Comparative Analysis of Polyphenolic Compounds in Different Amaranthus Species: Influence of Genotypes and Harvesting Year
Abstract
:1. Introduction
2. Materials and Methods
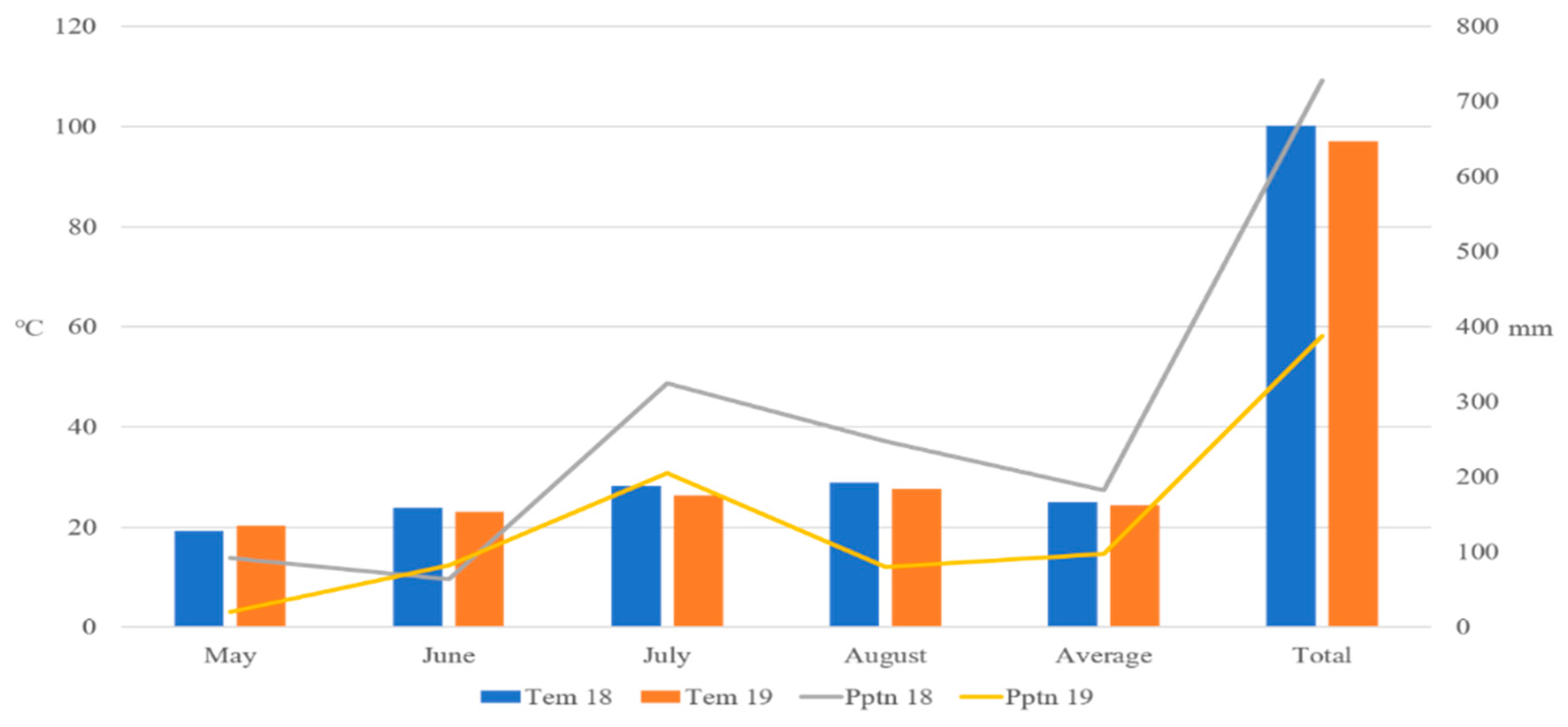
2.1. Plant Materials and Growth Conditions
2.2. Sampling and Content Extraction
2.3. Sample Analysis by UHPLC-PDA
2.4. Statistical Analysis
RPCI = Average of standard score.
3. Results
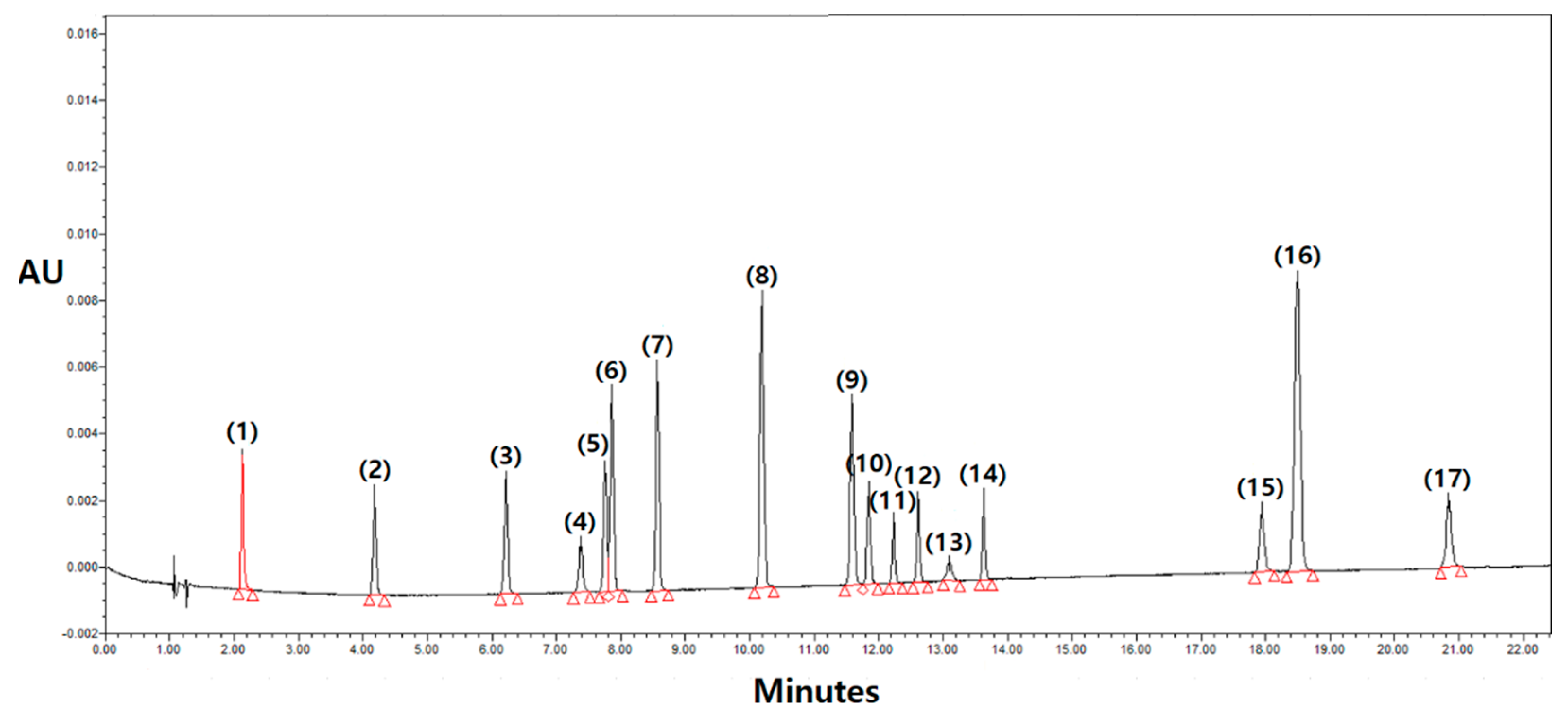
3.1. Polyphenolics Assessment Using the UPLC-PDA
3.1.1. Hydroxybenzoic Acid
3.1.2. Hydroxycinnamic Acid
3.1.3. Flavonoid
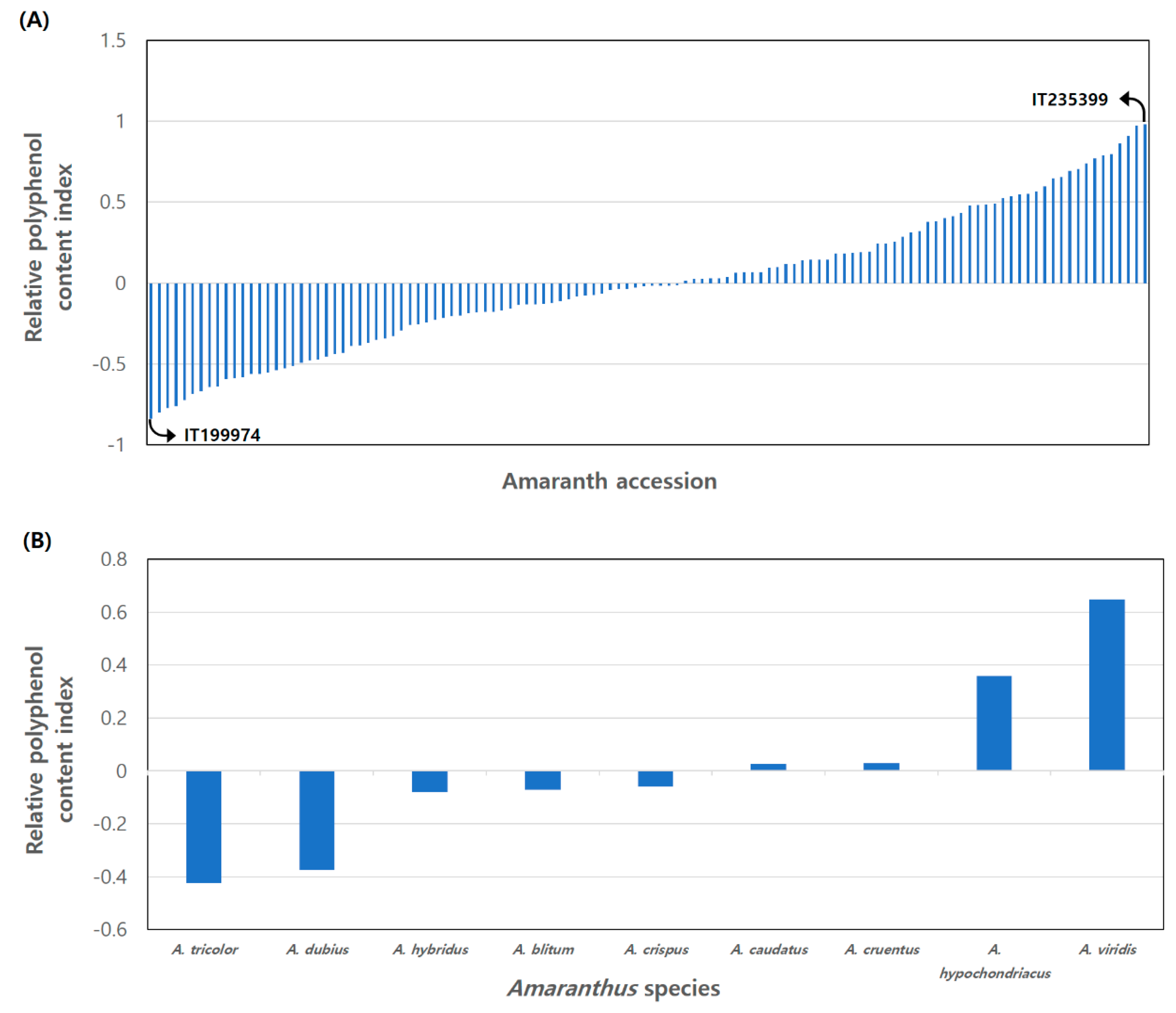
3.2. Relative Polyphenol Content Index
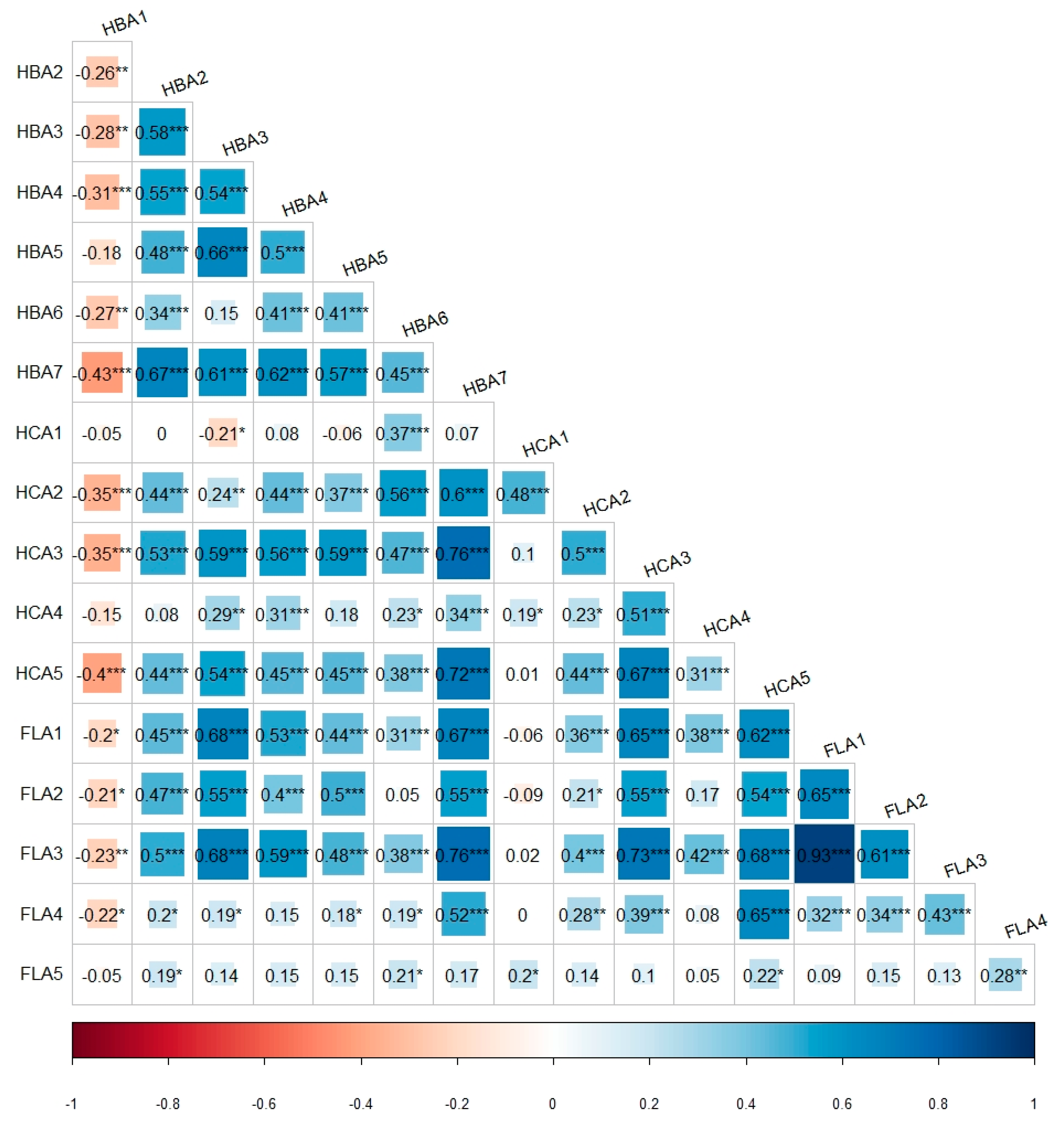
3.3. Correlation Analysis
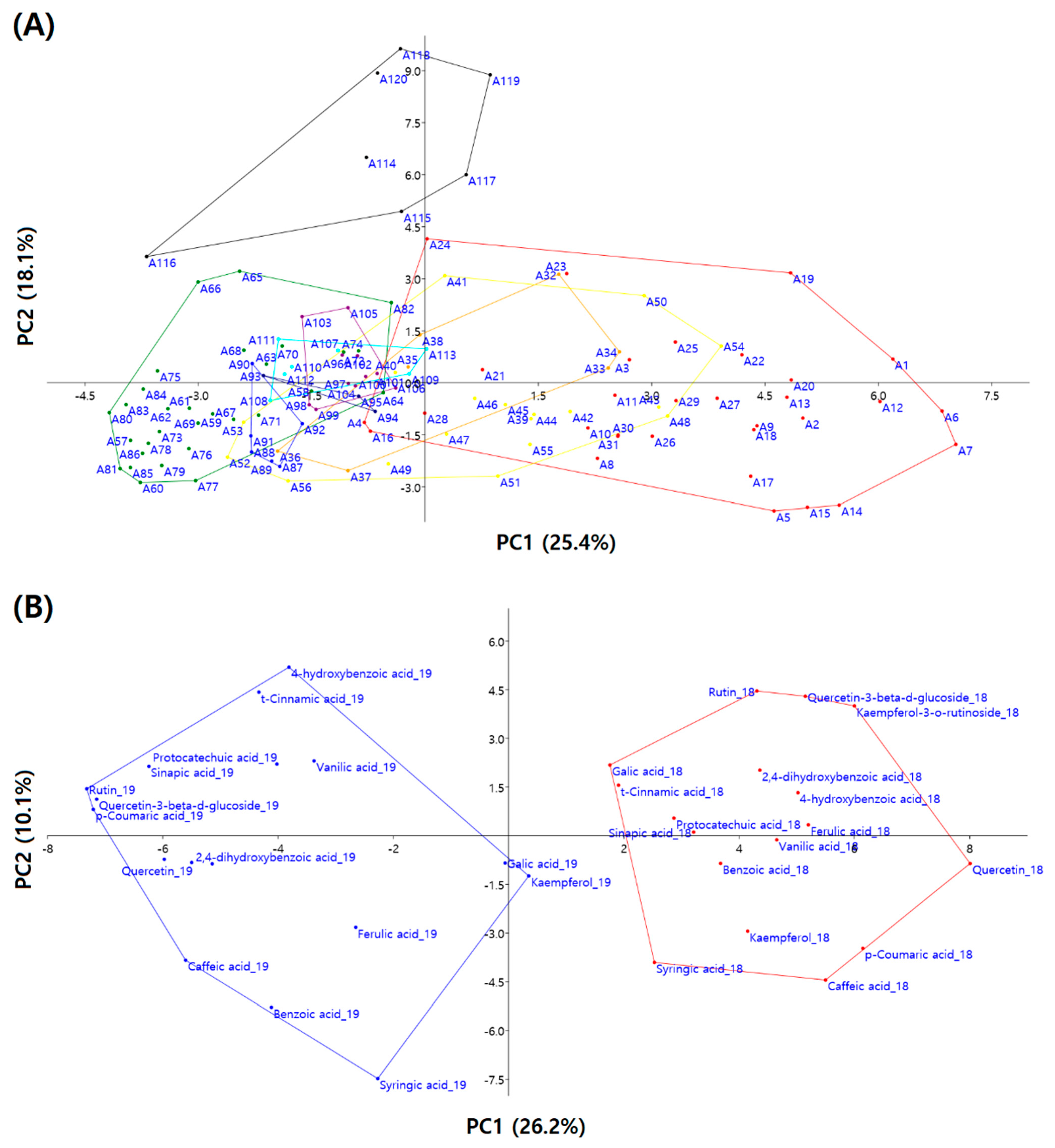
3.4. Principal Component Analysis
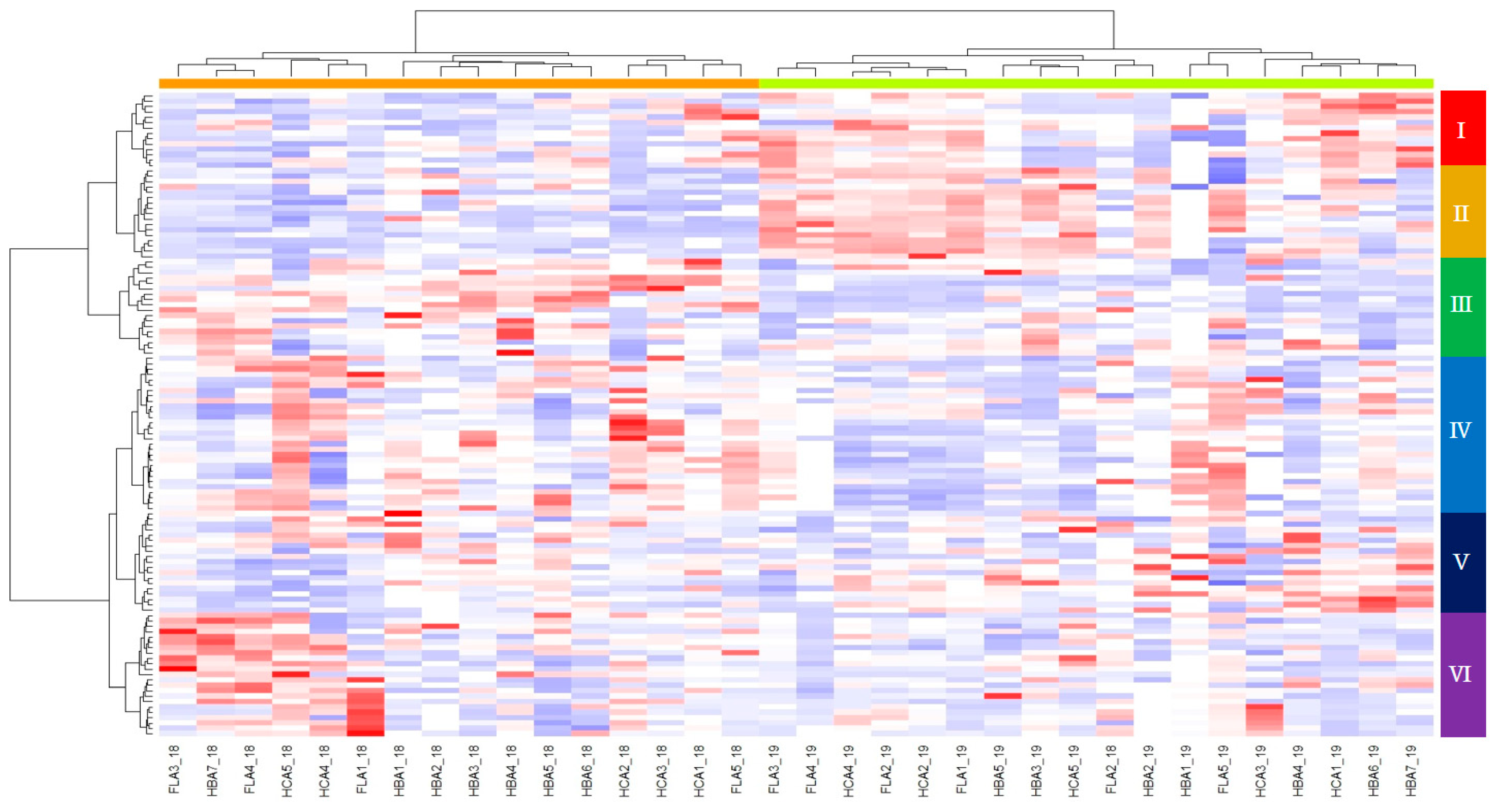
3.5. Heatmap Hierarchical Clustering
3.6. Statistical Analysis of Genotype, Year, and Genotype × Year across 17 Polyphenols
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zagoskina, N.V.; Zubova, M.Y.; Nechaeva, T.L.; Kazantseva, V.V.; Goncharuk, E.A.; Katanskaya, V.M.; Baranova, E.N.; Aksenova, M.A. Polyphenols in Plants: Structure, Biosynthesis, Abiotic Stress Regulation, and Practical Applications (Review). Int. J. Mol. Sci. 2023, 24, 13874. [Google Scholar] [CrossRef]
- Arts, I.C.; Hollman, P.C. Polyphenols and disease risk in epidemiologic studies. Am. J. Clin. Nutr. 2005, 81, 317s–325s. [Google Scholar] [CrossRef]
- Khanam, U.K.S.; Oba, S.; Yanase, E.; Murakami, Y. Phenolic acids, flavonoids and total antioxidant capacity of selected leafy vegetables. J. Funct. Foods 2012, 4, 979–987. [Google Scholar] [CrossRef]
- Lee, K.J.; Baek, D.Y.; Lee, G.A.; Cho, G.T.; So, Y.S.; Lee, J.R.; Ma, K.H.; Chung, J.W.; Hyun, D.Y. Phytochemicals and Antioxidant Activity of Korean Black Soybean (Glycine max L.) Landraces. Antioxidants 2020, 9, 213. [Google Scholar] [CrossRef] [PubMed]
- Mikulic Petkovsek, M.; Slatnar, A.; Stampar, F.; Veberic, R. The influence of organic/integrated production on the content of phenolic compounds in apple leaves and fruits in four different varieties over a 2-year period. J. Sci. Food Agric. 2010, 90, 2366–2378. [Google Scholar] [CrossRef]
- Neugart, S.; Baldermann, S.; Ngwene, B.; Wesonga, J.; Schreiner, M. Indigenous leafy vegetables of Eastern Africa—A source of extraordinary secondary plant metabolites. Food Res. Int. 2017, 100, 411–422. [Google Scholar] [CrossRef]
- Tang, Y.; Li, X.; Chen, P.X.; Zhang, B.; Liu, R.; Hernandez, M.; Draves, J.; Marcone, M.F.; Tsao, R. Assessing the Fatty Acid, Carotenoid, and Tocopherol Compositions of Amaranth and Quinoa Seeds Grown in Ontario and Their Overall Contribution to Nutritional Quality. J. Agric. Food Chem. 2016, 64, 1103–1110. [Google Scholar] [CrossRef]
- Das, S. Amaranths: The Crop of Great Prospect. In Amaranthus: A Promising Crop of Future; Springer: Singapore, 2016; pp. 13–48. [Google Scholar]
- Rastogi, A.; Shukla, S. Amaranth: A new millennium crop of nutraceutical values. Crit. Rev. Food Sci. Nutr. 2013, 53, 109–125. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.; Bhargava, A.; Chatterjee, A.; Pandey, A.C.; Mishra, B.K. Diversity in phenotypic and nutritional traits in vegetable amaranth (Amaranthus tricolor), a nutritionally underutilised crop. J. Sci. Food Agric. 2010, 90, 139–144. [Google Scholar] [CrossRef]
- Manyelo, T.G.; Sebola, N.A.; Mabelebele, M. Nutritional and Phenolic Profile of Early and Late Harvested Amaranth Leaves Grown Under Cultivated Conditions. Agriculture 2020, 10, 432. [Google Scholar] [CrossRef]
- López-Mejía, O.A.; López-Malo, A.; Palou, E. Antioxidant capacity of extracts from amaranth (Amaranthus hypochondriacus L.) seeds or leaves. Ind. Crop. Prod. 2014, 53, 55–59. [Google Scholar] [CrossRef]
- Narwade, S.G.; Pinto, S.V. Amaranth—A Functional Food. Concepts Dairy Vet. Sci. 2018, 1. [Google Scholar] [CrossRef]
- Jan, N.; Hussain, S.Z.; Naseer, B.; Bhat, T.A. Amaranth and quinoa as potential nutraceuticals: A review of anti-nutritional factors, health benefits and their applications in food, medicinal and cosmetic sectors. Food Chem. X 2023, 18, 100687. [Google Scholar] [CrossRef] [PubMed]
- Kalinova, J.; Dadakova, E. Rutin and total quercetin content in amaranth (Amaranthus spp.). Plant Foods Hum. Nutr. 2009, 64, 68–74. [Google Scholar] [CrossRef]
- Li, H.; Deng, Z.; Liu, R.; Zhu, H.; Draves, J.; Marcone, M.; Sun, Y.; Tsao, R. Characterization of phenolics, betacyanins and antioxidant activities of the seed, leaf, sprout, flower and stalk extracts of three Amaranthus species. J. Food Compos. Anal. 2015, 37, 75–81. [Google Scholar] [CrossRef]
- Karamać, M.; Gai, F.; Longato, E.; Meineri, G.; Janiak, M.A.; Amarowicz, R.; Peiretti, P.G. Antioxidant Activity and Phenolic Composition of Amaranth (Amaranthus caudatus) during Plant Growth. Antioxidants 2019, 8, 173. [Google Scholar] [CrossRef]
- Barba de la Rosa, A.P.; De León-Rodríguez, A.; Laursen, B.B.; Fomsgaard, I.S. Influence of the growing conditions on the flavonoids and phenolic acids accumulation in amaranth (Amaranthus hypochondriacus L.) leaves. Rev. Terra Latinoam. 2019, 37, 449–457. [Google Scholar] [CrossRef]
- Netshimbupfe, M.H.; Berner, J.; Van Der Kooy, F.; Oladimeji, O.; Gouws, C. Influence of Drought and Heat Stress on Mineral Content, Antioxidant Activity and Bioactive Compound Accumulation in Four African Amaranthus Species. Plants 2023, 12, 953. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Drought stress enhances nutritional and bioactive compounds, phenolic acids and antioxidant capacity of Amaranthus leafy vegetable. BMC Plant Biol. 2018, 18, 258. [Google Scholar] [CrossRef] [PubMed]
- Gerrano, A.S.; Rensburg, W.S.J.v.; Mavengahama, S.; Bairu, M.; Venter, S.; Adebola, P.O. Qualitative morphological diversity of Amaranthus species. J. Trop. Agric. 2017, 55, 12–20. [Google Scholar]
- Jeong, W.T.; Bang, J.-H.; Han, S.; Hyun, T.K.; Cho, H.; Lim, H.B.; Chung, J.-W. Establishment of a UPLC-PDA/ESI-Q-TOF/MS-Based Approach for the Simultaneous Analysis of Multiple Phenolic Compounds in Amaranth (A. cruentus and A. tricolor). Molecules 2020, 25, 5674. [Google Scholar] [CrossRef] [PubMed]
- Hammer, Ø.; Harper, D.A.T.; Ryan, P.D. Past: Paleontological Statistical Software Package for Education and Data Analysis. Palaeontol. Electron. 2001, 4, 1–9. [Google Scholar]
- Halliwell, B.; Aeschbach, R.; Löliger, J.; Aruoma, O.I. The characterization of antioxidants. Food Chem. Toxicol. 1995, 33, 601–617. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid antioxidants: Chemistry, metabolism and structure-activity relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef] [PubMed]
- Pasqualini, V.; Robles, C.; Garzino, S.; Greff, S.; Bousquet-Melou, A.; Bonin, G. Phenolic compounds content in Pinus halepensis Mill. needles: A bioindicator of air pollution. Chemosphere 2003, 52, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.; Wang, Y.; Ma, L.; Kang, L.; Liu, H.; Ma, X.; Zhao, D. Comparative Analysis of Polyphenols in Lycium barbarum Fruits Using UPLC-IM-QTOF-MS. Molecules 2023, 28, 4930. [Google Scholar] [CrossRef] [PubMed]
- Sytar, O.; Hemmerich, I.; Zivcak, M.; Rauh, C.; Brestic, M. Comparative analysis of bioactive phenolic compounds composition from 26 medicinal plants. Saudi J. Biol. Sci. 2018, 25, 631–641. [Google Scholar] [CrossRef]
- Zhang, X.; Bian, Z.; Li, S.; Chen, X.; Lu, C. Comparative Analysis of Phenolic Compound Profiles, Antioxidant Capacities, and Expressions of Phenolic Biosynthesis-Related Genes in Soybean Microgreens Grown under Different Light Spectra. J. Agric. Food Chem. 2019, 67, 13577–13588. [Google Scholar] [CrossRef]
- Tang, Y.; Tsao, R. Phytochemicals in quinoa and amaranth grains and their antioxidant, anti-inflammatory, and potential health beneficial effects: A review. Mol. Nutr. Food Res. 2017, 61, 1600767. [Google Scholar] [CrossRef]
- Sarker, U.; Oba, S. Antioxidant constituents of three selected red and green color Amaranthus leafy vegetable. Sci. Rep. 2019, 9, 18233. [Google Scholar] [CrossRef]
- Sun, T.; Tanumihardjo, S.A. An integrated approach to evaluate food antioxidant capacity. J. Food Sci. 2007, 72, R159–R165. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yang, L.; Yu, M.; Li, Z.; Ke, Z.; Qian, X.; Ruan, X.; He, L.; Wei, F.; Zhao, Y.; et al. Seasonal variation influences flavonoid biosynthesis path and content, and antioxidant activity of metabolites in Tetrastigma hemsleyanum Diels & Gilg. PLoS ONE 2022, 17, e0265954. [Google Scholar] [CrossRef]
- Lim, W.; Li, J. Synergetic effect of the Onion CHI gene on the PAP1 regulatory gene for enhancing the flavonoid profile of tomato skin. Sci. Rep. 2017, 7, 12377. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Li, M.; Zheng, G.; Wang, T.; Lin, J.; Wang, S.; Wang, X.; Chao, Q.; Cao, S.; Yang, Z.; et al. Metabolite Profiling of 14 Wuyi Rock Tea Cultivars Using UPLC-QTOF MS and UPLC-QqQ MS Combined with Chemometrics. Molecules 2018, 23, 104. [Google Scholar] [CrossRef] [PubMed]
- Gomes, A.F.; Almeida, M.P.; Leite, M.F.; Schwaiger, S.; Stuppner, H.; Halabalaki, M.; Amaral, J.G.; David, J.M. Seasonal variation in the chemical composition of two chemotypes of Lippia alba. Food Chem. 2019, 273, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Peters, K.; Gorzolka, K.; Bruelheide, H.; Neumann, S. Seasonal variation of secondary metabolites in nine different bryophytes. Ecol. Evol. 2018, 8, 9105–9117. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zheng, Z.-S.; Cheng, F.; Ruan, X.; Jiang, D.-A.; Pan, C.-D.; Wang, Q. Seasonal Dynamics of Metabolites in Needles of Taxus wallichiana var. mairei. Molecules 2016, 21, 1403. [Google Scholar] [CrossRef]
- Geber, M.A.; Griffen, L.R. Inheritance and Natural Selection on Functional Traits. Int. J. Plant Sci. 2003, 164, S21–S42. [Google Scholar] [CrossRef]
- Moore, B.D.; Andrew, R.L.; Külheim, C.; Foley, W.J. Explaining intraspecific diversity in plant secondary metabolites in an ecological context. New Phytol. 2014, 201, 733–750. [Google Scholar] [CrossRef]
- Zangerl, A.R.; Berenbaum, M.R. Furanocoumarin Induction in Wild Parsnip: Genetics and Population Variation. Ecology 1990, 71, 1933–1940. [Google Scholar] [CrossRef]
| Parameters | Conditions | |
|---|---|---|
| UPLC-PDA conditions below | ||
| Injection volume | 5 μL | |
| Column temperature | 40 °C | |
| Flow rate | 0.25 mL/min | |
| Column type | BEH C18 column (2.1 mm × 100 mm, 1.7 μm) | |
| Gradient/mobile phase Time (min) | Solvent A (%) | Solvent B (%) |
| 0 | 98 | 2 |
| 20 | 75 | 25 |
| 24 | 40 | 60 |
| 27 | 10 | 90 |
| 28 | 10 | 90 |
| 30 | 98 | 2 |
| 35 | re-equilibration | |
| Phenolic Compound | Year | Species | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A. hypo | A. crue | A. caud | A. tric | A. dubi | A. blit | A. cris | A. hybr | A. viri | Average | ||
| Hydroxybenzoic acid (μg g−1) | |||||||||||
| HBA1 | 2018 | 3.0 ± 1.5 ab 1 | 4.5 ± 3.9 a | 2.8 ± 1.6 ab | 2.3 ± 0.5 b | 1.8 ± 0.3 b | 1.9 ± 0.4 b | 2.0 ± 0.5 b | 4.5 ± 2.0 a | 4.3 ± 0.5 a | 2.0 ± 1.8 g |
| 2019 | 24.2 a | 7.7 ± 8.7 b | 7.5 ± 7.5 b | 9.2 ± 0.5 b | 8.6 b | 9.0 ± 0.7 b | 8.8 ± 0.3 b | 9.5 ± 0.9 b | 8.9 ± 0.2 b | 4.3 ± 5.3 fg | |
| HBA2 | 2018 | 6.2 ± 3.9 bc | 6.1 ± 5.1 bc | 5.4 ± 2.3 bc | 3.0 ± 1.3 c | 1.9 ± 0.4 c | - | 2.5 ± 1.0 c | 7.5 ± 5.2 b | 16.9 ± 4.6 a | 4.1 ± 4.8 fg |
| 2019 | 14.8 ± 6.0 a | 9.0 ± 4.5 b | 8.5 ± 6.1 b | 3.5 ± 1.4 b | 4.2 ± 0.9 b | - | 2.9 ± 0.2 b | 5.6 ± 0.3 b | 3.9 ± 1.2 b | 6.5 ± 6.9 fg | |
| HBA3 | 2018 | 11.1 ± 4.8 bc | 13.3 ± 4.9 b | 10.1 ± 9.9 bc | 6.9 ± 4.5 bc | 10.2 ± 7.4 bc | 5.4 ± 1.3 c | 6.9 ± 3.9 bc | 7.1 ± 2.6 bc | 31.9 ± 11.0 a | 10.3 ± 8.4 ef |
| 2019 | 108.3 ± 43.5 a | 78.9 ± 33.7 ab | 36.1 ± 33.6 cd | 13.0 ± 12.4 d | 6.3 ± 1.2 d | 37.2 ± 16.7 cd | 61.8 ± 18.5 bc | 27.9 ± 23.5 d | 38.8 ± 28.1 cd | 51.1 ± 47.6 b | |
| HBA4 | 2018 | 24.6 ± 28.4 b | 18.4 ± 6.4 b | 16.1 ± 11.8 b | 9.2 ± 14.1 b | 13.7 ± 10.1 b | 13.6 ± 13.4 b | 17.6 ± 7.7 b | 18.1 ± 7.9 b | 49.4 ± 14.2 a | 18.3 ± 19.5 d |
| 2019 | 42.7 ± 15.9 a | 19.8 ± 15.6 b | 38.1 ± 14.2 a | 10.6 ± 12.3 b | 12.4 ± 8.1 b | 21.8 ± 3.6 b | 21.1 ± 10.2 b | 23.6 ± 19.7 b | 16.8 ± 10.6 b | 25.7 ± 19.0 c | |
| HBA5 | 2018 | 16.6 ± 5.6 bc | 22.7 ± 5.9 b | 19.4 ± 6.7 bc | 13.5 ± 8.6 cd | 4.1 ± 0.5 e | 13.7 ± 14.9 cd | 5.9 ± 3.8 de | 19.8 ± 8.9 bc | 46.5 ± 14.3 a | 16.8 ± 11.5 de |
| 2019 | 52.2 ± 12.9 a | 37.4 ± 24.0 ab | 40.1 ± 41 ab | 9.4 ± 5.5 cd | 6.5 ± 1.9 d | 19.0 ± 3.4 bcd | 33.0 ± 26.1 abc | 18.3 ± 15.4 bcd | 36.7 ± 31.9 ab | 30.9 ± 27.2 c | |
| HBA6 | 2018 | 4.9 ± 4.3 b | 6.0 ± 5.9 b | 6.0 ± 3.5 b | 2.9 ± 0.9 b | 2.7 ± 1.2 b | 4.5 ± 1.4 b | 4.6 ± 1.6 b | 4.5 ± 1.4 b | 10.6 ± 5.7 a | 4.5 ± 3.8 fg |
| 2019 | 13.4 ± 10.6 ab | 6.2 ± 1.6 bc | 20.2 ± 6.8 a | 5.0 ± 3.4 bc | 6.3 ± 2.2 bc | 6.8 ± 4.0 bc | 6.9 ± 3.5 bc | 4.1 ± 1.0 c | 5.3 ± 3.1 bc | 9.5 ± 8.7 fg | |
| HBA7 | 2018 | 61.7 ± 22.2 b | 64.3 ± 25.8 b | 64.2 ± 33.2 b | 39.7 ± 21.7 bc | 27.5 ± 4.0 c | 33.3 ± 9.2 bc | 39.5 ± 28.5 bc | 52.3 ± 13.2 bc | 142.5 ± 65.7 a | 56.5 ± 36.6 b |
| 2019 | 173.5 ± 59.2 a | 110.5 ± 50.3 b | 109.9 ± 55.3 b | 32.5 ± 24.0 c | 74.2 ± 19.2 bc | 57.9 ± 3.1 c | 61.2 ± 16.1 c | 74.8 ± 18.2 bc | 44.7 ± 21.7 c | 93.6 ± 68.1 a | |
| Hydroxycinnamic acid (μg g−1) | |||||||||||
| HCA1 | 2018 | 5.2 ± 4.4 c | 5.1 ± 2.2 c | 4.5 ± 4.2 c | 9.5 ± 10.3 bc | 18.4 ± 16.3 ab | 14.4 ± 3.7 bc | 13.8 ± 4.9 bc | 10.8 ± 7.2 bc | 27.1 ± 23.0 a | 9.3 ± 10.6 cd |
| 2019 | 14.6 ± 12.8 ab | 9.4 ± 7.8 b | 20.9 ± 13.2 a | 8.2 ± 4.6 b | 12.4 ± 5.2 ab | 7.9 ± 0.7 b | 8.9 ± 5.2 b | 9.0 ± 4.3 b | 9.2 ± 3.3 b | 12.1 ± 10.0 c | |
| HCA2 | 2018 | 6.3 ± 4.3 b | 7.8 ± 4.1 b | 7.1 ± 5.1 b | 7.8 ± 4.9 b | 5.0 ± 1.3 b | 7.0 ± 0.6 b | 5.3 ± 1.5 b | 5.2 ± 0.7 b | 24.9 ± 12.7 a | 7.4 ± 6.7 d |
| 2019 | 17.8 ± 8.0 a | 10.6 ± 8.5 b | 18.1 ± 11.2 a | 4.0 ± 1.9 b | 8.5 ± 1.5 b | 5.6 ± 0.6 b | 4.3 ± 0.8 b | 6.0 ± 1.6 b | 5.8 ± 2.6 b | 10.5 ± 9.0 cd | |
| HCA3 | 2018 | 17.5 ± 7.1 bc | 20.3 ± 9.2 bc | 17.7 ± 9.8 bc | 15.6 ± 10.9 c | 17.0 ± 10.5 bc | 12.8 ± 10.3 c | 26.1 ± 6.2 b | 19.3 ± 8.5 bc | 35.6 ± 10.2 a | 19.0 ± 10.2 b |
| 2019 | 41.0 ± 16.9 a | 25.7 ± 7.1 bc | 28.3 ± 9.9 b | 9.8 ± 6.8 d | 18.9 ± 1.1 bcd | 18.7 ± 8.6 bcd | 19.9 ± 1.9 bcd | 19.0 ± 5.8 bcd | 15.0 ± 6.4 cd | 24.0 ± 15.6 a | |
| HCA4 | 2018 | 17.6 ± 9.6 b | 21.1 ± 13.8 b | 15.8 ± 11.5 b | 15.8 ± 26.2 b | 14.0 ± 6.8 b | 51.5 ± 41.9 a | 55.4 ± 29.2 a | 14.4 ± 13.4 b | 29.6 ± 15.6 b | 20.3 ± 22.0 b |
| 2019 | 33.8 ± 14.2 ab | 29.1 ± 22.6 ab | 24.2 ± 14.0 b | 14.5 ± 16.2 b | 33.8 ± 9.5 ab | 47.8 ± 22.7 a | 33.0 ± 19.0 ab | 21.8 ± 9.3 b | 24.1 ± 30.3 b | 24.3 ± 19.2 a | |
| HCA5 | 2018 | 2.4 ± 0.6 a | 2.6 ± 0.6 a | 2.5 ± 0.6 a | 2.3 ± 0.8 a | 2.0 ± 0.7 a | 2.8 ± 0.9 a | 2.4 ± 0.6 a | 2.4 ± 0.7 a | 3.4 ± 2.6 a | 1.6 ± 1.4 e |
| 2019 | 6.3 ± 2.8 a | 3.9 ± 1.7 ab | 4.1 ± 1.6 ab | 2.6 ± 0.7 b | 1.8 ± 0.2 b | 2.8 ± 0.5 b | 2.3 ± 0.5 b | 2.3 ± 0.4 b | 1.9 ± 0.3 b | 3.1 ± 2.8 e | |
| Flavonoid (μg g−1) | |||||||||||
| FLA1 | 2018 | 1072.3 ± 799.9 ab | 788.1 ± 767.4 abc | 614.6 ± 485.4 bc | 831.3 ± 854.1 abc | 95.7 ± 199.2 c | 973.3 ± 391.0 ab | 1162.0 ± 495.8 ab | 1116.6 ± 411.5 ab | 1485.1 ± 679.5 a | 910.4 ± 736.9 b |
| 2019 | 3166.5 ± 1317.4 a | 2433.9 ± 576.4 ab | 1875.5 ± 816.0 bc | 821.6 ± 710.0 d | 1360.0 ± 348.6 cd | 1915.7 ± 941.4 bc | 1716.7 ± 434.6 bcd | 1368.6 ± 427.3 cd | 1201.0 ± 589.4 cd | 1869.9 ± 1234.9 a | |
| FLA2 | 2018 | 74.4 ± 49.2 bc | 97.5 ± 86.2 bc | 44.7 ± 30.8 bc | 48.5 ± 52.2 bc | 23.2 ± 33.4 c | 55.2 ± 26.4 bc | 129.2 ± 160.2 b | 123.8 ± 104.3 b | 224.0 ± 157.2 a | 78.4 ± 89.4 cd |
| 2019 | 310.1 ± 118.1 a | 229.0 ± 154.8 ab | 112.9 ± 56.2 c | 72.6 ± 68.5 c | 140.7 ± 65.7 bc | 137.7 ± 51.2 bc | 181.4 ± 111.3 bc | 177.3 ± 95.8 bc | 182.2 ± 117.6 bc | 176.6 ± 130.4 c | |
| FLA3 | 2018 | 54.2 ± 33.2 bc | 47.3 ± 30.0 bc | 35.3 ± 21.6 c | 51.5 ± 33.1 bc | 34.4 ± 15.5 c | 57.5 ± 16.3 bc | 71.0 ± 27.6 ab | 57.6 ± 19.7 bc | 100.0 ± 41.2 a | 53.8 ± 32.7 cd |
| 2019 | 161.1 ± 61.7 a | 112.0 ± 35.1 b | 109.3 ± 43.4 b | 46.9 ± 26.9 c | 71.6 ± 10.6 bc | 105.0 ± 45.4 b | 84.1 ± 14.8 bc | 68.8 ± 17.7 bc | 57.6 ± 26.9 c | 97.6 ± 59.2 cd | |
| FLA4 | 2018 | 9.3 ± 6.0 c | 10.5 ± 10.2 c | 13.9 ± 8.5 bc | 23.7 ± 10.7 a | 22.7 ± 1.5 ab | 28.0 ± 0.7 a | 24.1 ± 4.2 a | 22.5 ± 3.9 ab | 31.5 ± 19.1 a | 18.1 ± 11.3 d |
| 2019 | 56.2 ± 28.1 a | 23.2 ± 19.7 b | 41.4 ± 24.1 ab | 28.8 ± 4.2 b | 30.6 ± 2.3 b | 32.0 ± 0.7 b | 30.7 ± 2.3 b | 32.4 ± 3.4 b | 29.7 ± 3.0 b | 38.1 ± 21.1 d | |
| FLA5 | 2018 | 3.7 ± 3.3 b | 2.2 ± 0.7 b | 4.2 ± 2.6 b | 3.6 ± 1.3 b | 2.9 ± 0.4 b | 3.0 ± 0.3 b | 2.8 ± 0.1 b | 3.3 ± 1.4 b | 9.3 ± 5.0 a | 3.5 ± 2.8 d |
| 2019 | 16.2 ± 10.6 a 1 | 12.9 ± 7.1 a | 13.1 ± 7.7 a | 14.3 ± 3.0 a | 16.0 ± 0.6 a | 16.6 ± 0.4 a | 16.5 ± 0.5 a | 13.8 ± 3.4 a | 13.8 ± 3.3 a | 14.7 ± 6.8 d | |
| Average | 2018 | 81.82 ± 58.15 a | 66.93 ± 61.35 a | 52.02 ± 38.18 a | 63.95 ± 62.12 a | 17.48 ± 19.31 a | 79.87 ± 33.29 a | 92.42 ± 48.34 a | 87.63 ± 37.75 a | 133.68 ± 63.65 ab | |
| 2019 | 250.16 ± 108.67 b | 185.84 ± 57.60 b | 147.54 ± 68.34 b | 65.09 ± 53.08 a | 106.64 ± 29.93 b | 152.59 ± 68.98 ab | 134.91 ± 39.16 ab | 110.75 ± 38.12 ab | 99.73 ± 51.72 a | ||
| Principal Components | PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | PC7 | PC8 |
|---|---|---|---|---|---|---|---|---|
| Eigen value | 7.98 | 5.68 | 2.42 | 1.66 | 1.37 | 1.27 | 1.09 | 1.00 |
| % of variance | 25.44 | 18.13 | 7.71 | 5.30 | 4.37 | 4.06 | 3.49 | 3.19 |
| Cumulative % | 25.44 | 43.57 | 51.28 | 56.59 | 60.96 | 65.02 | 68.50 | 71.69 |
| Component matrix | ||||||||
| gallic acid_18 | 0.052 | 0.099 | −0.135 | −0.218 | 0.199 | 0.042 | 0.093 | −0.016 |
| 3,4-dihydroxybenzoic acid_18 | 0.054 | 0.179 | 0.039 | −0.176 | 0.281 | 0.091 | −0.045 | 0.086 |
| 4-hydroxybenzoic acid_18 | 0.065 | 0.299 | 0.035 | −0.112 | 0.261 | −0.051 | 0.204 | −0.179 |
| 2,4-dihydroxybenzoic acid_18 | 0.060 | 0.262 | −0.048 | 0.003 | 0.040 | −0.102 | 0.100 | 0.335 |
| vanillic acid_18 | 0.067 | 0.284 | 0.142 | −0.351 | 0.024 | −0.002 | 0.126 | 0.044 |
| caffeic acid_18 | −0.095 | 0.123 | 0.154 | 0.376 | 0.342 | 0.108 | −0.252 | 0.040 |
| syringic acid_18 | 0.079 | 0.186 | 0.328 | 0.174 | 0.014 | −0.229 | −0.096 | 0.214 |
| p-coumaric acid_18 | −0.025 | 0.247 | 0.236 | 0.105 | 0.291 | 0.177 | −0.077 | 0.045 |
| ferulic acid_18 | 0.056 | 0.296 | 0.031 | 0.212 | −0.080 | 0.058 | 0.118 | −0.419 |
| sinapic acid_18 | −0.011 | 0.115 | −0.102 | 0.519 | −0.038 | 0.018 | 0.295 | −0.259 |
| rutin_18 | 0.085 | 0.285 | −0.215 | 0.109 | −0.309 | −0.028 | −0.041 | 0.280 |
| quercetin-3-β-D-glucoside_18 | 0.041 | 0.283 | −0.199 | −0.187 | −0.210 | 0.037 | −0.129 | −0.012 |
| benzoic acid_18 | 0.113 | 0.282 | 0.175 | 0.000 | 0.048 | 0.030 | 0.095 | 0.018 |
| kaempferol-3-O-β-rutinoside_18 | 0.039 | 0.323 | −0.186 | 0.171 | −0.228 | −0.054 | −0.129 | 0.153 |
| quercetin_18 | −0.149 | 0.201 | −0.061 | −0.030 | −0.259 | 0.319 | −0.185 | −0.160 |
| cinnamic acid_18 | 0.026 | 0.139 | −0.056 | −0.078 | −0.169 | 0.073 | −0.046 | −0.351 |
| kaempferol_18 | 0.020 | 0.212 | 0.263 | −0.166 | 0.018 | −0.055 | −0.291 | −0.060 |
| gallic acid_19 | −0.004 | −0.025 | −0.036 | −0.014 | −0.049 | 0.104 | 0.122 | 0.280 |
| 3,4-dihydroxybenzoic acid_19 | 0.185 | −0.073 | −0.144 | 0.000 | 0.106 | 0.069 | 0.152 | 0.058 |
| 4-hydroxybenzoic acid_19 | 0.261 | 0.051 | −0.261 | 0.037 | 0.173 | −0.119 | 0.149 | 0.130 |
| 2,4-dihydroxybenzoic acid_19 | 0.249 | −0.035 | 0.083 | −0.015 | −0.070 | −0.087 | 0.091 | 0.099 |
| vanillic acid_19 | 0.234 | 0.043 | −0.080 | −0.070 | 0.165 | −0.121 | 0.349 | −0.225 |
| caffeic acid_19 | 0.115 | −0.044 | 0.421 | −0.005 | −0.340 | 0.040 | 0.182 | 0.025 |
| syringic acid_19 | 0.178 | −0.065 | 0.303 | −0.047 | −0.225 | 0.124 | 0.291 | −0.036 |
| p-coumaric acid_19 | 0.251 | −0.059 | 0.284 | −0.050 | −0.160 | −0.066 | −0.068 | 0.024 |
| ferulic acid_19 | 0.309 | −0.075 | −0.016 | 0.025 | 0.049 | −0.077 | −0.074 | −0.069 |
| sinapic acid_19 | 0.109 | −0.057 | 0.101 | 0.355 | 0.058 | 0.072 | 0.002 | 0.094 |
| rutin_19 | 0.306 | −0.026 | −0.089 | 0.133 | −0.073 | −0.090 | −0.144 | 0.040 |
| quercetin-3-β-D-glucoside_19 | 0.265 | 0.032 | −0.219 | −0.055 | −0.003 | 0.006 | −0.198 | −0.059 |
| benzoic acid_19 | 0.326 | −0.063 | −0.042 | −0.010 | 0.022 | 0.042 | −0.163 | −0.066 |
| kaempferol-3-O-β-rutinoside_19 | 0.325 | −0.056 | −0.036 | 0.124 | −0.047 | −0.002 | −0.114 | −0.006 |
| quercetin_19 | 0.252 | −0.085 | 0.029 | −0.059 | 0.037 | 0.322 | −0.274 | −0.198 |
| cinnamic acid_19 | 0.201 | −0.107 | 0.043 | −0.004 | 0.169 | 0.246 | −0.147 | 0.022 |
| kaempferol_19 | 0.050 | 0.009 | −0.069 | 0.016 | −0.019 | 0.709 | 0.251 | 0.274 |
| Phenolic Compounds | Group 1 | Group 2 | Group 3 | Group 4 | Group 5 | Group 6 |
|---|---|---|---|---|---|---|
| Hydroxybenzoic acid (μg g−1) | ||||||
| HBA1 | 1.7 ± 4.6 a 1 | 1.2 ± 1.9 bc | 2.4 ± 3.3 a | 4.2 ± 3.7 d | 4.0 ± 5.7 ab | 4.1 ± 3.6 cd |
| HBA2 | 5.1 ± 4.0 c | 11.4 ± 7.2 b | 8.3 ± 6.5 a | 1.8 ± 2.0 c | 6.5 ± 6.7 bc | 1.9 ± 3.0 bc |
| HBA3 | 27.4 ± 33.9 b | 64.2 ± 58.8 b | 56.0 ± 50.1 a | 7.3 ± 4.9 b | 22.0 ± 22.0 b | 25.0 ± 25.0 b |
| HBA4 | 31.2 ± 18.3 b | 26.1 ± 16.7 b | 37.8 ± 28.3 a | 7.5 ± 6.6 b | 22.3 ± 16.1 b | 19.1 ± 13.8 b |
| HBA5 | 29.6 ± 13.9 b | 36.7 ± 23.0 bc | 40.3 ± 33.4 a | 9.8 ± 7.4 d | 22.3 ± 13.7 bc | 17.1 ± 17.4 cd |
| HBA6 | 15.7 ± 11.6 a | 7.1 ± 6.9 b | 7.5 ± 5.6 a | 3.3 ± 2.1 b | 7.8 ± 7.2 b | 5.1 ± 3.4 b |
| HBA7 | 115.2 ± 50.0 b | 133.9 ± 84.2 bc | 98.0 ± 51.9 a | 34.4 ± 23.0 d | 60.0 ± 31.2 cd | 52.8 ± 22.2 cd |
| Hydroxycinnamic acid (μg g−1) | ||||||
| HCA1 | 16.4 ± 15.0 b | 8.1 ± 6.8 b | 10.2 ± 13.4 a | 10.6 ± 9.4 ab | 10.0 ± 10.3 b | 10.03 ± 5.9 ab |
| HCA2 | 16.8 ± 12.1 b | 11.3 ± 7.5 bc | 11.8 ± 10.3 a | 5.6 ± 4.1 bc | 7.3 ± 5.8 c | 5.8 ± 2.5 bc |
| HCA3 | 28.5 ± 9.2 b | 32.3 ± 21.5 bc | 25.9 ± 9.9 a | 12.3 ± 8.7 c | 18.2 ± 8.9 c | 20.1 ± 8.6 b |
| HCA4 | 25.6 ± 11.7 bc | 25.6 ± 15.8 c | 27.6 ± 17.6 ab | 10.4 ± 15.7 c | 17.5 ± 14.7 c | 32.8 ± 30.5 a |
| HCA5 | 3.7 ± 2.4 a | 4.5 ± 3.8 a | 2.6 ± 2.2 a | 0.9 ± 1.2 a | 1.6 ± 1.3 a | 2.1 ± 1.0 a |
| Flavonoid (μg g−1) | ||||||
| FLA1 | 1931.1 ± 1483.9 b | 2115.2 ± 1579.9 bc | 1785.0 ± 812.5 a | 553.5 ± 535.4 d | 979.7 ± 788.4 cd | 1609.9 ± 599.1 a |
| FLA2 | 108.5 ± 78.9 b | 221.0 ± 174.1 b | 191.0 ± 107.9 a | 43.6 ± 49.5 b | 93.0 ± 98.3 b | 154.7 ± 109.5 a |
| FLA3 | 103.6 ± 73.3 b | 112.6 ± 77.8 b | 92.3 ± 37.7 a | 41.9 ± 23.9 b | 53.0 ± 33.2 b | 79.7 ± 24.3 a |
| FLA4 | 36.7 ± 27.2 cd | 41.6 ± 34.5 d | 23.7 ± 16.5 bc | 24.8 ± 4.9 ab | 17.4 ± 11.1 d | 29.4 ± 8.5 a |
| FLA5 | 9.3 ± 7.2 a | 9.9 ± 11.6 b | 10.7 ± 8.0 a | 8.8 ± 6.2 b | 7.0 ± 6.5 b | 9.3 ± 6.4 b |
| Type of Polyphenol | Genotype | Year | Genotype × Year |
|---|---|---|---|
| gallic acid | 1.958 *** | 8.907 *** | 1.484 *** |
| 3,4-dihydroxybenzoic acid | 10.93 *** | 9.652 *** | 6.756 *** |
| 4-hydroxybenzoic acid | 326.4 *** | 2996.4 *** | 293.1 *** |
| 2,4-dihydroxybenzic acid | 78.37 *** | 98.77 *** | 41.32 *** |
| vanillic acid | 104.8 *** | 356.7 *** | 63.9 *** |
| syringic acid | 9.53 *** | 45.32 *** | 7.18 *** |
| benzoic acid | 910.6 *** | 2483.8 *** | 686.2 *** |
| caffeic acid | 6.228 *** | 14.131 *** | 18.443 *** |
| p-coumaric acid | 9.933 *** | 17.154 *** | 17.368 *** |
| ferulic acid | 34.32 *** | 45.07 *** | 39.23 *** |
| sinapic acid | 102.93 *** | 30.22 * | 33.32 *** |
| cinnamic acid | 0.885 *** | 3.742 *** | 1.163 *** |
| rutin | 221,815 *** | 1,656,986 *** | 168,219 *** |
| quercetin-3-β-D-glucoside | 2878 *** | 17,369 *** | 1785 *** |
| kaempferol-3-O-β-rutinoside | 431 *** | 3445 *** | 532 *** |
| quercetin | 13 *** | 716.8 *** | 72 *** |
| kaempferol | 0.71 * | 222.69 *** | 1.17 *** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bang, J.-H.; Jo, I.-H.; Sebastin, R.; Jeong, W.T.; Oh, S.; Heo, T.-Y.; Sung, J.; Hyun, T.k.; So, Y.-S.; Yu, J.-K.; et al. Comparative Analysis of Polyphenolic Compounds in Different Amaranthus Species: Influence of Genotypes and Harvesting Year. Antioxidants 2024, 13, 501. https://doi.org/10.3390/antiox13040501
Bang J-H, Jo I-H, Sebastin R, Jeong WT, Oh S, Heo T-Y, Sung J, Hyun Tk, So Y-S, Yu J-K, et al. Comparative Analysis of Polyphenolic Compounds in Different Amaranthus Species: Influence of Genotypes and Harvesting Year. Antioxidants. 2024; 13(4):501. https://doi.org/10.3390/antiox13040501
Chicago/Turabian StyleBang, Jun-Hyoung, Ick-Hyun Jo, Raveendar Sebastin, Won Tea Jeong, Sangtaek Oh, Tae-Young Heo, Jeehye Sung, Tae kyung Hyun, Yoon-Sup So, Ju-Kyung Yu, and et al. 2024. "Comparative Analysis of Polyphenolic Compounds in Different Amaranthus Species: Influence of Genotypes and Harvesting Year" Antioxidants 13, no. 4: 501. https://doi.org/10.3390/antiox13040501





