis of Tomato Samples
2.1.2. Determination of Phytonutrients in Golden and Red Tomatoes by HPLC System
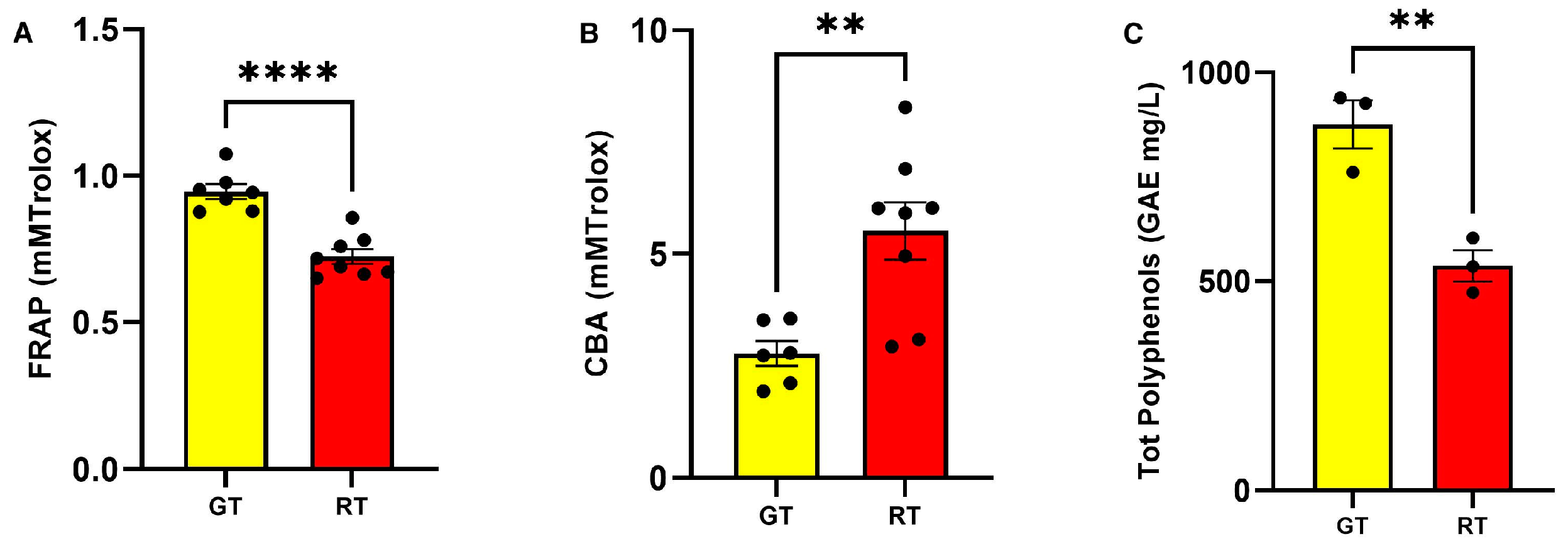
2.1.3. Determination of Total Polyphenolic Content and Antioxidant Properties
2.2. Animals
Experimental Groups
2.3. Preparation of the Orally Administered Tomato Solutions
2.4. Biometric, Biochemical, and Oxidative Homeostasis Parameters Induced by MetS
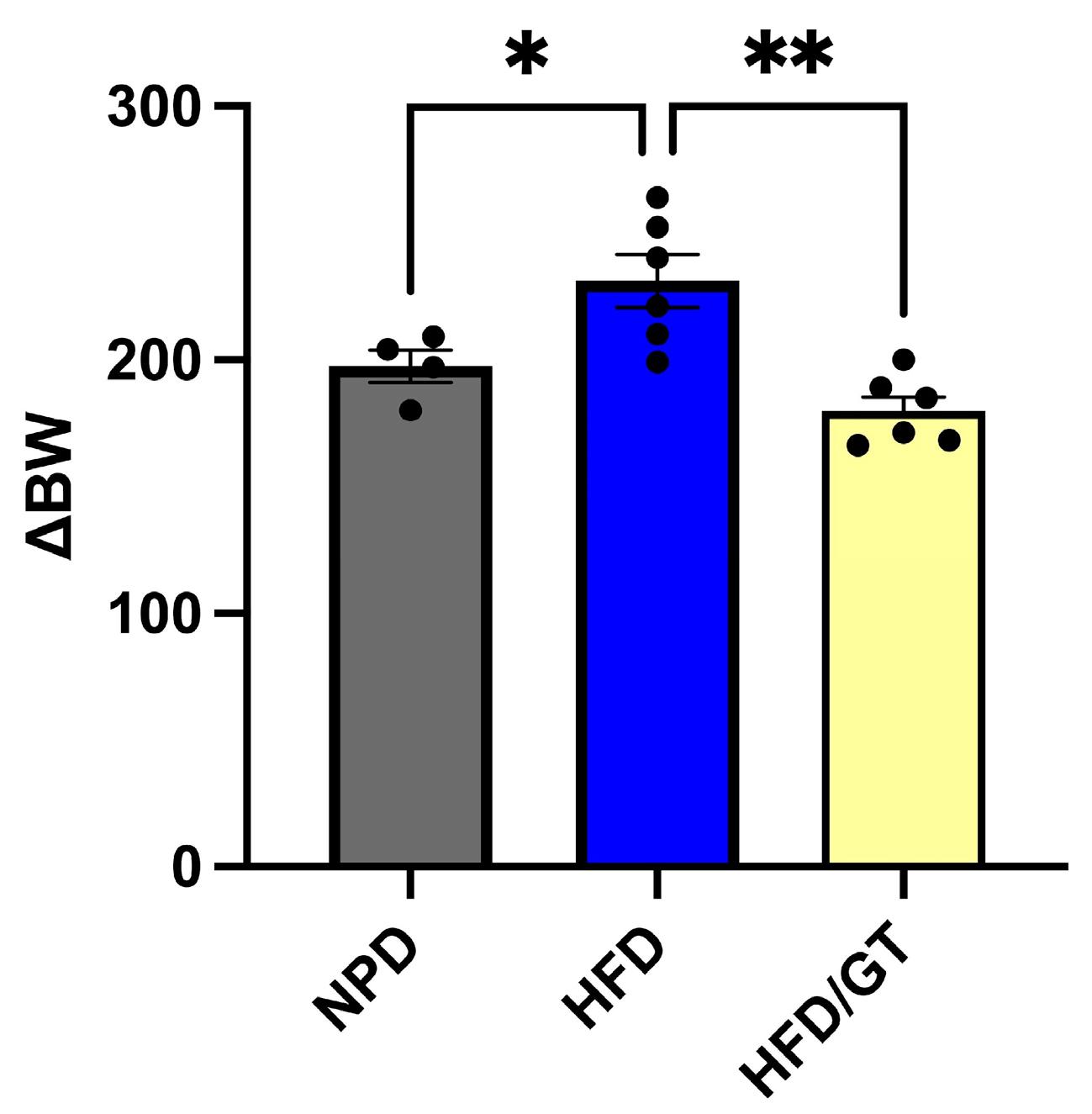
2.4.1. Body Weight Gain
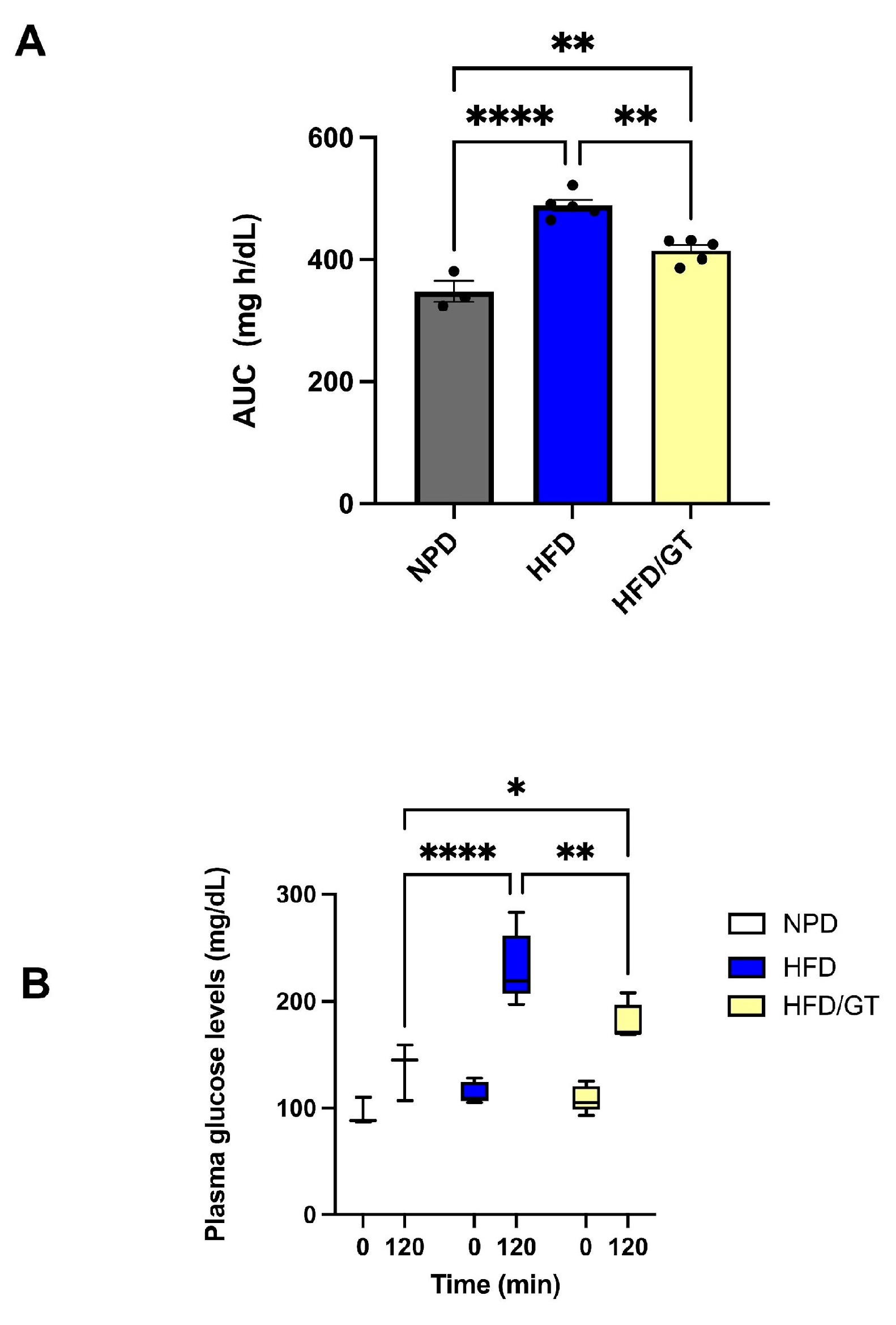
2.4.2. Glucose and Lipid Homeostasis assays
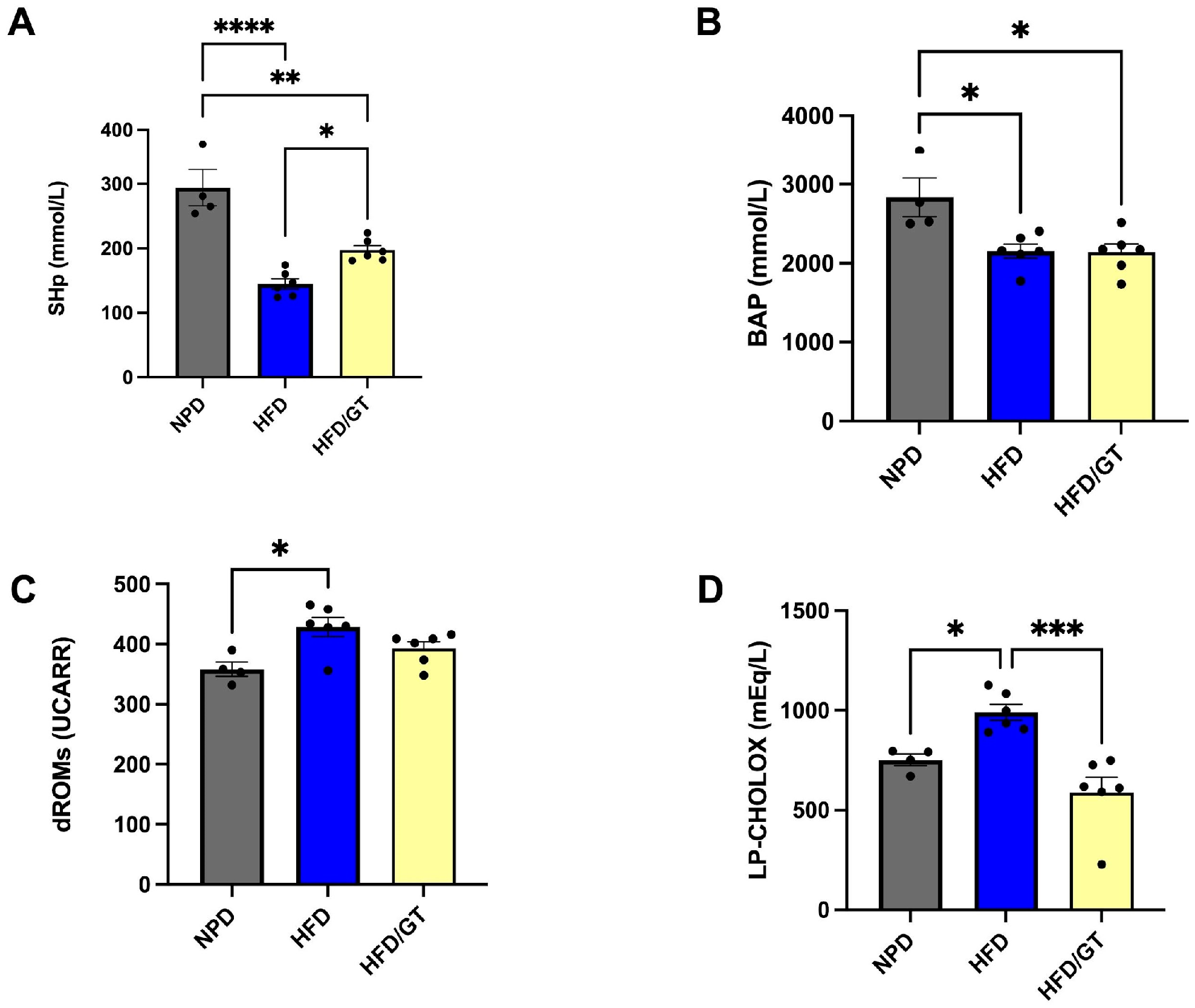
2.4.3. Oxidative Stress Parameters and Plasma Antioxidant Status
2.4.4. MDA Assay
2.4.5. RONS Assay
2.4.6. GSH Measurements
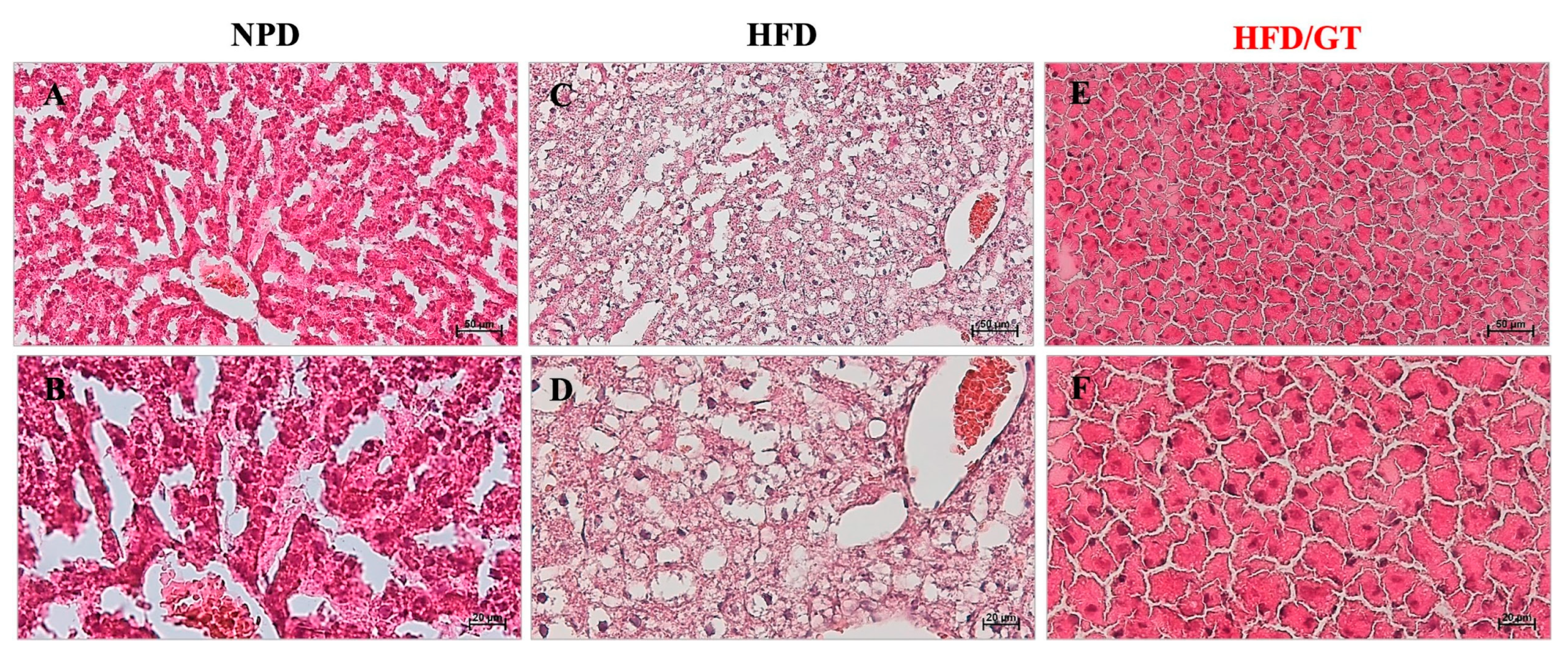
2.5. Histological Analyses
2.6. Statistical Analyses
3. Results
3.1. Analytical, Nutritional, and Antioxidant Composition of Tomato Food Matrices
3.2. Effects of GT Treatment on Body Weight in MetS
3.3. Effects of GT Treatment on Glucose and Lipid Homeostasis in MetS
3.4. Effects of GT on Plasma Redox Homeostasis Biomarkers in MetS
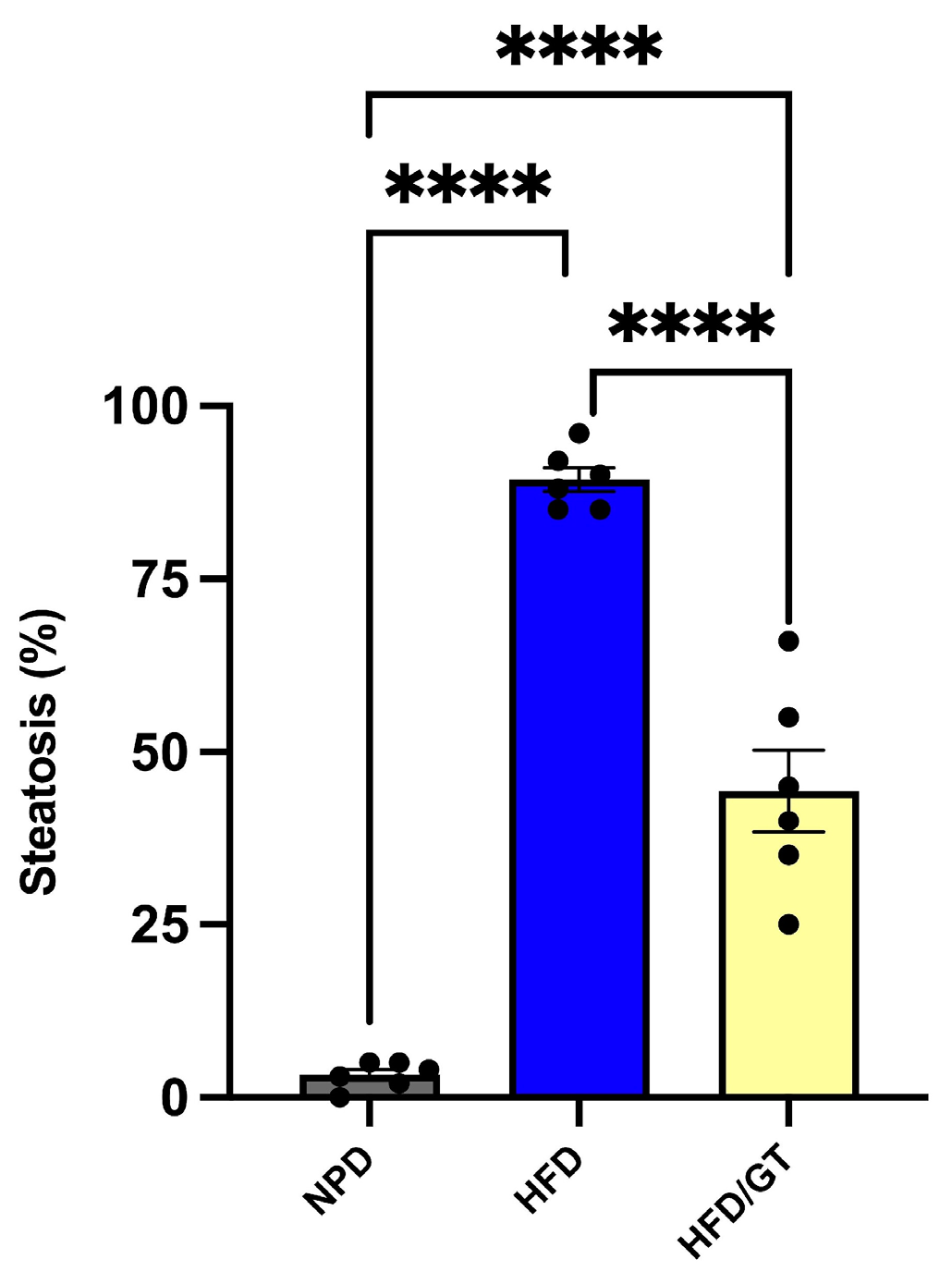
3.5. Effects of GT on Hepatic Steatosis
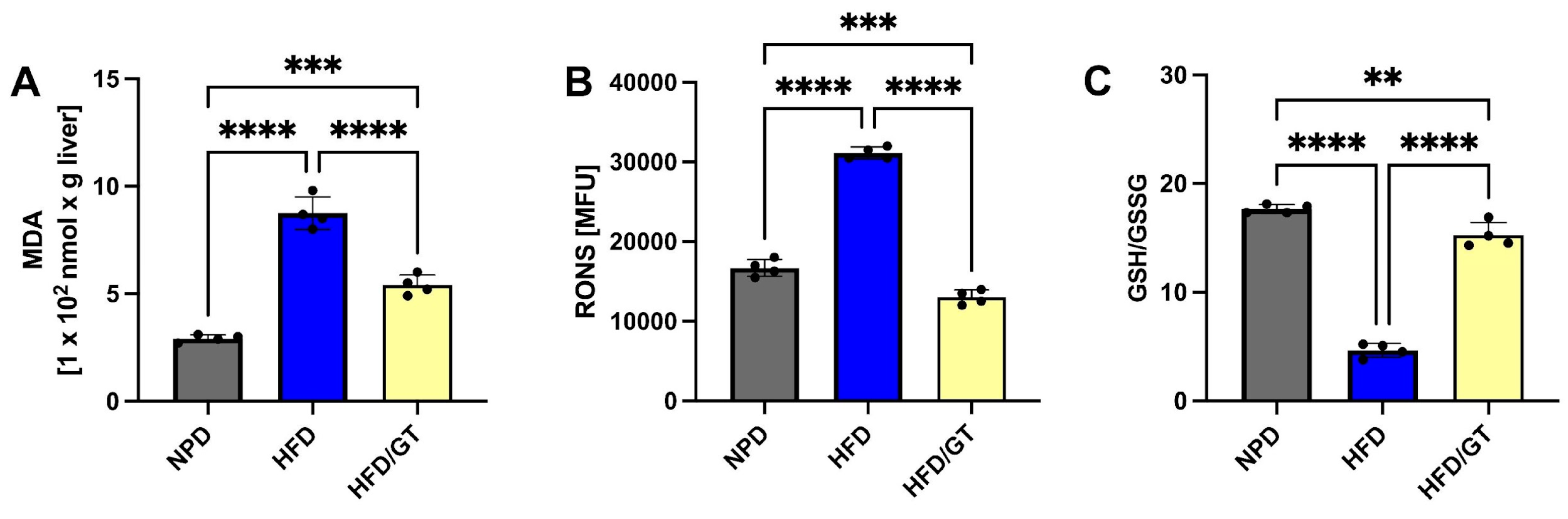
3.6. Effects of GT on MetS-Induced Hepatic Oxidative Stress
3.7. Effects of RT on MetS-Induced Systemic and Hepatic Oxidative Stress
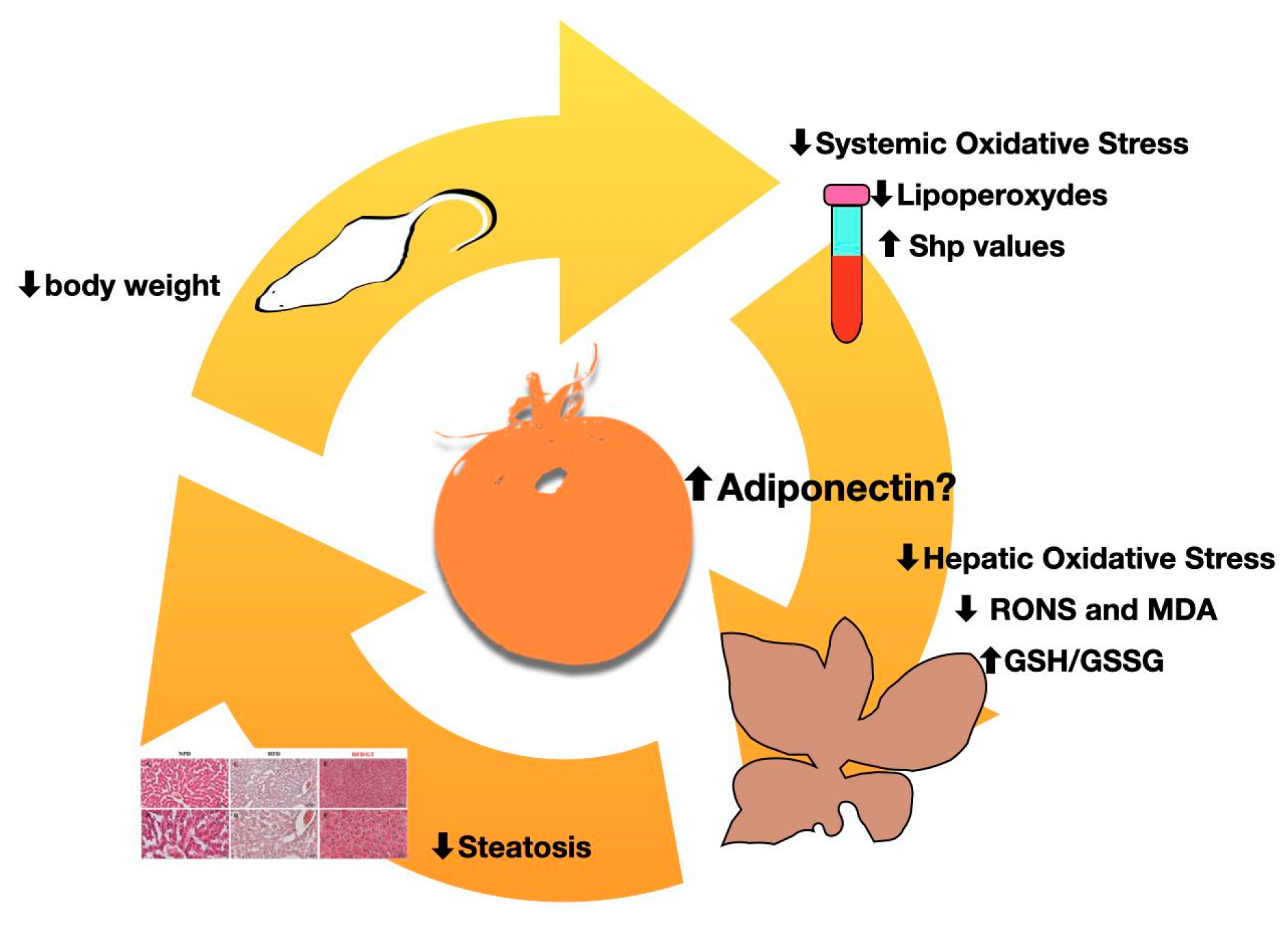
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cruz-Carrión, Á.; Ruiz de Azua, M.J.; Bravo, F.I.; Aragonès, G.; Muguerza, B.; Suárez, M.; Arola-Arnal, A. Tomatoes consumed in-season prevent oxidative stress in Fischer 344 rats: Impact of geographical origin. Food Funct. 2021, 12, 8340–8350. [Google Scholar] [CrossRef] [PubMed]
- Willcox, J.K.; Catignani, G.L.; Lazarus, S. Tomatoes and Cardiovascular Health. Crit. Rev. Food Sci. Nutr. 2003, 43, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.; Siervo, M.; Lara, J. Tomato and lycopene supplementation and cardiovascular risk factors: A systematic review and meta-analysis. Atherosclerosis 2017, 257, 100–108. [Google Scholar] [CrossRef] [PubMed]
- Islam, N.; Akhter, N. Comparative Study of Protective Effect of Tomato Juice and N-Hexane Extract of Tomato on Blood Lipids and Oxidative Stress in Cholesterol-Fed Rats. Anwer Khan Mod. Med. Coll. J. 2017, 8, 30–37. [Google Scholar] [CrossRef]
- Khayat Nouri, M.H.; Abad, A.N.A. Comparative Study of Tomato and Tomato Paste Supplementation on the Level of Serum Lipids and Lipoproteins Levels in Rats Fed With High Cholesterol. Iran. Red Crescent Med. J. 2013, 15, 287–291. [Google Scholar] [CrossRef]
- Bose, K.S.C.; Agrawal, B.K. Effect of long term supplementation of tomatoes (cooked) on levels of antioxidant enzymes, lipid peroxidation rate, lipid profile and glycated haemoglobin in Type 2 diabetes mellitus. West Indian Med. J. 2006, 55, 274–278. [Google Scholar] [CrossRef]
- Blum, A.; Merei, M.; Karem, A.; Blum, N.; Ben-Arzi, S.; Wirsansky, I.; Khazim, K. Effects of tomatoes on the lipid profile. Clin. Investig. Med. 2006, 29, 298–300. [Google Scholar]
- Cuevas-Ramos, D.; Almeda-Valdés, P.; Chávez-Manzanera, E.; Meza-Arana, C.E.; Brito-Córdova, G.; Mehta, R.R.; Pérez-Méndez, O.O.; Gómez-Pérez, F.F.J. Effect of tomato consumption on high-density lipoprotein cholesterol level: A randomized, single-blinded, controlled clinical trial. Diabetes Metab. Syndr. Obes. Targets Ther. 2013, 6, 263–273. [Google Scholar] [CrossRef]
- Thies, F.; Masson, L.F.; Rudd, A.; Vaughan, N.; Tsang, C.; Brittenden, J.; Simpson, W.G.; Duthie, S.; Horgan, G.W.; Duthie, G. Effect of a tomato-rich diet on markers of cardiovascular disease risk in moderately overweight, disease-free, middle-aged adults: A randomized controlled trial. Am. J. Clin. Nutr. 2012, 95, 1013–1022. [Google Scholar] [CrossRef]
- Gambino, G.; Giglia, G.; Schiera, G.; Di Majo, D.; Epifanio, M.S.; La Grutta, S.; Baido, R.L.; Ferraro, G.; Sardo, P. Haptic Perception in Extreme Obesity: qEEG Study Focused on Predictive Coding and Body Schema. Brain Sci. 2020, 10, 908. [Google Scholar] [CrossRef]
- Di Majo, D.; Cacciabaudo, F.; Accardi, G.; Gambino, G.; Giglia, G.; Ferraro, G.; Candore, G.; Sardo, P. Ketogenic and Modified Mediterranean Diet as a Tool to Counteract Neuroinflammation in Multiple Sclerosis: Nutritional Suggestions. Nutrients 2022, 14, 2384. [Google Scholar] [CrossRef]
- Giammanco, M.; Lantieri, L.; Leto, G.; Plescia, F.; Di Majo, D. Nutrition, obesity and hormones. J. Biol. Res. 2018, 91. [Google Scholar] [CrossRef]
- Spahis, S.; Borys, J.-M.; Levy, E. Metabolic Syndrome as a Multifaceted Risk Factor for Oxidative Stress. Antioxid. Redox Signal. 2017, 26, 445–461. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.; Feldman, E.L. Insulin resistance as a key link for the increased risk of cognitive impairment in the metabolic syndrome. Exp. Mol. Med. 2015, 47, e149. [Google Scholar] [CrossRef]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef]
- Alberti, K.G.M.M.; Zimmet, P.; Shaw, J. Metabolic syndrome—A new world-wide definition. A Consensus Statement from the International Diabetes Federation. Diabet. Med. 2006, 23, 469–480. [Google Scholar] [CrossRef] [PubMed]
- DeVallance, E.; Li, Y.; Jurczak, M.J.; Cifuentes-Pagano, E.; Pagano, P.J. The Role of NADPH Oxidases in the Etiology of Obesity and Metabolic Syndrome: Contribution of Individual Isoforms and Cell Biology. Antioxid. Redox Signal. 2019, 31, 687–709. [Google Scholar] [CrossRef]
- Vatashchuk, M.V.; Bayliak, M.M.; Hurza, V.V.; Storey, K.B.; Lushchak, V.I. Metabolic Syndrome: Lessons from Rodent and Drosophila Models. BioMed Res. Int. 2022, 2022, 5850507. [Google Scholar] [CrossRef]
- Rodríguez-Correa, E.; González-Pérez, I.; Clavel-Pérez, P.I.; Contreras-Vargas, Y.; Carvajal, K. Biochemical and nutritional overview of diet-induced metabolic syndrome models in rats: What is the best choice? Nutr. Diabetes 2020, 10, 24. [Google Scholar] [CrossRef]
- Di Majo, D.; Sardo, P.; Giglia, G.; Di Liberto, V.; Zummo, F.P.; Zizzo, M.G.; Caldara, G.F.; Rappa, F.; Intili, G.; van Dijk, R.M.; et al. Correlation of Metabolic Syndrome with Redox Homeostasis Biomarkers: Evidence from High-Fat Diet Model in Wistar Rats. Antioxidants 2022, 12, 89. [Google Scholar] [CrossRef]
- Li, P.; Lu, B.; Gong, J.; Li, L.; Chen, G.; Zhang, J.; Chen, Y.; Tian, X.; Han, B.; Guo, Y.; et al. Chickpea Extract Ameliorates Metabolic Syndrome Symptoms via Restoring Intestinal Ecology and Metabolic Profile in Type 2 Diabetic Rats. Mol. Nutr. Food Res. 2021, 65, e2100007. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Cai, H.; Jiang, Z.; Li, Y.; Zhong, H.; Zhang, H.; Feng, F. Glycerol-Monolaurate-Mediated Attenuation of Metabolic Syndrome is Associated with the Modulation of Gut Microbiota in High-Fat-Diet-Fed Mice. Mol. Nutr. Food Res. 2019, 63, e1801417. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Y.; Ibn Sina, A.A.I.; Khandker, S.S.; Neesa, L.; Tanvir, E.M.; Kabir, A.; Khalil, M.I.; Gan, S.H. Nutritional Composition and Bioactive Compounds in Tomatoes and Their Impact on Human Health and Disease: A Review. Foods 2020, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, A.; Zhao, L.; Bhagavathula, A.S.; Amirthalingam, P.; Rahmani, J.; Salehisahlabadi, A.; Abdulazeem, H.M.; Adebayo, O.; Yin, X. Effect of tomato consumption on fasting blood glucose and lipid profiles: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2020, 34, 1956–1965. [Google Scholar] [CrossRef]
- Moncada, A.; Vetrano, F.; Esposito, A.; Miceli, A. Effects of NAA and Ecklonia maxima Extracts on Lettuce and Tomato Transplant Production. Agronomy 2022, 12, 329. [Google Scholar] [CrossRef]
- McGuire, R.G. Reporting of Objective Color Measurements. Hortscience 1992, 27, 1254–1255. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration. Methods Enzymol. 1999, 299, 15–27. [Google Scholar] [CrossRef]
- Phachonpai, W.; Muchimapura, S.; Tong-Un, T.; Wattanathorn, J.; Thukhammee, W.; Thipkaew, C.; Sripanidkulchai, B.; Wannanon, P. Acute Toxicity Study of Tomato Pomace Extract in Rodent. OnLine J. Biol. Sci. 2013, 13, 28–34. [Google Scholar] [CrossRef]
- Aborehab, N.M.; El Bishbishy, M.H.; Waly, N.E. Resistin mediates tomato and broccoli extract effects on glucose homeostasis in high fat diet-induced obesity in rats. BMC Complement. Altern. Med. 2016, 16, 1–10. [Google Scholar] [CrossRef]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [PubMed]
- Bowe, J.E.; Franklin, Z.J.; Hauge-Evans, A.C.; King, A.J.; Persaud, S.J.; Jones, P.M. Metabolic Phenotyping Guidelines: Assessing Glucose Homeostasis in Rodent Models. J. Endocrinol. 2014, 222, G13–G25. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hashimoto, J.; Suzuki, T.; Satoh, A. The effects of exercise load during development on oxidative stress levels and antioxidant potential in adulthood. Free. Radic. Res. 2017, 51, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Macri, A.; Scanarotti, C.; Bassi, A.M.; Giuffrida, S.; Sangalli, G.; Traverso, C.E.; Iester, M. Evaluation of oxidative stress levels in the conjunctival epithelium of patients with or without dry eye, and dry eye patients treated with preservative-free hyaluronic acid 0.15 % and vitamin B12 eye drops. Graefe’s Arch. Clin. Exp. Ophthalmol. 2015, 253, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Mancini, S.; Mariani, F.; Sena, P.; Benincasa, M.; Roncucci, L. Myeloperoxidase expression in human colonic mucosa is related to systemic oxidative balance in healthy subjects. Redox Rep. 2017, 22, 399–407. [Google Scholar] [CrossRef]
- Jansen, E.; Ruskovska, T. Serum Biomarkers of (Anti)Oxidant Status for Epidemiological Studies. Int. J. Mol. Sci. 2015, 16, 27378–27390. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Terzo, S.; Attanzio, A.; Calvi, P.; Mulè, F.; Tesoriere, L.; Allegra, M.; Amato, A. Indicaxanthin from Opuntia ficus-indica Fruit Ameliorates Glucose Dysmetabolism and Counteracts Insulin Resistance in High-Fat-Diet-Fed Mice. Antioxidants 2021, 11, 80. [Google Scholar] [CrossRef]
- Auberval, N.; Dal, S.; Bietiger, W.; Pinget, M.; Jeandidier, N.; Maillard-Pedracini, E.; Schini-Kerth, V.; Sigrist, S. Metabolic and oxidative stress markers in Wistar rats after 2 months on a high-fat diet. Diabetol. Metab. Syndr. 2014, 6, 130. [Google Scholar] [CrossRef]
- Di Majo, D.; La Neve, L.; La Guardia, M.; Casuccio, A.; Giammanco, M. The influence of two different pH levels on the antioxidant properties of flavonols, flavan-3-ols, phenolic acids and aldehyde compounds analysed in synthetic wine and in a phosphate buffer. J. Food Compos. Anal. 2011, 24, 265–269. [Google Scholar] [CrossRef]
- Alam, M.A.; Subhan, N.; Rahman, M.M.; Uddin, S.J.; Reza, H.M.; Sarker, S.D. Effect of Citrus Flavonoids, Naringin and Naringenin, on Metabolic Syndrome and Their Mechanisms of Action. Adv. Nutr. 2014, 5, 404–417. [Google Scholar] [CrossRef]
- Santana-Gálvez, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. Chlorogenic Acid: Recent Advances on Its Dual Role as a Food Additive and a Nutraceutical against Metabolic Syndrome. Molecules 2017, 22, 358. [Google Scholar] [CrossRef]
- Giammanco, M.; Aiello, S.; Casuccio, A.; La Guardia, M.; Cicero, L.; Puleio, R.; Vazzana, I.; Tomasello, G.; Cassata, G.; Leto, G.; et al. Effects of 3,5-Diiodo-L-Thyronine on the Liver of High Fat Diet Fed Rats. J. Biol. Res. 2016, 89. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Sun, Y.; Zhang, G.; Bai, J.; Guo, J.; Su, X.; Du, H.; Cao, X.; Yang, J.; et al. Naringenin improves insulin sensitivity in gestational diabetes mellitus mice through AMPK. Nutr. Diabetes 2019, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Zygmunt, K.; Faubert, B.; MacNeil, J.; Tsiani, E. Naringenin, a citrus flavonoid, increases muscle cell glucose uptake via AMPK. Biochem. Biophys. Res. Commun. 2010, 398, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Gao, M.; Liu, D. Chlorogenic Acid Improves High Fat Diet-Induced Hepatic Steatosis and Insulin Resistance in Mice. Pharm. Res. 2015, 32, 1200–1209. [Google Scholar] [CrossRef]
- Jin, S.; Chang, C.; Zhang, L.; Liu, Y.; Huang, X.; Chen, Z. Chlorogenic Acid Improves Late Diabetes through Adiponectin Receptor Signaling Pathways in db/db Mice. PLoS ONE 2015, 10, e0120842. [Google Scholar] [CrossRef]
- Wan, C.-W.; Wong, C.N.-Y.; Pin, W.-K.; Wong, M.H.-Y.; Kwok, C.-Y.; Chan, R.Y.-K.; Yu, P.H.-F.; Chan, S.-W. Chlorogenic Acid Exhibits Cholesterol Lowering and Fatty Liver Attenuating Properties by Up-regulating the Gene Expression of PPAR-α in Hypercholesterolemic Rats Induced with a High-Cholesterol Diet. Phytother. Res. 2013, 27, 545–551. [Google Scholar] [CrossRef]
- Lee, M.-K.; Moon, S.-S.; Lee, S.-E.; Bok, S.-H.; Jeong, T.-S.; Park, Y.B.; Choi, M.-S. Naringenin 7-O-cetyl ether as inhibitor of HMG-CoA reductase and modulator of plasma and hepatic lipids in high cholesterol-fed rats. Bioorg. Med. Chem. 2003, 11, 393–398. [Google Scholar] [CrossRef]
- Andrade-Pavón, D.; Gómez-García, O.; Villa-Tanaca, L. Molecular Recognition of Citroflavonoids Naringin and Naringenin at the Active Site of the HMG-CoA Reductase and DNA Topoisomerase Type II Enzymes of Candida spp. and Ustilago maydis. Indian J. Microbiol. 2022, 62, 79–87. [Google Scholar] [CrossRef]
- Cho, A.-S.; Jeon, S.-M.; Kim, M.-J.; Yeo, J.; Seo, K.-I.; Choi, M.-S.; Lee, M.-K. Chlorogenic acid exhibits anti-obesity property and improves lipid metabolism in high-fat diet-induced-obese mice. Food Chem. Toxicol. 2010, 48, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Huang, K.; Liang, X.-C.; Zhong, Y.-L.; He, W.-Y.; Wang, Z. 5-Caffeoylquinic acid decreases diet-induced obesity in rats by modulating PPARα and LXRα transcription. J. Sci. Food Agric. 2015, 95, 1903–1910. [Google Scholar] [CrossRef] [PubMed]
- Paolisso, G.; Gambardella, A.; Tagliamonte, M.R.; Saccomanno, F.; Salvatore, T.; Gualdiero, P.; D’Onofrio, M.V.; Howard, B.V. Does free fatty acid infusion impair insulin action also through an increase in oxidative stress? J. Clin. Endocrinol. Metab. 1996, 81, 4244–4248. [Google Scholar] [CrossRef]
- McKeegan, K.; Mason, S.A.; Trewin, A.J.; Keske, M.A.; Wadley, G.D.; Della Gatta, P.A.; Nikolaidis, M.G.; Parker, L. Reactive oxygen species in exercise and insulin resistance: Working towards personalized antioxidant treatment. Redox Biol. 2021, 44, 102005. [Google Scholar] [CrossRef]
- Allegra, M.; Gentile, C.; Tesoriere, L.; Livrea, M.A. Protective effect of melatonin against cytotoxic actions of malondialdehyde: An in vitro study on human erythrocytes. J. Pineal Res. 2002, 32, 187–193. [Google Scholar] [CrossRef]
- Li, N.; Wu, X.; Zhuang, W.; Xia, L.; Chen, Y.; Wu, C.; Rao, Z.; Du, L.; Zhao, R.; Yi, M.; et al. Tomato and lycopene and multiple health outcomes: Umbrella review. Food Chem. 2021, 343, 128396. [Google Scholar] [CrossRef]
- Kakimoto, P.A.; Kowaltowski, A.J. Effects of high fat diets on rodent liver bioenergetics and oxidative imbalance. Redox Biol. 2016, 8, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wang, T.; Liu, P.; Yang, F.; Wang, X.; Zheng, W.; Sun, W. Hesperetin ameliorates hepatic oxidative stress and inflammation via the PI3K/AKT-Nrf2-ARE pathway in oleic acid-induced HepG2 cells and a rat model of high-fat diet-induced NAFLD. Food Funct. 2021, 12, 3898–3918. [Google Scholar] [CrossRef]
- Elvira-Torales, L.I.; Navarro-González, I.; González-Barrio, R.; Martín-Pozuelo, G.; Doménech, G.; Seva, J.; García-Alonso, J.; Periago-Castón, M.J. Tomato Juice Supplementation Influences the Gene Expression Related to Steatosis in Rats. Nutrients 2018, 10, 1215. [Google Scholar] [CrossRef]
- Alam, P.; Raka, M.A.; Khan, S.; Sarker, J.; Ahmed, N.; Nath, P.D.; Hasan, N.; Mohib, M.M.; Tisha, A.; Taher Sagor, M.A. A clinical review of the effectiveness of tomato (Solanum lycopersicum) against cardiovascular dysfunction and related metabolic syndrome. J. Herb. Med. 2019, 16, 100235. [Google Scholar] [CrossRef]
- Ferron, A.J.T.; Aldini, G.; Francisqueti-Ferron, F.V.; Silva, C.C.V.d.A.; Bazan, S.G.Z.; Garcia, J.L.; de Campos, D.H.S.; Ghiraldeli, L.; Kitawara, K.A.H.; Altomare, A.; et al. Protective Effect of Tomato-Oleoresin Supplementation on Oxidative Injury Recoveries Cardiac Function by Improving β-Adrenergic Response in a Diet-Obesity Induced Model. Antioxidants 2019, 8, 368. [Google Scholar] [CrossRef] [PubMed]
- Araújo, M.C.; Soczek, S.H.S.; Pontes, J.P.; Marques, L.A.C.; Santos, G.S.; Simão, G.; Bueno, L.R.; Maria-Ferreira, D.; Muscará, M.N.; Fernandes, E.S. An Overview of the TRP-Oxidative Stress Axis in Metabolic Syndrome: Insights for Novel Therapeutic Approaches. Cells 2022, 11, 1292. [Google Scholar] [CrossRef] [PubMed]
- Holzer, P.; Izzo, A.A. The pharmacology of TRP channels. Br. J. Pharmacol. 2014, 171, 2469–2473. [Google Scholar] [CrossRef] [PubMed]
- Gambino, G.; Gallo, D.; Covelo, A.; Ferraro, G.; Sardo, P.; Giglia, G. TRPV1 channels in nitric oxide-mediated signalling: Insight on excitatory transmission in rat CA1 pyramidal neurons. Free. Radic. Biol. Med. 2022, 191, 128–136. [Google Scholar] [CrossRef]
- Gambino, G.; Rizzo, V.; Giglia, G.; Ferraro, G.; Sardo, P. Cannabinoids, TRPV and Nitric Oxide: The Three Ring Circus of Neuronal Excitability. Brain Struct. Funct. 2020, 225, 1–15. [Google Scholar] [CrossRef]
- De Vadder, F.; Kovatcheva-Datchary, P.; Goncalves, D.; Vinera, J.; Zitoun, C.; Duchampt, A.; Bäckhed, F.; Mithieux, G. Microbiota-Generated Metabolites Promote Metabolic Benefits via Gut-Brain Neural Circuits. Cell 2014, 156, 84–96. [Google Scholar] [CrossRef]
- Wang, P.-X.; Deng, X.-R.; Zhang, C.-H.; Yuan, H.-J. Gut microbiota and metabolic syndrome. Chin. Med. J. 2020, 133, 808–816. [Google Scholar] [CrossRef]
- Dabke, K.; Hendrick, G.; Devkota, S. The gut microbiome and metabolic syndrome. J. Clin. Investig. 2019, 129, 4050–4057. [Google Scholar] [CrossRef]
- Tralongo, P.; Tomasello, G.; Sinagra, E.; Damiani, P.; Leone, A.; Palumbo, V.; Giammanco, M.; Di Majo, D.; Abruzzo, A.; Bruno, A.; et al. The Role of Butyric Acid as a Oprotective Agent against Inflammatory Bowel Disease. Euromediterranean Biomed. J. 2014, 9, 24–35. [Google Scholar] [CrossRef]
- Tomasello, G.; Zeenny, M.; Giammanco, M.; Di Majo, D.; Traina, G.; Sinagra, E.; Damiani, P.; Zein, R.; Jurjus, A.; Leone, A. Intestinal Microbiota Mutualism and Gastrointestinal Diseases. Euromediterr. Biomed. J. 2015, 10, 65–75. [Google Scholar] [CrossRef]
- Spooner, H.C.; Derrick, S.A.; Maj, M.; Manjarín, R.; Hernandez, G.V.; Tailor, D.S.; Bastani, P.S.; Fanter, R.K.; Fiorotto, M.L.; Burrin, D.G.; et al. High-Fructose, High-Fat Diet Alters Muscle Composition and Fuel Utilization in a Juvenile Iberian Pig Model of Non-Alcoholic Fatty Liver Disease. Nutrients 2021, 13, 4195. [Google Scholar] [CrossRef] [PubMed]
- Gambino, G.; Brighina, F.; Allegra, M.; Marrale, M.; Collura, G.; Gagliardo, C.; Attanzio, A.; Tesoriere, L.; Di Majo, D.; Ferraro, G.; et al. Modulation of Human Motor Cortical Excitability and Plasticity by Opuntia Ficus Indica Fruit Consumption: Evidence from a Preliminary Study through Non-Invasive Brain Stimulation. Nutrients 2022, 14, 4915. [Google Scholar] [CrossRef] [PubMed]
- Palermo, A.; Giglia, G.; Vigneri, S.; Cosentino, G.; Fierro, B.; Brighina, F. Does habituation depend on cortical inhibition? Results of a rTMS study in healthy subjects. Exp. Brain Res. 2011, 212, 101–107. [Google Scholar] [CrossRef] [PubMed]


| Pellet HFD (PF4215) | NPDSND (PF1609) | |
|---|---|---|
| Energy (Kcal/Kg) | 5500–6000 | 3947 |
| Fat Total (g/100 g) | 60 | 3.50 |
| SFA (g/60 g) | 30 | 0.7 |
| MUFA (g/60 g) | 23 | 0.8 |
| PUFA (g/60 g) | 7 | 2 |
| Crude protein (g/100 g) | 23 | 22 |
| Carbohydrates (Starch g/100 g) | - | 35.18 |
| Sugar (g/100 g) | - | 5.66 |
| Fiber (g/100 g) | 5 | 4.5 |
| Ash (g/100 g) | 5.50 | 7.5 |
| Vitamin A (IU) | 8400 | 19.533 |
| Vitamin D3 (IU) | 2100 | 1260 |
| Analytical Parameters | Red Tomato | Golden Tomato |
|---|---|---|
| Moisture (% g) | 94.2 | 91.5 |
| Ash (%g) | 0.8 | 0.9 |
| Brix degree | 5.8 | 5.3 |
| Acidity (mg %) | 0.5 | 0.6 |
| pH | 4.55 | 4.36 |
| Antioxidant Components | Golden Tomato Concentration mg/100 gDW | Red Tomato Concentration mg/100 gDW |
|---|---|---|
| Vitamin C | 170.4 | 311.2 |
| 𝛽-carotene | 39.08 | 126.22 |
| Lycopene | 333.0 | 1971.0 |
| Phytoene | 6.5 | 16.8 |
| Lutein | 7.78 | 6.37 |
| Naringenin | 38.31 | 16.5 |
| Gallic acid | 2.0 | <0.2 |
| Chlorogenic acid | 9.8 | 1.9 |
| Rutin | 4.68 | 7.1 |
| Experimental Groups | Triglycerides (mg/dL) | TC (mg/dL) | LDL Cholesterol (mg/dL) | HDL Cholesterol (mg/dL) |
|---|---|---|---|---|
| NPD | 153.59 ± 61.49 | 60.90 ± 12.57 | 5.91 ± 3.15 | 34.35 ± 4.33 |
| HFD | 170.05 ± 57.44 | 86.57 ± 11.62 # | 14.08 ± 3.13 # | 30.02 ± 6.19 |
| HFD/GT | 183.58 ± 18.04 | 124.80 ± 23.12 *,# | 9.98 ± 0.99 *,# | 56.80 ± 9.01 *,# |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gambino, G.; Giglia, G.; Allegra, M.; Di Liberto, V.; Zummo, F.P.; Rappa, F.; Restivo, I.; Vetrano, F.; Saiano, F.; Palazzolo, E.; et al. “Golden” Tomato Consumption Ameliorates Metabolic Syndrome: A Focus on the Redox Balance in the High-Fat-Diet-Fed Rat. Antioxidants 2023, 12, 1121. https://doi.org/10.3390/antiox12051121
Gambino G, Giglia G, Allegra M, Di Liberto V, Zummo FP, Rappa F, Restivo I, Vetrano F, Saiano F, Palazzolo E, et al. “Golden” Tomato Consumption Ameliorates Metabolic Syndrome: A Focus on the Redox Balance in the High-Fat-Diet-Fed Rat. Antioxidants. 2023; 12(5):1121. https://doi.org/10.3390/antiox12051121
Chicago/Turabian StyleGambino, Giuditta, Giuseppe Giglia, Mario Allegra, Valentina Di Liberto, Francesco Paolo Zummo, Francesca Rappa, Ignazio Restivo, Filippo Vetrano, Filippo Saiano, Eristanna Palazzolo, and et al. 2023. "“Golden” Tomato Consumption Ameliorates Metabolic Syndrome: A Focus on the Redox Balance in the High-Fat-Diet-Fed Rat" Antioxidants 12, no. 5: 1121. https://doi.org/10.3390/antiox12051121









