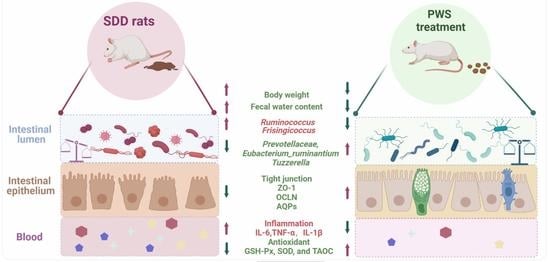
Pingwei San Ameliorates Spleen Deficiency-Induced Diarrhea through Intestinal Barrier Protection and Gut Microbiota Modulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of PWS, Rhubarb, and Shenling Baizhu San (SLG)
2.2. Analysis of the Main Components of PWS
2.3. Animal Models and Treatment
2.4. Record of Body Weights and Fecal Moisture Content
2.5. D-Xylose Absorption and Excretion Assay
2.6. Histopathological Observation and Alcian Blue-Periodic Acid Schiff (AB-PAS) Staining
2.7. Immunohistochemistry (IHC)
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Measurement for T-AOC, GSH-Px, and Superoxide Dismutase (SOD) Activities in the Colon, Serum of Rats
2.10. The Permeability Test of Intestinal Mucosal Barrier
2.11. Quantitative Reverse-Transcription (qRT)-PCR for the Gene Expression
2.12. Determination of Fecal Flora Diversity
2.13. Statistical Analysis
3. Results
3.1. Chemical Profile of PWS
3.2. PWS Ameliorates SDD Symptoms in Rats
3.3. PWS Suppresses Inflammation and Inflammation-Induced Oxidation in SDD Rats
3.4. PWS Restored Flora Diversity to Curb SDD
3.5. PWS Increases the Expression of Aquaporin3 (AQP3), Aquaporin4 (AQP4), and Aquaporin8 (AQP8)
3.6. PWS Relieved SDD by Maintaining Goblet Cell Function and Restoring the Intestinal Mucus Barrier
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.M.; Li, X.B.; Peng, Y. Impact of Qi-invigorating traditional Chinese medicines on intestinal flora: A basis for rational choice of prebiotics. Chin. J. Nat. Med. 2017, 15, 241–254. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Feng, W.; Zhang, S.; Chen, L.; Sheng, Y.; Tang, F.; He, J.; Xu, X.; Ao, H.; Peng, C. Ameliorative effect of Atractylodes macrocephala essential oil combined with Panax ginseng total saponins on 5-fluorouracil induced diarrhea is associated with gut microbial modulation. J. Ethnopharmacol. 2019, 238, 111887. [Google Scholar] [CrossRef]
- Shi, K.; Qu, L.; Lin, X.; Xie, Y.; Tu, J.; Liu, X.; Zhou, Z.; Cao, G.; Li, S.; Liu, Y. Deep-Fried Atractylodis Rhizoma Protects against Spleen Deficiency-Induced Diarrhea through Regulating Intestinal Inflammatory Response and Gut Microbiota. Int. J. Mol. Sci. 2019, 21, 124. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, K.; Zhu, S.Y.; Deng, X.L.; Chen, X.Y.; Fu, N.L.; Chen, J. Shenling Baizhu Powder Ameliorates Pi (Spleen)-Deficiency-Induced Functional Diarrhea in Rats. Chin. J. Integr. Med. 2021, 27, 206–211. [Google Scholar] [CrossRef] [PubMed]
- Xue, D.H.; Liu, Y.Q.; Cai, Q.; Liang, K.; Zheng, B.Y.; Li, F.X.; Pang, X. Comparison of Bran-Processed and Crude Atractylodes Lancea Effects on Spleen Deficiency Syndrome in Rats. Pharmacogn. Mag. 2018, 14, 214–219. [Google Scholar]
- Camilleri, M.; Carlson, P.; Chedid, V.; Vijayvargiya, P.; Burton, D.; Busciglio, I. Aquaporin Expression in Colonic Mucosal Biopsies From Irritable Bowel Syndrome With Diarrhea. Clin. Transl. Gastroenterol. 2019, 10, e00019. [Google Scholar] [CrossRef]
- Wu, Y.; Tang, L.; Wang, B.; Sun, Q.; Zhao, P.; Li, W. The role of autophagy in maintaining intestinal mucosal barrier. J. Cell Physiol. 2019, 234, 19406–19419. [Google Scholar] [CrossRef]
- Andoh, A.; Nishida, A. Alteration of the Gut Microbiome in Inflammatory Bowel Disease. Digestion 2023, 104, 16–23. [Google Scholar] [CrossRef]
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens 2019, 8, 126. [Google Scholar] [CrossRef]
- Awad, W.A.; Hess, C.; Hess, M. Enteric Pathogens and Their Toxin-Induced Disruption of the Intestinal Barrier through Alteration of Tight Junctions in Chickens. Toxins 2017, 9, 60. [Google Scholar] [CrossRef]
- Luo, Z.; Liu, C.; Hu, Y.; Xia, T.; Zhang, B.; Chen, F.; Tan, X.; Zheng, Z. Gegen Qinlian decoction restores the intestinal barrier in bacterial diarrhea piglets by promoting Lactobacillus growth and inhibiting the TLR2/MyD88/NF-kappaB pathway. Biomed. Pharmacother. 2022, 155, 113719. [Google Scholar] [CrossRef] [PubMed]
- Salvo Romero, E.; Alonso Cotoner, C.; Pardo Camacho, C.; Casado Bedmar, M.; Vicario, M. The intestinal barrier function and its involvement in digestive disease. Rev. Esp. Enferm. Dig. 2015, 107, 686–696. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Piao, X.; Niu, W.; Zhang, Q.; Ma, C.; Wu, T.; Gu, Q.; Cui, T.; Li, S. Kuijieyuan Decoction Improved Intestinal Barrier Injury of Ulcerative Colitis by Affecting TLR4-Dependent PI3K/AKT/NF-kappaB Oxidative and Inflammatory Signaling and Gut Microbiota. Front. Pharmacol. 2020, 11, 1036. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Guo, J.; Chang, X.; Gui, S. Painong-San extract alleviates dextran sulfate sodium-induced colitis in mice by modulating gut microbiota, restoring intestinal barrier function and attenuating TLR4/NF-kappaB signaling cascades. J. Pharm. Biomed. Anal. 2022, 209, 114529. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, S.; Lin, X.; Huang, Y.; Wei, X.; Zhou, J.; Li, R.; Deng, B.; Fu, C. Metabolomics of Spleen-Yang deficiency syndrome and the therapeutic effect of Fuzi Lizhong pill on regulating endogenous metabolism. J. Ethnopharmacol. 2021, 278, 114281. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; He, X.; Yan, X.; Xiao, G.; Cao, L.; Han, K. Effects of Lingzhu Zhixie Oral Liquid on Morphology, Permeability and mRNA Expression of Tightlunction Proteins Claudin-1,0ccludin and Z0-1 in Colonic Mucosa of Rats with Splenasthenic Diarrhea. Chin. J. Vet. Med. 2021, 47, 580–586. [Google Scholar]
- Ji, H.J.; Kang, N.; Chen, T.; Lv, L.; Ma, X.X.; Wang, F.Y.; Tang, X.D. Shen-ling-bai-zhu-san, a spleen-tonifying Chinese herbal formula, alleviates lactose-induced chronic diarrhea in rats. J. Ethnopharmacol. 2019, 231, 355–362. [Google Scholar] [CrossRef]
- Zhang, Z.; Shen, P.; Xie, W.; Cao, H.; Liu, J.; Cao, Y.; Zhang, N. Pingwei San ameliorates dextran sulfate sodium-induced chronic colitis in mice. J. Ethnopharmacol. 2019, 236, 91–99. [Google Scholar] [CrossRef]
- Riedlinger, J.E.; Tan, P.W.; Lu, W. Ping wei san, a Chinese medicine for gastrointestinal disorders. Ann. Pharmacother. 2001, 35, 228–235. [Google Scholar] [CrossRef]
- Xu, B.; Yan, Y.; Huang, J.; Yin, B.; Pan, Y.; Ma, L. Cortex Phellodendri extract’s anti-diarrhea effect in mice related to its modification of gut microbiota. Biomed. Pharmacother. 2020, 123, 109720. [Google Scholar] [CrossRef]
- Liu, C.; Song, C.; Wang, Y.; Xiao, Y.; Zhou, Z.; Cao, G.; Sun, X.; Liu, Y. Deep-fried Atractylodes lancea rhizome alleviates spleen deficiency diarrhea-induced short-chain fatty acid metabolic disorder in mice by remodeling the intestinal flora. J. Ethnopharmacol. 2023, 303, 115967. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q. Research on Syndrome of Dampness in Middle Jiao Model Rats Affecting Water-Glucose Synergistic Transporter of Duodenumg; Chengdu University of Traditional Chinese Medicine: Chengdu, China, 2017. [Google Scholar]
- Park, C.J.; Armenia, S.J.; Shaughnessy, M.P.; Greig, C.J.; Cowles, R.A. Potentiation of serotonin signaling leads to increased carbohydrate and lipid absorption in the murine small intestine. J. Pediatr. Surg. 2019, 54, 1245–1249. [Google Scholar] [CrossRef]
- Park, C.J.; Armenia, S.J.; Zhang, L.; Cowles, R.A. The 5-HT4 Receptor Agonist Prucalopride Stimulates Mucosal Growth and Enhances Carbohydrate Absorption in the Ileum of the Mouse. J. Gastrointest. Surg. 2019, 23, 1198–1205. [Google Scholar] [CrossRef]
- Wei, Y.Y.; Zhang, Y.N.; Wang, H.; Ga, Y.; Fan, Y.; Wang, Q.; Gu, J.H.; Zhang, X.Y.; Gong, X.H.; Hao, Z.H. Mori fructus aqueous extracts attenuate carbon tetrachloride-induced renal injury via the Nrf2 pathway and intestinal flora. Ecotoxicol. Environ. Saf. 2022, 245, 114118. [Google Scholar] [CrossRef] [PubMed]
- Xiao, C.; Wang, M.; Yang, X.; Sun, J.; Weng, L.; Qiu, Z. Rice Water-Fried Atractylodis Rhizoma Relieves Spleen Deficiency Diarrhea by Regulating the Intestinal Microbiome. Oxid. Med. Cell Longev. 2023, 2023, 1983616. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Lu, Q.; Jiang, W.; Pei, X.; Sun, Y.; Hao, H.; Hao, K. Pharmacokinetics and pharmacodynamics of rhubarb anthraquinones extract in normal and disease rats. Biomed. Pharmacother. 2017, 91, 425–435. [Google Scholar] [CrossRef]
- Esposito, F.; Carli, I.; Del Vecchio, C.; Xu, L.; Corona, A.; Grandi, N.; Piano, D.; Maccioni, E.; Distinto, S.; Parolin, C.; et al. Sennoside A, derived from the traditional chinese medicine plant Rheum L., is a new dual HIV-1 inhibitor effective on HIV-1 replication. Phytomedicine 2016, 23, 1383–1391. [Google Scholar] [CrossRef]
- Ma, Y.L.; Wu, Z.M.; Liu, X.; Lan, J.E.; Zai, W.J.; Jin, X.; Xie, H.; Mu, Q.; Liu, H.R. Antidiarrheal activity of the extracts of Valeriana jatamansi Jones on castor oil-induced diarrhea mouse by regulating multiple signal pathways. J. Ethnopharmacol. 2022, 298, 115560. [Google Scholar] [CrossRef]
- Zhao, M.; Zhao, Q.; Guan, Z.; Liu, Q.; Zhou, H.; Huang, Q.; Huo, B. Effect of Panax ginseng and Fructus Mume on Intestinal Barrier and Gut Microbiota in Rats with Diarrhea. J. Med. Food 2023, 26, 165–175. [Google Scholar] [CrossRef]
- Grondin, J.A.; Kwon, Y.H.; Far, P.M.; Haq, S.; Khan, W.I. Mucins in Intestinal Mucosal Defense and Inflammation: Learning From Clinical and Experimental Studies. Front. Immunol. 2020, 11, 2054. [Google Scholar] [CrossRef]
- Lee, S.H. Intestinal permeability regulation by tight junction: Implication on inflammatory bowel diseases. Intest. Res. 2015, 13, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T. Regulation of the intestinal barrier by nutrients: The role of tight junctions. Anim. Sci. J. 2020, 91, e13357. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.; Li, Y.; Xue, C.; Dong, N.; Bi, C.; Shan, A. Aquaporin: Targets for dietary nutrients to regulate intestinal health. J. Anim. Physiol. Anim. Nutr. 2022, 106, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Pan, H.H.; Zhou, X.X.; Ma, Y.Y.; Pan, W.S.; Zhao, F.; Yu, M.S.; Liu, J.Q. Resveratrol alleviates intestinal mucosal barrier dysfunction in dextran sulfate sodium-induced colitis mice by enhancing autophagy. World J. Gastroenterol. 2020, 26, 4945–4959. [Google Scholar] [CrossRef]
- Wang, L.; Hou, Y.; Yi, D.; Ding, B.; Zhao, D.; Wang, Z.; Zhu, H.; Liu, Y.; Gong, J.; Assaad, H.; et al. Beneficial roles of dietary oleum cinnamomi in alleviating intestinal injury. Front. Biosci. -Landmark 2015, 20, 814–828. [Google Scholar]
- Luo, D.; Huang, Z.; Jia, G.; Zhao, H.; Liu, G.; Chen, X. Naringin mitigates LPS-induced intestinal barrier injury in mice. Food Funct. 2023, 14, 1617–1626. [Google Scholar] [CrossRef] [PubMed]
- Raka, R.N.; Xiao, J.; Wu, H.; Lv, W.; Ding, Z.; Cao, Y.; Li, X.; Sun, J.; Luan, K. Pingyin Rose Essential Oil Restores Intestinal Barrier Integrity in DSS-induced Mice Colitis Model. Food Res. Int. 2023, 164, 112362. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.Y.; Guan, Y.M.; Zhao, H.M.; Yan, D.M.; Tong, W.T.; Wan, P.T.; Zhu, W.F.; Liu, H.N.; Liang, X.L. The protective and healing effects of Si Shen Wan in trinitrobenzene sulphonic acid-induced colitis. J. Ethnopharmacol. 2012, 143, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Li, H.; Li, Y.; Qiao, J. Lactobacillus salivarius, a Potential Probiotic to Improve the Health of LPS-Challenged Piglet Intestine by Alleviating Inflammation as Well as Oxidative Stress in a Dose-Dependent Manner During Weaning Transition. Front. Vet. Sci. 2020, 7, 547425. [Google Scholar] [CrossRef] [PubMed]
- Deng, M.; Chen, H.; Long, J.; Song, J.; Xie, L.; Li, X. Atractylenolides (I, II, and III): A review of their pharmacology and pharmacokinetics. Arch. Pharm. Res. 2021, 44, 633–654. [Google Scholar] [CrossRef]
- Cicalau, G.I.P.; Babes, P.A.; Calniceanu, H.; Popa, A.; Ciavoi, G.; Iova, G.M.; Ganea, M.; Scrobota, I. Anti-Inflammatory and Antioxidant Properties of Carvacrol and Magnolol, in Periodontal Disease and Diabetes Mellitus. Molecules 2021, 26, 6899. [Google Scholar] [CrossRef]
- Rickert, U.; Cossais, F.; Heimke, M.; Arnold, P.; Preusse-Prange, A.; Wilms, H.; Lucius, R. Anti-inflammatory properties of Honokiol in activated primary microglia and astrocytes. J. Neuroimmunol. 2018, 323, 78–86. [Google Scholar] [CrossRef]
- Tejada, S.; Pinya, S.; Martorell, M.; Capo, X.; Tur, J.A.; Pons, A.; Sureda, A. Potential Anti-inflammatory Effects of Hesperidin from the Genus Citrus. Curr. Med. Chem. 2018, 25, 4929–4945. [Google Scholar] [CrossRef]
- Qu, L.; Shi, K.; Xu, J.; Liu, C.; Ke, C.; Zhan, X.; Xu, K.; Liu, Y. Atractylenolide-1 targets SPHK1 and B4GALT2 to regulate intestinal metabolism and flora composition to improve inflammation in mice with colitis. Phytomedicine 2022, 98, 153945. [Google Scholar] [CrossRef]
- Deng, Y.; Han, X.; Tang, S.; Xiao, W.; Tan, Z.; Zhou, C.; Wang, M.; Kang, J. Magnolol and honokiol regulate the calcium-activated potassium channels signaling pathway in Enterotoxigenic Escherichia coli-induced diarrhea mice. Eur. J. Pharmacol. 2015, 755, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Fujisaka, S.; Watanabe, Y.; Tobe, K. The gut microbiome: A core regulator of metabolism. J. Endocrinol. 2023, 256, e220111. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E. Gut microbiota in 2015: Prevotella in the gut: Choose carefully. Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 69–70. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, Y.; Wang, Y.; Chen, X.; Wang, C.; Chen, X.; Yuan, X.; Liu, L.; Yang, J.; Zhou, X. Prevotellaceae produces butyrate to alleviate PD-1/PD-L1 inhibitor-related cardiotoxicity via PPARalpha-CYP4X1 axis in colonic macrophages. J. Exp. Clin. Cancer Res. 2022, 41, 1. [Google Scholar] [CrossRef] [PubMed]
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short chain fatty acids in human gut and metabolic health. Benef. Microbes 2020, 11, 411–455. [Google Scholar] [CrossRef]
- Mukherjee, A.; Lordan, C.; Ross, R.P.; Cotter, P.D. Gut microbes from the phylogenetically diverse genus Eubacterium and their various contributions to gut health. Gut Microbes 2020, 12, 1802866. [Google Scholar] [CrossRef] [PubMed]
- He, R.; Wang, S.; Yang, S.; Liu, R.; Nan, N.; Lu, X.; Gong, M.; Li, J. Shaoyao-Gancao-Tang regulates the T-helper-type 1/T-helper-type 2 ratio in the lung and gut and alters gut microbiota in rats with ovalbumin-induced asthma. J. Ethnopharmacol. 2023, 309, 116300. [Google Scholar] [CrossRef] [PubMed]






| Time | Flow Rate (mL/min) | %A | %B |
|---|---|---|---|
| 0 | 0.3 | 100 | 0 |
| 10 | 0.3 | 70 | 30 |
| 25 | 0.3 | 60 | 40 |
| 30 | 0.3 | 50 | 50 |
| 40 | 0.3 | 30 | 70 |
| 45 | 0.3 | 0 | 100 |
| 60 | 0.3 | 0 | 100 |
| 60.5 | 0.3 | 100 | 0 |
| 70 | 0.3 | 100 | 0 |
| Gene | Primer Sequence (5′–3′) | Gene Accession Number |
|---|---|---|
| Zo-1 | F: CCATCTTTGGACCGATTGCTG R: TAATGCCCGAGCTCCGATG | NM_001106266.1 |
| Ocln | F: GTCTTGGGAGCCTTGACATCTTG R: GCATTGGTCGAACGTGCATC | NM_031329.3 |
| Aqp3 | F: CCCAATGGCACAGCTGGTA R: GTCAACAATGGCCAGCACAC | NM_031703.2 |
| Aqp4 | F: CCATGGAACAACGCCAACTG R: CAGTGTATGGACCACCTCGAAAC | NM_001142366.2 |
| Aqp8 | F: GGCCTCAAGACCATGCTGCTA R: ACCTGCTCCTGCTCCTGGACTA | NM_019158.2 |
| Gapdh | F: GCAAGTTCAACGGCACAG R: GCCAGTAGACTCCACGACAT | NM_017008.4 |
| Constituents | RT (min) | Calc.MW | Formula | Group Area | Reference Ion |
|---|---|---|---|---|---|
| Magnolol | 38.606 | 266.1306 | C18 H18 O2 | 1.3 × 1010 | [M − H] − 1 |
| Hesperidin | 22.517 | 610.18966 | C28 H34 O15 | 1.16 × 1010 | [M − H] − 1 |
| Liquiritin | 21.484 | 418.12654 | C21 H22 O9 | 5.79 × 109 | [M − H] − 1 |
| Honokiol | 36.539 | 266.1306 | C18 H18 O2 | 7.3 × 108 | [M − H] − 1 |
| Glycyrrhizic acid | 31.424 | 822.40331 | C42 H62 O16 | 1.22 × 108 | [M + H] + 1 |
| Atractylenolide III | 33.455 | 248.14131 | C15 H20 O3 | 1.05 × 108 | [M + H] + 1 |
| Atractylenolide II | 37.76 | 232.14646 | C15 H20 O2 | 85,619,188 | [M + H] + 1 |
| Atractylenolide I | 40.772 | 230.1308 | C15 H18 O2 | 47,974,522 | [M + H] + 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, Y.; Zhao, Q.; Wei, Y.; Wang, H.; Ga, Y.; Zhang, Y.; Hao, Z. Pingwei San Ameliorates Spleen Deficiency-Induced Diarrhea through Intestinal Barrier Protection and Gut Microbiota Modulation. Antioxidants 2023, 12, 1122. https://doi.org/10.3390/antiox12051122
Fan Y, Zhao Q, Wei Y, Wang H, Ga Y, Zhang Y, Hao Z. Pingwei San Ameliorates Spleen Deficiency-Induced Diarrhea through Intestinal Barrier Protection and Gut Microbiota Modulation. Antioxidants. 2023; 12(5):1122. https://doi.org/10.3390/antiox12051122
Chicago/Turabian StyleFan, Yimeng, Qingyu Zhao, Yuanyuan Wei, Huiru Wang, Yu Ga, Yannan Zhang, and Zhihui Hao. 2023. "Pingwei San Ameliorates Spleen Deficiency-Induced Diarrhea through Intestinal Barrier Protection and Gut Microbiota Modulation" Antioxidants 12, no. 5: 1122. https://doi.org/10.3390/antiox12051122