Uncovering the Cardioprotective Potential of Diacerein in Doxorubicin Cardiotoxicity: Mitigating Ferritinophagy-Mediated Ferroptosis via Upregulating NRF2/SLC7A11/GPX4 Axis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drugs and Chemicals
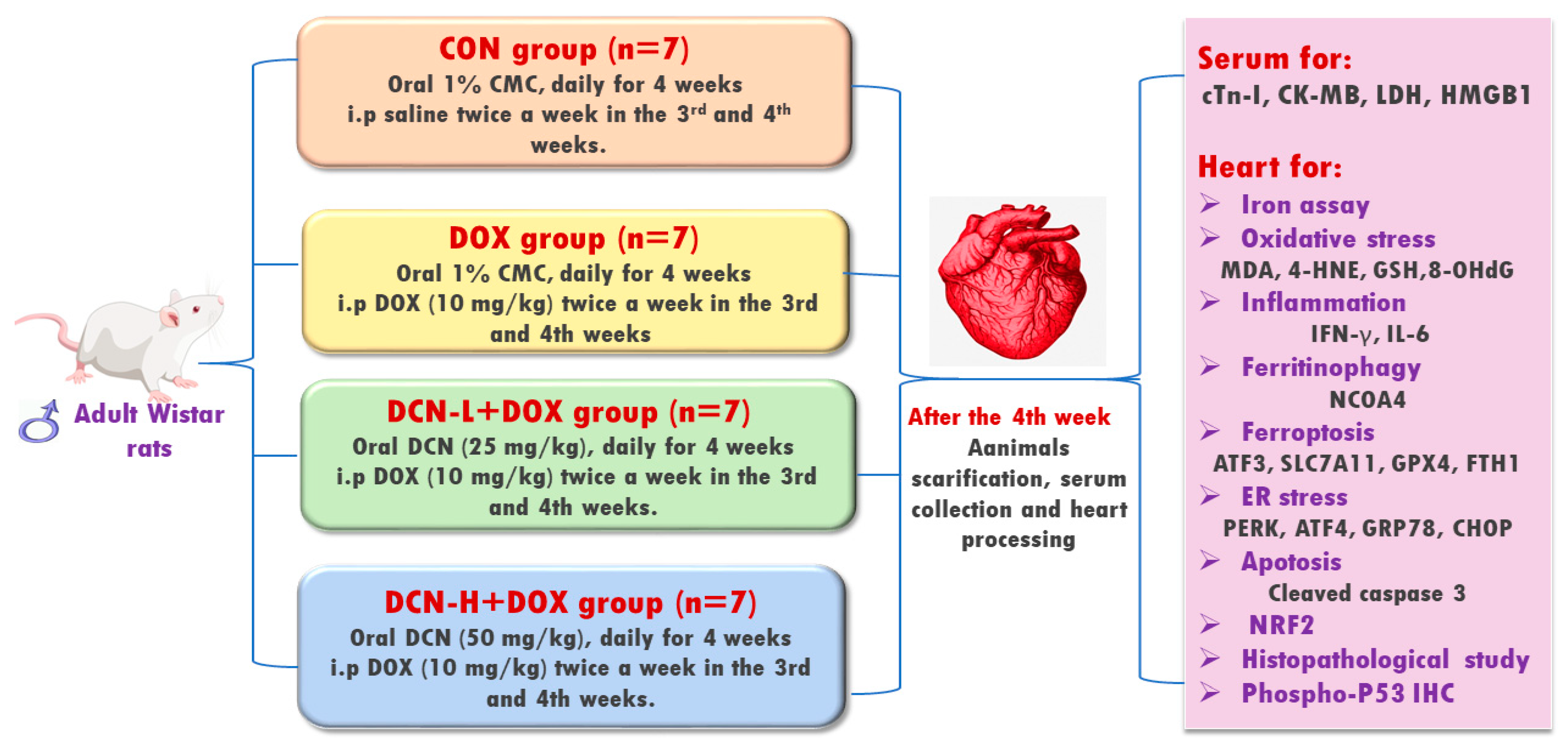
2.2. Experimental Design
2.3. Sampling
2.3.1. Blood Sampling and Serum Preparation
2.3.2. Collection of Heart and Calculating Heart-to-Body Weight (HW/BW) Ratio
2.4. Biochemical Assay
2.4.1. Assay of Cardiac Toxicity Biomarkers
2.4.2. Determination of Cardiac Activating Transcription Factor (ATF) 3 and Fe2+
2.4.3. Detection of Cardiac Redox Status Biomarkers
2.4.4. Evaluation of Inflammation-Related Biomarkers
2.4.5. Assay of Cardiac NRF2 DNA-Binding Activity, ER Stress Sensors, and Cleaved Caspase-3
2.5. Molecular Assay of NCOA4, FTH1, SLC7A11, and Protein Kinase-Like ER Kinase (PERK) by Quantitative RT-PCR Analysis
2.6. Histopathological Examination
2.7. Immunohistochemical Analysis
2.8. Morphometric Analysis
2.9. Statistical Analysis
3. Results
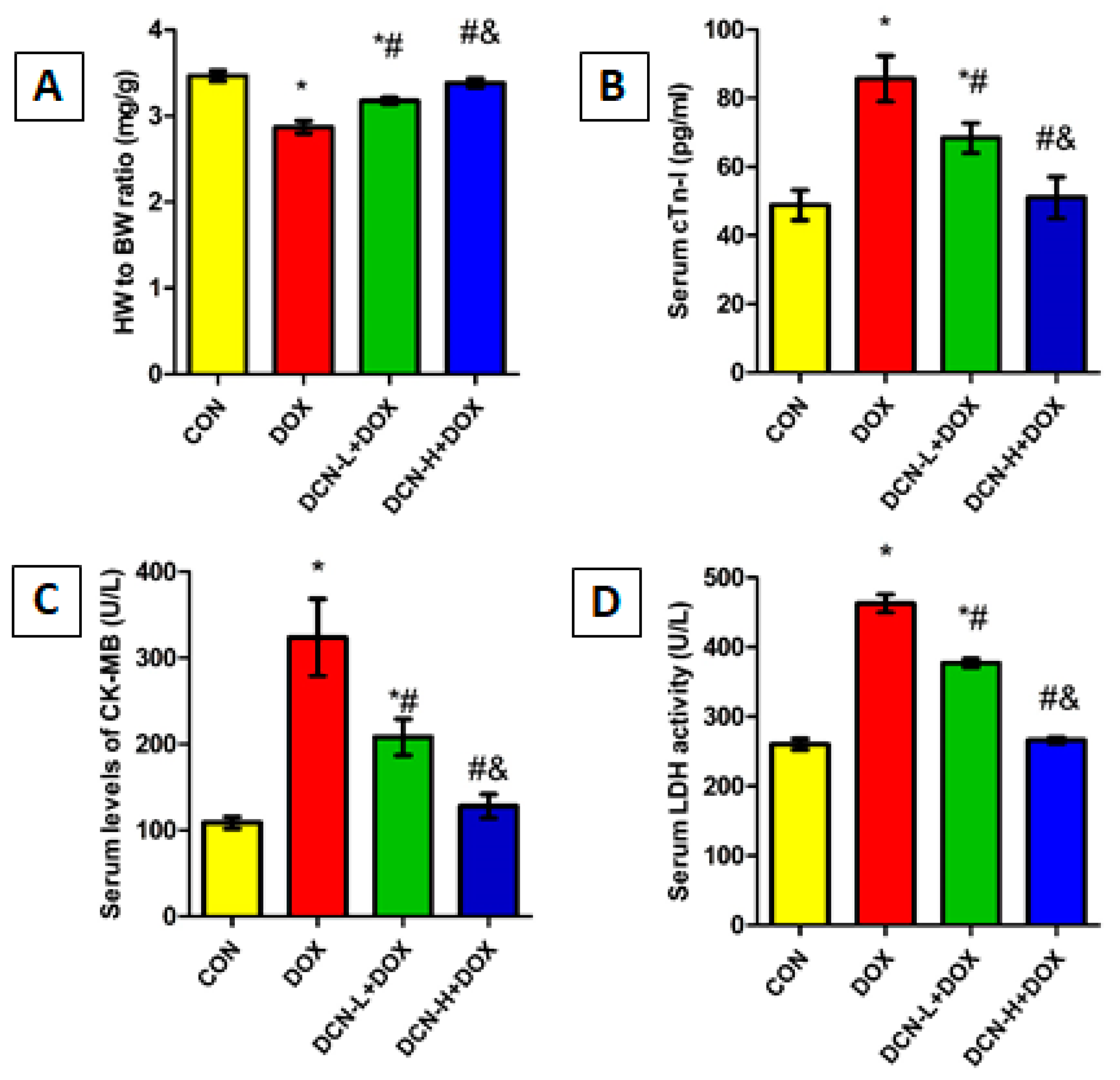
3.1. DCN-Attenuated DOX-Induced Cardiomyocyte Injury
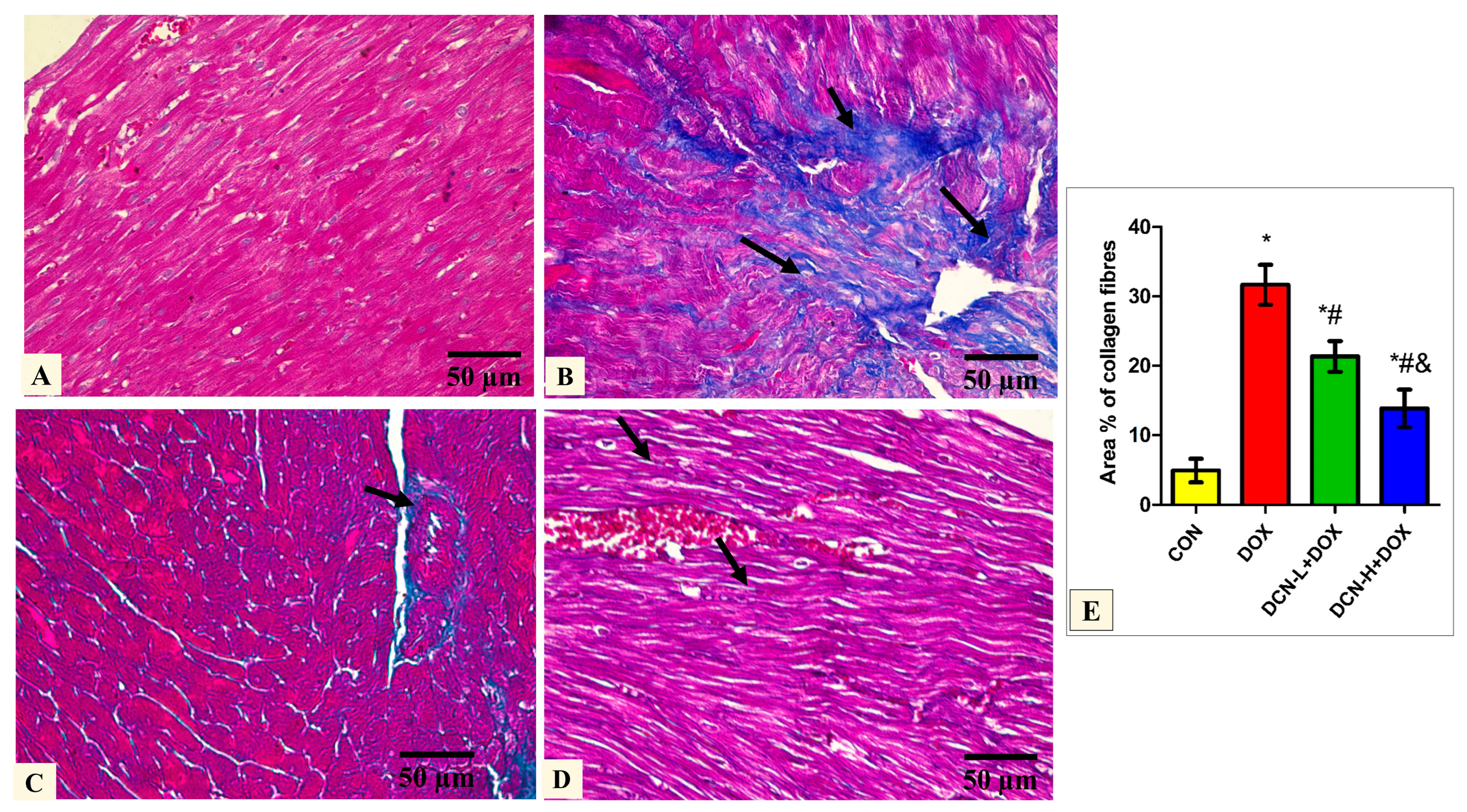
3.2. DCN-Alleviated DOX-Provoked Cytomorphological Abnormalities in Heart Tissue
3.3. Modulatory Effects of DCN on Cardiac Collagen Fiber Content
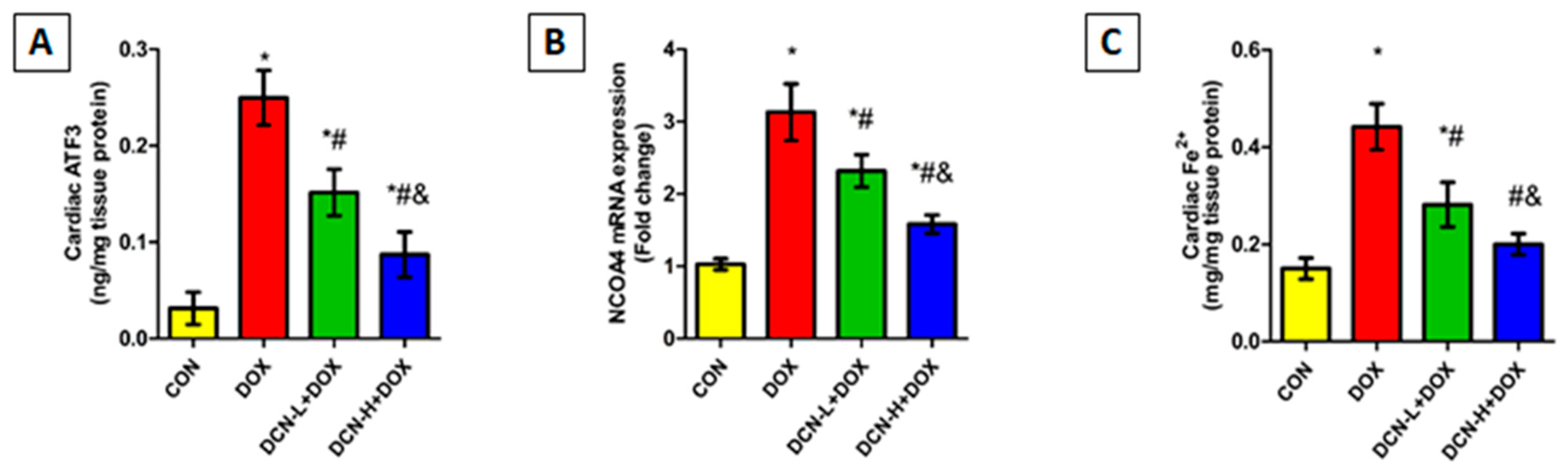
3.4. DCN Effect on Cardiac ATF3, NCOA4-Mediated Ferritinophagy, and Ferrous Iron Content
3.5. DCN Abolished the DOX-Provoked Cardiac OS and Inflammation
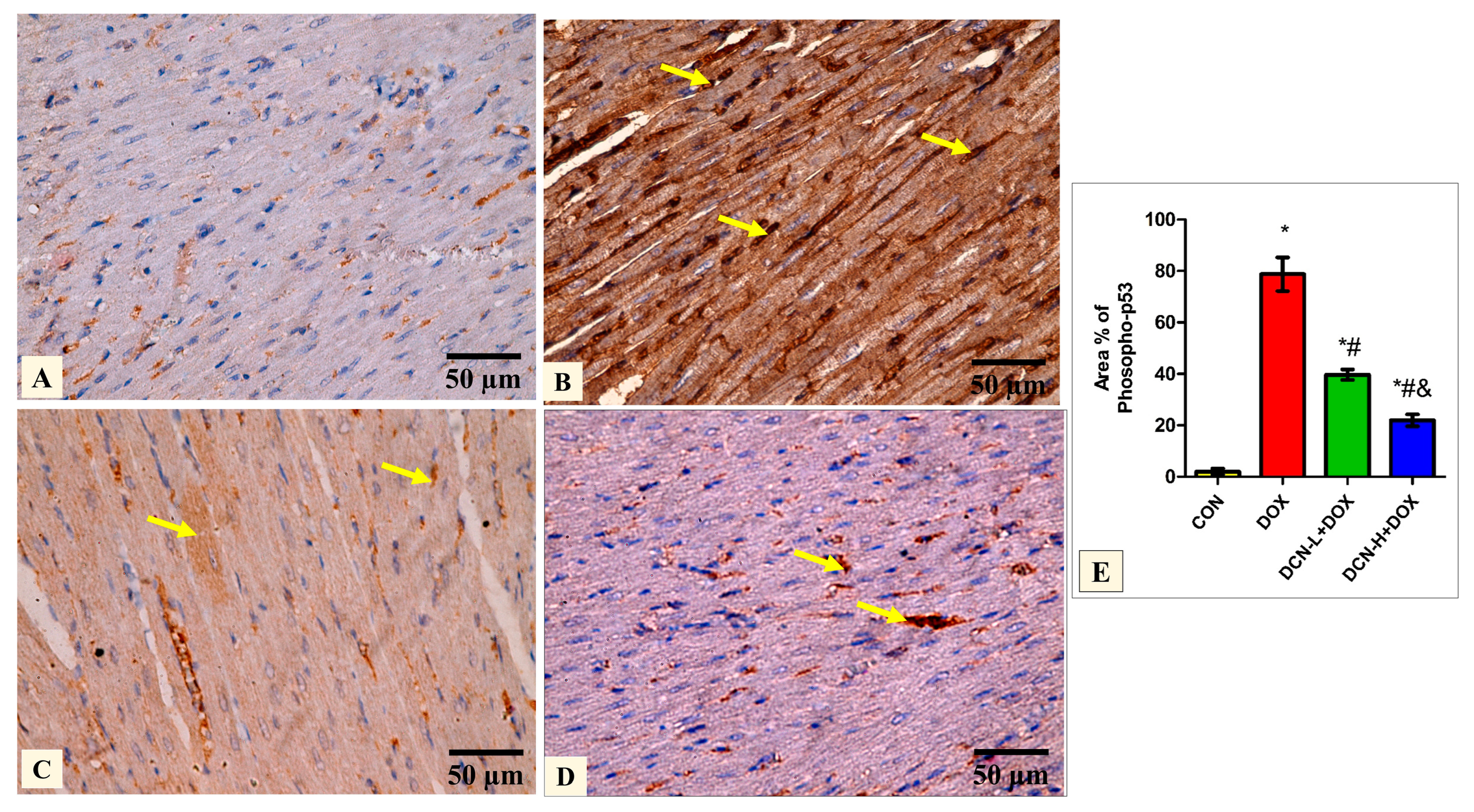
3.6. DCN-Abolished DOX-Aggravated Phospho-P53 Immunoreactivity in Rats’ Cardiac Tissue
3.7. DCN Reinforced the Cardiomyocytes’ Resistance to Ferroptosis by Activating the NRF2 Signaling
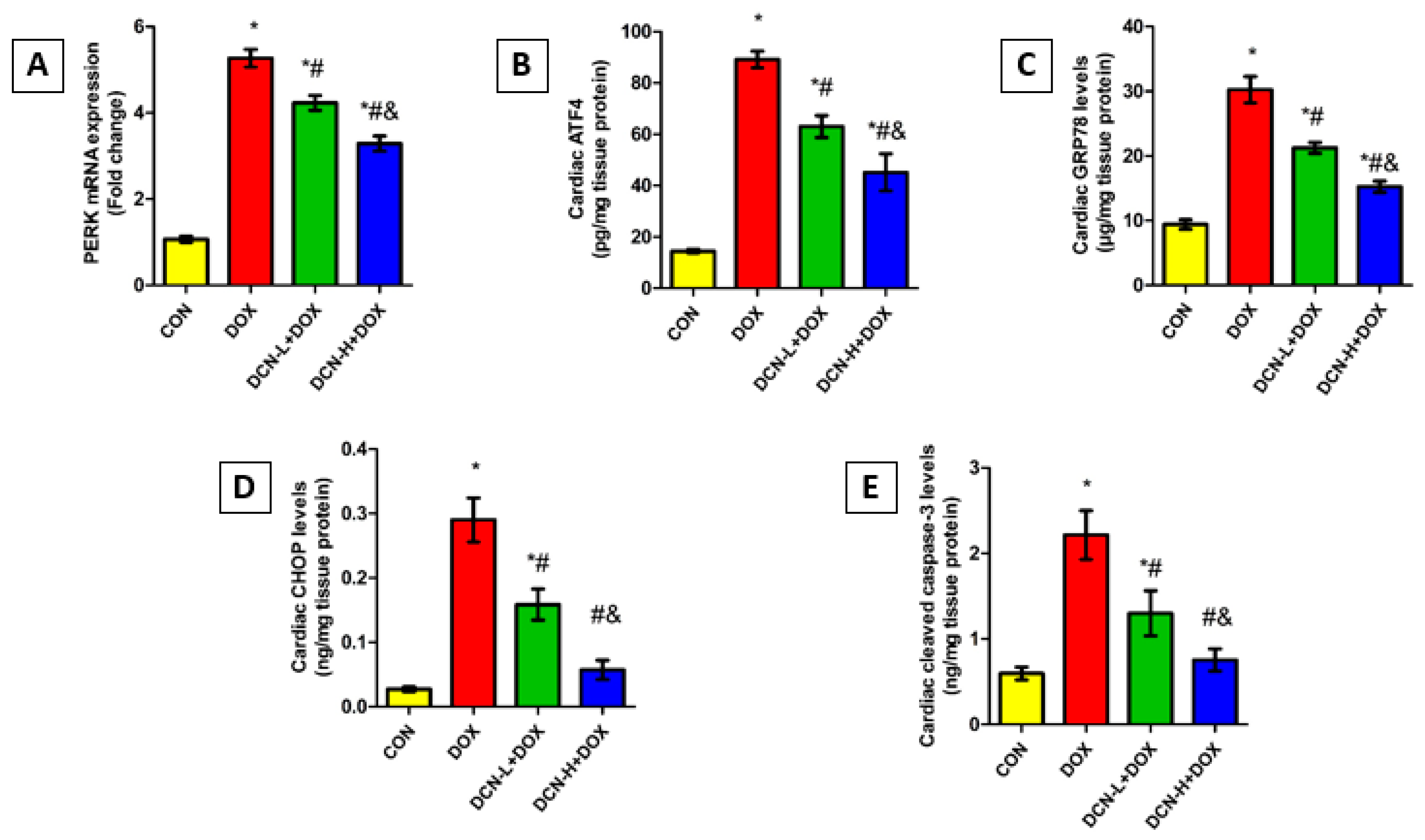
3.8. DCN Mitigated DOX-Evoked Cardiac ER Stress and Apoptosis
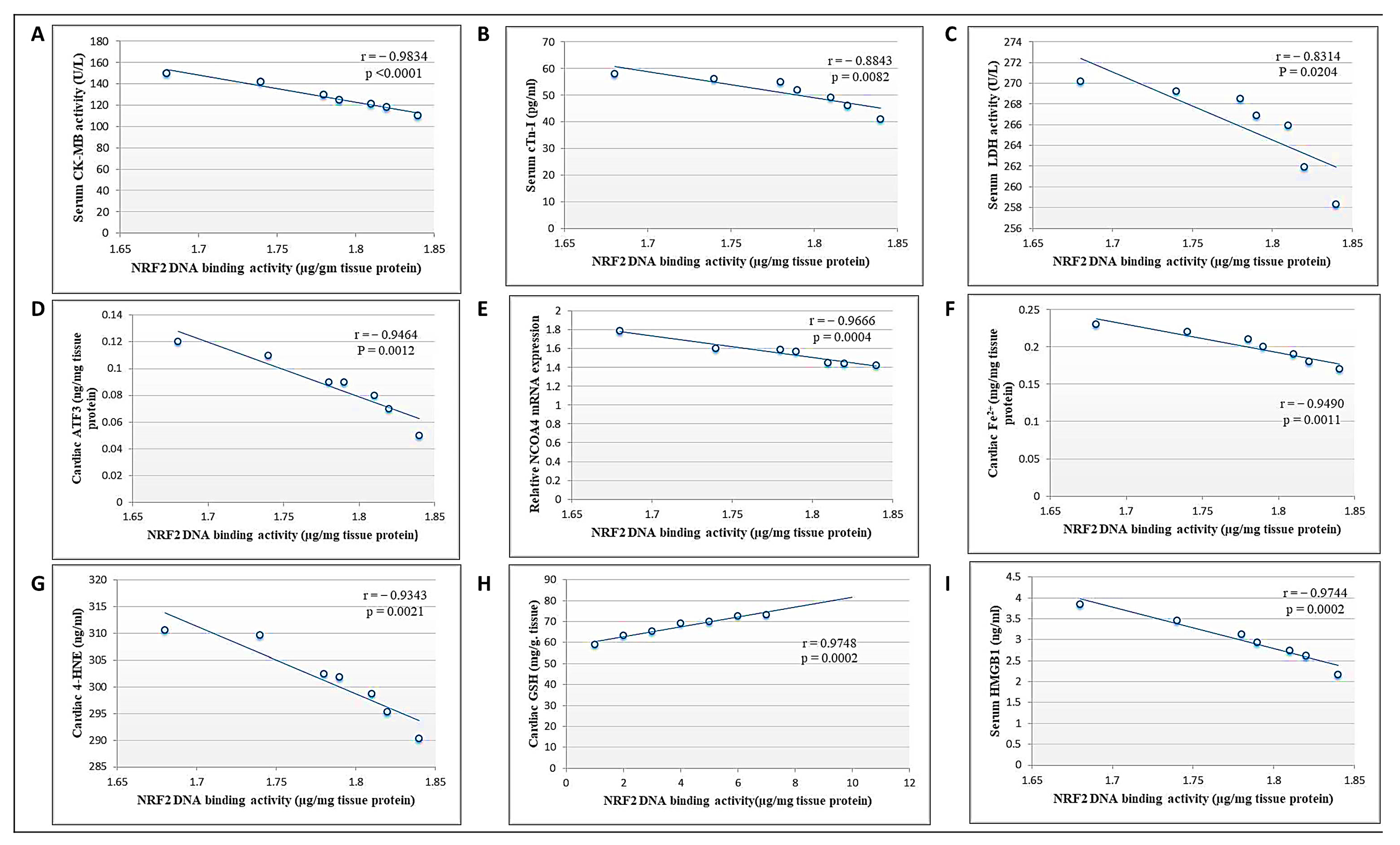
3.9. Correlation between Cardiac NRF2 and Other Measured Parameters in the DCN High Dose-Treated Group
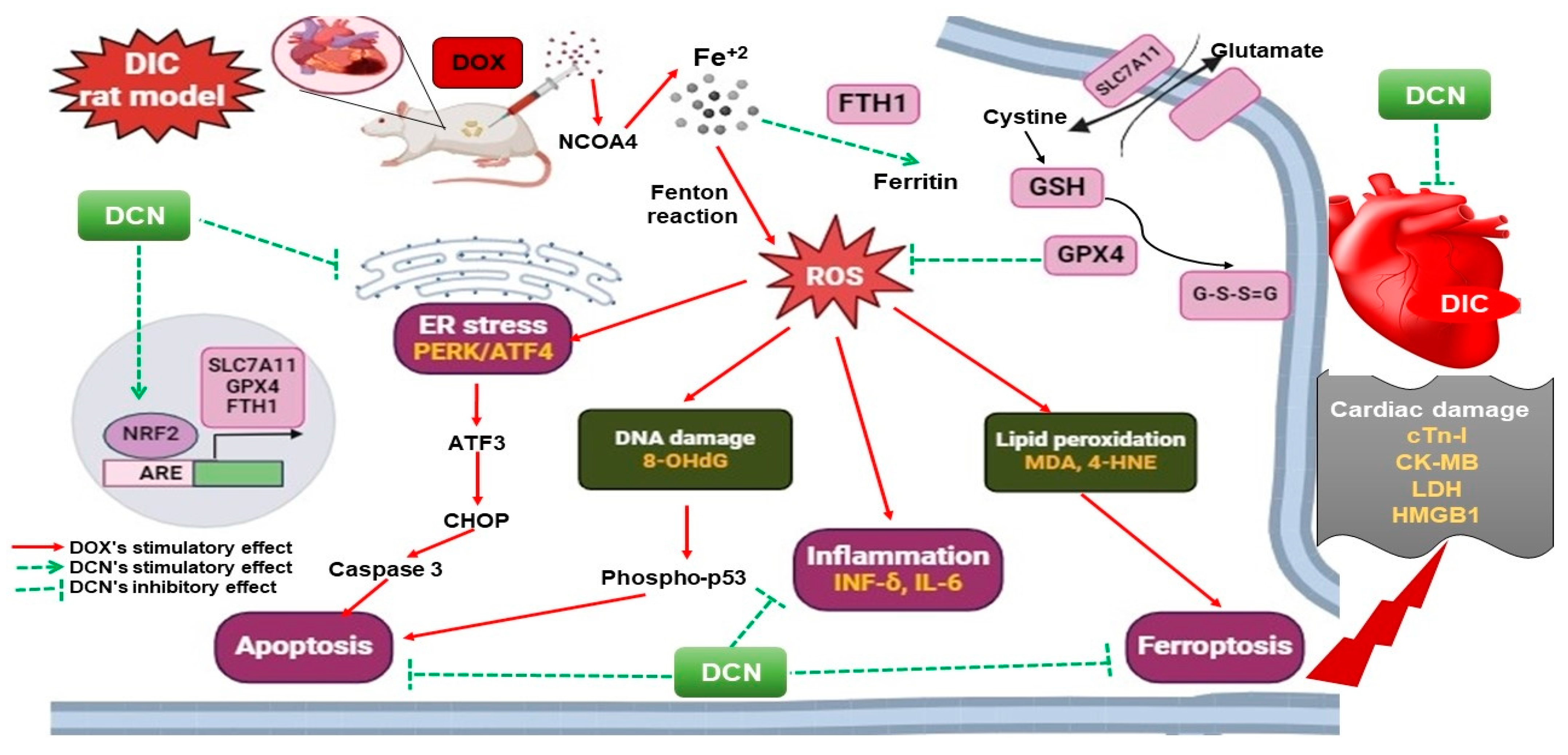
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, B.B.; Leung, K.T.; Poon, E.N. Mitochondrial-Targeted Therapy for Doxorubicin-Induced Cardiotoxicity. Int. J. Mol. Sci. 2022, 23, 1912. [Google Scholar] [CrossRef] [PubMed]
- Yarmohammadi, F.; Rezaee, R.; Haye, A.W.; Karimi, G. Endoplasmic reticulum stress in doxorubicin-induced cardiotoxicity may be therapeutically targeted by natural and chemical compounds: A review. Pharmacol. Res. 2021, 164, 105383. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Wang, L.; Qiao, Y.; Yang, B.; Yin, D.; He, M. Epigallocatechin-3-gallate pretreatment alleviates doxorubicin-induced ferroptosis and cardiotoxicity by upregulating AMPKα2 and activating adaptive autophagy. Redox Biol. 2021, 48, 102185. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Qiao, Y.; Wang, D.; Tang, C.; Yan, G. Ferritinophagy and ferroptosis in cardiovascular disease: Mechanisms and potential applications. Biomed. Pharmacother. 2021, 141, 111872. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Du, M.; Yu, R.; Cheng, Y.; Wu, B.; Fu, J.; Tan, W.; Zhou, Q.; Balawi, E.; Liao, Z. A novel mechanism linking ferroptosis and endoplasmic reticulum stress via the circPtpn14/miR-351-5p/5-LOX signaling in melatonin-mediated treatment of traumatic brain injury. Free. Radic. Biol. Med. 2022, 178, 271–294. [Google Scholar] [CrossRef] [PubMed]
- Belavgeni, A.; Meyer, C.; Stumpf, J.; Hugo, C.; Linkermann, A. Ferroptosis and Necroptosis in the Kidney. Cell Chem. Biol. 2020, 27, 448–462. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Xia, Y.; Jin, S.; Xue, C.; Wang, Y.; Hu, R.; Jiang, H. Nrf2 attenuates ferroptosis-mediated IIR-ALI by modulating TERT and SLC7A11. Cell Death Dis. 2021, 12, 1027. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Liu, X.; Shi, J.; Wu, X. Involvement of Nrf2 in myocardial ischemia and reperfusion injury. Int. J. Biol. Macromol. 2019, 125, 496–502. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Qian, J.; Rahman, S.M.J.; Siska, P.J.; Zou, Y.; Harris, B.K.; Hoeksema, M.D.; Trenary, I.A.; Heidi, C.; Eisenberg, R.; et al. xCT (SLC7A11)-mediated metabolic reprogramming promotes non-small cell lung cancer progression. Oncogene 2018, 37, 5007–5019. [Google Scholar] [CrossRef]
- Anandhan, A.; Dodson, M.; Schmidlin, C.J.; Liu, P.; Zhang, D.D. Breakdown of an Ironclad Defense System: The Critical Role of NRF2 in Mediating Ferroptosis. Cell Chem. Biol. 2020, 27, 436–447. [Google Scholar] [CrossRef]
- Abdelfattah, A.M.; Mahmoud, S.S.; El-Wafaey, D.I.; Abdelgeleel, H.M.; Abdelhamid, A.M. Diacerein ameliorates cholestasis-induced liver fibrosis in rat via modulating HMGB1/RAGE/NF-κB/JNK pathway and endoplasmic reticulum stress. Sci. Rep. 2023, 13, 11455. [Google Scholar] [CrossRef] [PubMed]
- Youssef, N.S.; Elzaitony, A.S.; Abdel Baky, N.A. Diacerein attenuate LPS-induced acute lung injury via inhibiting ER stress and apoptosis: Impact on the crosstalk between SphK1/S1P, TLR4/NFκB/STAT3, and NLRP3/IL-1β signaling pathways. Life Sci. 2022, 308, 120915. [Google Scholar] [CrossRef] [PubMed]
- Leite, N.C.; Viegas, B.B.; Villela-Nogueira, C.A.; Carlos, F.O.; Cardoso, C.R.L.; Salles, G.F. Efficacy of diacerein in reducing liver steatosis and fibrosis in patients with type 2 diabetes and non-alcoholic fatty liver disease: A randomized, placebo-controlled trial. Diabetes Obes. Metab. 2019, 21, 1266–1270. [Google Scholar] [CrossRef] [PubMed]
- Ali, D.M.; Mahmoud, M.H.; Rifaai, R.A.; Fawzy, M.A.; Atta, M.; Welson, N.N.; Batiha, G.E.; Alexiou, A.; Papadakis, M.; Abdelzaher, W.Y. Diacerein modulates TLR4/ NF-κB/IL-1β and TRPC1/CHOP signalling pathways in gentamicin-induced parotid toxicity in rats. J. Cell. Mol. Med. 2023, 27, 1735–1744. [Google Scholar] [CrossRef]
- Abdel-Latif, R.T.; Wadie, W.; Abdel-Mottaleb, Y.; Abdallah, D.M.; El-Maraghy, N.N.; El-Abhar, H.S. Reposition of the anti-inflammatory drug diacerein in an in-vivo colorectal cancer model. Saudi Pharm. J. 2022, 30, 72–90. [Google Scholar] [CrossRef]
- Barakat, N.; Barakat, L.A.; Zakaria, M.M.; Khirallah, S.M. Diacerein ameliorates kidney injury induced by cisplatin in rats by activation of Nrf2/Ho-1 pathway and Bax down-regulation. Saudi J. Biol. Sci. 2021, 28, 7219–7226. [Google Scholar] [CrossRef]
- Teng, Y.-N.; Kao, M.-C.; Huang, S.-Y.; Wu, T.-S.; Lee, T.-E.; Kuo, C.-Y.; Hung, C.-C. Novel application of rhein and its prodrug diacerein for reversing cancer-related multidrug resistance through the dual inhibition of P-glycoprotein efflux and STAT3-mediated P-glycoprotein expression. Biomed. Pharmacother. 2022, 150, 112995. [Google Scholar] [CrossRef]
- Tian, W.; Yang, L.; Liu, Y.; He, J.; Yang, L.; Zhang, Q.; Liu, F.; Li, J.; Liu, J.; Sumi, S.; et al. Resveratrol attenuates doxorubicin-induced cardiotoxicity in rats by up-regulation of vascular endothelial growth factor B. J. Nutr. Biochem. 2020, 79, 108132. [Google Scholar] [CrossRef]
- Abd-Ellatif, R.N.; Hegab, I.I.; Atef, M.M.; Sadek, M.T.; Hafez, Y.M. Diacerein protects against glycerol-induced acute kidney injury: Modulating oxidative stress, inflammation, apoptosis and necroptosis. Chem. Biol. Interact. 2019, 306, 47–53. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Uchiyama, M.; Mihara, M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal. Biochem. 1978, 86, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Paglia, D.E.; Valentine, W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967, 70, 158–169. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, J.D.; Gamble, M. Theory and Practice of Histological Techniques, 8th ed.; Elsevier: London, UK, 2019; pp. 121–132. [Google Scholar]
- Guo, R.; Hua, Y.; Ren, J.; Bornfeldt, K.E.; Nair, S. Cardiomyocyte-specific disruption of Cathepsin K protects against doxorubicin-induced cardiotoxicity. Cell Death Dis. 2018, 9, 692, Corrected in Cell Death Dis. 2019, 10, 933. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Kaushik, A.S.; Rehman, M.; Chaudhary, R.; Jawaid, T.; Kamal, M.; Mishra, V. Interleukin-6 expression and its modulation by diacerein in a rat model of chronic stress induced cardiac dysfunction. Heliyon 2021, 7, e08522. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Luo, W.; Yu, T.; Liang, S.; Zou, C.; Sun, J.; Li, G.; Liang, G. Diacerein alleviates Ang II-induced cardiac inflammation and remodeling by inhibiting the MAPKs/c-Myc pathway. Phytomedicine 2022, 106, 154387. [Google Scholar] [CrossRef]
- Zhao, S.-Y.; Zhao, H.-H.; Wang, B.-H.; Shao, C.; Pan, W.-J.; Li, S.-M. Rhein alleviates advanced glycation end products (AGEs)-induced inflammatory injury of diabetic cardiomyopathy in vitro and in vivo models. J. Nat. Med. 2023, 77, 898–915. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Liu, X.; Pi, W.; Zhang, Y.; Yu, L.; Xu, C.; Sun, Z.; Jiang, J. Fisetin Attenuates Doxorubicin-Induced Cardiomyopathy In Vivo and In Vitro by Inhibiting Ferroptosis Through SIRT1/Nrf2 Signaling Pathway Activation. Front. Pharmacol. 2022, 12, 808480. [Google Scholar] [CrossRef]
- Hanna, M.; Seddiek, H.; Aboulhoda, B.E.; Morcos, G.N.B.; Akabawy, A.M.A.; Elbaset, M.A.; Ibrahim, A.A.; Khalifa, M.M.; Khalifah, I.M.; Fadel, M.S.; et al. Synergistic cardioprotective effects of melatonin and deferoxamine through the improvement of ferritinophagy in doxorubicin-induced acute cardiotoxicity. Front. Physiol. 2022, 13, 1050598. [Google Scholar] [CrossRef]
- Li, B.; Gong, J.; Sheng, S.; Lu, M.; Guo, S.; Zhao, X.; Zhang, H.; Wang, H.; Tian, Z.; Tian, Y. Increased hepcidin in hemorrhagic plaques correlates with iron-stimulated IL-6/STAT3 pathway activation in macrophages. Biochem. Biophys. Res. Commun. 2019, 515, 394–400. [Google Scholar] [CrossRef] [PubMed]
- Charlebois, E.; Pantopoulos, K. Iron overload inhibits BMP/SMAD and IL-6/STAT3 signaling to hepcidin in cultured hepatocytes. PLoS ONE 2021, 16, e0253475. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.-T.; Lin, Y.-W.; Ho, C.-H.; Chen, Z.-C.; Liu, P.-Y.; Shih, J.-Y. Dapagliflozin suppresses ER stress and protects doxorubicin-induced cardiotoxicity in breast cancer patients. Arch. Toxicol. 2021, 95, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Chipurupalli, S.; Samavedam, U.; Robinson, N. Crosstalk Between ER Stress, Autophagy and Inflammation. Front. Med. 2021, 8, 758311. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lv, Y.; Zhao, N.; Guan, G.; Wang, J. Protein kinase R-like ER kinase and its role in endoplasmic reticulum stress-decided cell fate. Cell Death Dis. 2015, 6, e1822. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, X.-Z.; He, C.; Wang, L.; Liang, S.-P.; Wang, C.-C.; Li, C.; Luo, T.-F.; Feng, C.-S.; Wang, Z.-C.; et al. ATF3 contributes to brucine-triggered glioma cell ferroptosis via promotion of hydrogen peroxide and iron. Acta Pharmacol. Sin. 2021, 42, 1690–1702. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yan, J.; Zhao, Q.; Zhang, Y.; Zhang, Y. ATF3 promotes ferroptosis in sorafenib-induced cardiotoxicity by suppressing Slc7a11 expression. Front. Pharmacol. 2022, 13, 904314. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Oliveira, T.; Ferreira, L.L.; Coelho, A.R.; Deus, C.M.; Oliveira, P.J. Doxorubicin triggers bioenergetic failure and p53 activation in mouse stem cell-derived cardiomyocytes. Toxicol. Appl. Pharmacol. 2018, 348, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Tang, Y.; Lu, G.; Gu, J. p53 at the Crossroads between Doxorubicin-Induced Cardiotoxicity and Resistance: A Nutritional Balancing Act. Nutrients 2023, 15, 2259. [Google Scholar] [CrossRef]
- Zeng, C.; Tang, H.; Chen, H.; Li, M.; Xiong, D. Ferroptosis: A new approach for immunotherapy. Cell Death Discov. 2020, 6, 122. [Google Scholar] [CrossRef]
- El-Sherbiny, M.; El-Shafey, M.; El-Agawy, M.S.E.-D.; Mohamed, A.S.; Eisa, N.H.; Elsherbiny, N.M. Diacerein ameliorates testosterone-induced benign prostatic hyperplasia in rats: Effect on oxidative stress, inflammation and apoptosis. Int. Immunopharmacol. 2021, 100, 108082. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zheng, L. A Review on Bioactive Anthraquinone and Derivatives as the Regulators for ROS. Molecules 2023, 28, 8139. [Google Scholar] [CrossRef] [PubMed]
- Adinolfi, S.; Patinen, T.; Deen, A.J.; Pitkänen, S.; Härkönen, J.; Kansanen, E.; Küblbeck, J.; Levonen, A.-L. The KEAP1-NRF2 pathway: Targets for therapy and role in cancer. Redox Biol. 2023, 63, 102726. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Jin, H.; Huo, X.; Meng, Q.; Wang, C.; Sun, P.; Ma, X.; Sun, H.; Dong, D.; Wu, J.; et al. Protective effect of Rhein against vancomycin-induced nephrotoxicity through regulating renal transporters and Nrf2 pathway. Phytotherapy Res. 2022, 36, 4244–4262. [Google Scholar] [CrossRef]
- Chen, W.; Jiang, T.; Wang, H.; Tao, S.; Lau, A.; Fang, D.; Zhang, D.D. Does Nrf2 Contribute to p53-Mediated Control of Cell Survival and Death? Antioxid. Redox Signal. 2012, 17, 1670–1675. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhu, X.; Dong, J.; Chen, F.; Gao, Q.; Zhang, L.; Cai, D.; Dong, H.; Ruan, B.; Wang, Y.; et al. Reversal of Epigenetic Peroxisome Proliferator-Activated Receptor-γ Suppression by Diacerein Alleviates Oxidative Stress and Osteoarthritis in Mice. Antioxid. Redox Signal. 2022, 37, 40–53. [Google Scholar] [CrossRef]
- Anandhan, A.; Dodson, M.; Shakya, A.; Chen, J.; Liu, P.; Wei, Y.; Tan, H.; Wang, Q.; Jiang, Z.; Yang, K.; et al. NRF2 controls iron homeostasis and ferroptosis through HERC2 and VAMP8. Sci. Adv. 2023, 9, eade9585. [Google Scholar] [CrossRef]
- Zhao, X.; Tian, Z.; Sun, M.; Dong, D. Nrf2: A dark horse in doxorubicin-induced cardiotoxicity. Cell Death Discov. 2023, 9, 261. [Google Scholar] [CrossRef]
- Jyotsana, N.; Ta, K.T.; DelGiorno, K.E. The Role of Cystine/Glutamate Antiporter SLC7A11/xCT in the Pathophysiology of Cancer. Front. Oncol. 2022, 12, 858462. [Google Scholar] [CrossRef]
- Kciuk, M.; Gielecińska, A.; Mujwar, S.; Kołat, D.; Kałuzińska-Kołat, Ż.; Celik, I.; Kontek, R. Doxorubicin—An Agent with Multiple Mechanisms of Anticancer Activity. Cells 2023, 12, 659. [Google Scholar] [CrossRef]
- Tsang, W.P.; Chau, S.P.; Kong, S.K.; Fung, K.P.; Kwok, T.T. Reactive oxygen species mediate doxorubicin induced p53-independent apoptosis. Life Sci. 2003, 73, 2047–2058. [Google Scholar] [CrossRef] [PubMed]
- Schuler, M.; Green, D.R. Mechanisms of p53-dependent apoptosis. Biochem. Soc. Trans. 2001, 29, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Tian, M.; Ding, C.; Yu, S. The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection. Front. Immunol. 2019, 9, 3083. [Google Scholar] [CrossRef] [PubMed]


| Gene | Accession Number | Forward (5′-3′) | Reverse (5′-3′) |
|---|---|---|---|
| NCOA4 | NM_001034007.1 | TGAAGTGCAGTGCTCACACA | TTCGCTGCTGCTGACAGTTA |
| FTH1 | NM_012848.2 | CCCTTTGCAACTTCGTCGCT | CTCCGAGTCCTGGTGGTAGT |
| SLC7A11 | NM_001107673.3 | GAGGCGCTGTAGCCACATTA | GGCATTCAACCAGGTGATCC |
| PERK | NM_031599.2 | GAAGTCGAGAGGCGTGGGGG | GCCCGTCTCGCCGCTAGGAG |
| GAPDH | NM_017008.4 | GGCATCGTGGAAGGGCTC | GACCTTGCCCACAGCCTT |
| Parameter/Groups | CON | DOX | DCN-L+DOX | DCN-H+DOX |
|---|---|---|---|---|
| Cardiac GSH (mg/g tissue) | 86.29 ± 4.09 | 25.27 ± 3.11 * | 47.86 ± 4.43 *# | 67.54 ± 5.23 *#& |
| Cardiac MDA (nmol/g. tissue) | 5.043 ± 1.54 | 19.89 ± 1.10 * | 16.09 ± 1.14 *# | 12.64 ± 1.83 *#& |
| Cardiac 4-HNE (pg/g tissue) | 114.9 ± 6.42 | 566.2 ± 9.94 * | 430.0 ± 9.92 *# | 301.3 ± 7.33 *#& |
| Cardiac 8-OHdG (ng/g tissue) | 4.614 ± 1.20 | 41.00 ± 4.93 * | 30.64 ± 4.11 *# | 14.20± 4.13 *#& |
| Cardiac IL-6 (pg/mg tissue) | 5.18 ± 0.97 | 31.81 ± 3.26 * | 25.09 ± 1.24 *# | 20.46 ± 1.25 *#& |
| Cardiac IFN-γ (pg/mg tissue) | 25.13 ± 3.56 | 97.66 ± 1.69 * | 58.17 ± 3.70 *# | 38.81 ± 5.69 *#& |
| Serum HMGB1 (ng/mL) | 1.821 ± 0.103 | 7.587 ± 0.73 * | 4.764 ± 0.61 *# | 2.983 ± 0.55 *#& |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Gohary, R.M.; Okasha, A.H.; Abd El-Azeem, A.H.; Abdel Ghafar, M.T.; Ibrahim, S.; Hegab, I.I.; Farghal, E.E.; Shalaby, S.A.F.; Elshora, O.A.; ElMehy, A.E.; et al. Uncovering the Cardioprotective Potential of Diacerein in Doxorubicin Cardiotoxicity: Mitigating Ferritinophagy-Mediated Ferroptosis via Upregulating NRF2/SLC7A11/GPX4 Axis. Antioxidants 2024, 13, 493. https://doi.org/10.3390/antiox13040493
El-Gohary RM, Okasha AH, Abd El-Azeem AH, Abdel Ghafar MT, Ibrahim S, Hegab II, Farghal EE, Shalaby SAF, Elshora OA, ElMehy AE, et al. Uncovering the Cardioprotective Potential of Diacerein in Doxorubicin Cardiotoxicity: Mitigating Ferritinophagy-Mediated Ferroptosis via Upregulating NRF2/SLC7A11/GPX4 Axis. Antioxidants. 2024; 13(4):493. https://doi.org/10.3390/antiox13040493
Chicago/Turabian StyleEl-Gohary, Rehab M., Asmaa H. Okasha, Alaa H. Abd El-Azeem, Muhammad T. Abdel Ghafar, Sarah Ibrahim, Islam I. Hegab, Eman E. Farghal, Soha Abdel Fattah Shalaby, Ola A. Elshora, Aisha E. ElMehy, and et al. 2024. "Uncovering the Cardioprotective Potential of Diacerein in Doxorubicin Cardiotoxicity: Mitigating Ferritinophagy-Mediated Ferroptosis via Upregulating NRF2/SLC7A11/GPX4 Axis" Antioxidants 13, no. 4: 493. https://doi.org/10.3390/antiox13040493





