Inhibiting AGS Cancer Cell Proliferation through the Combined Application of Aucklandiae Radix and Hyperthermia: Investigating the Roles of Heat Shock Proteins and Reactive Oxygen Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of AR Extract
2.2. Liquid Chromatography (LC)–Mass Spectrometry (MS) Analysis
2.3. Cell Culture
2.4. HT Treatment
2.5. MTT Assay
2.6. Trypan Blue Assay
2.7. Morphology Assay
2.8. Wound Healing Assay
2.9. Colony Formation Assay
2.10. Western Blot Analysis
2.11. Apoptosis Assay
2.12. Cell Cycle Analysis
2.13. Analysis of ROS
2.14. Statistical Analysis
3. Results
3.1. Chemical Components of AR Identified by UPLC-ESI-QTOF-MS/MS
3.2. Co-Treatment with AR and 43 °C HT Synergistically Inhibited AGS Cell Proliferation
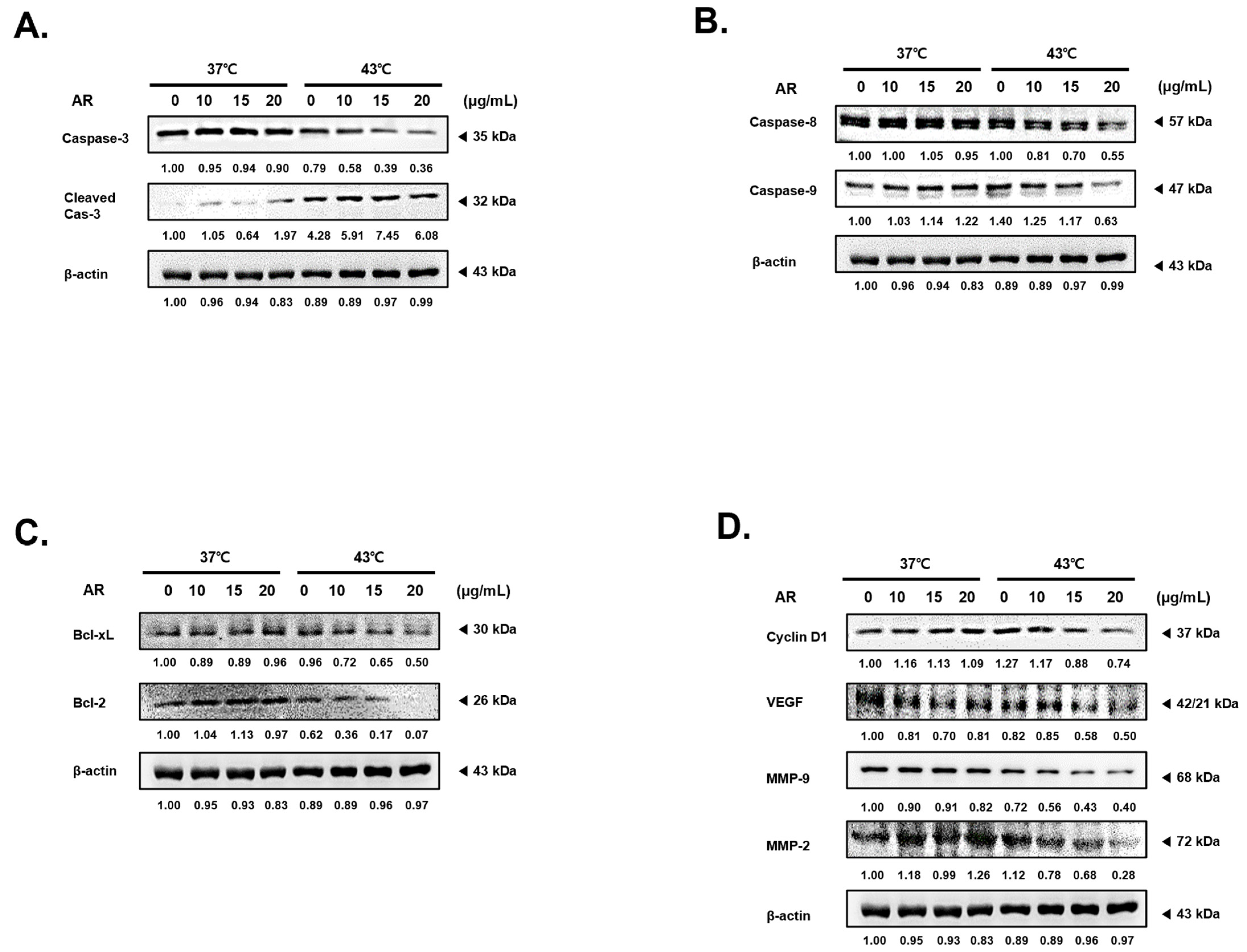
3.3. The Combined Application of AR and 43 °C HT Enhanced the Manifestation of Factors Associated with Apoptosis and Suppressed Factors Related to Protection and Proliferation in AGS Cells
3.4. The Combined Application of AR and 43 °C HT Induced Apoptosis and Cell Cycle Arrest in AGS Cells
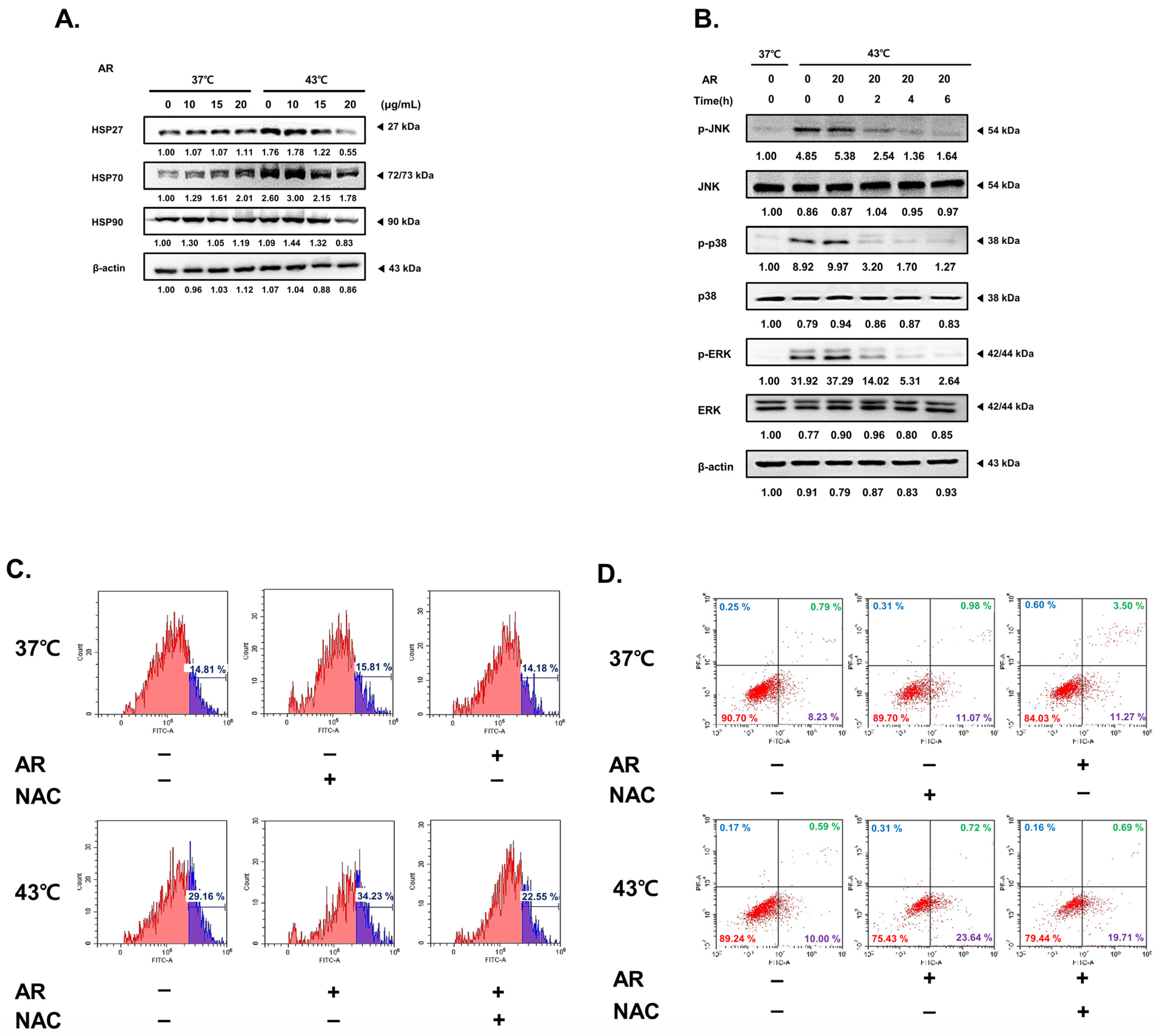
3.5. The Combined Application of AR and 43 °C HT Synergistically Inhibited HSP Expression, Leading to ROS-Dependent Apoptosis
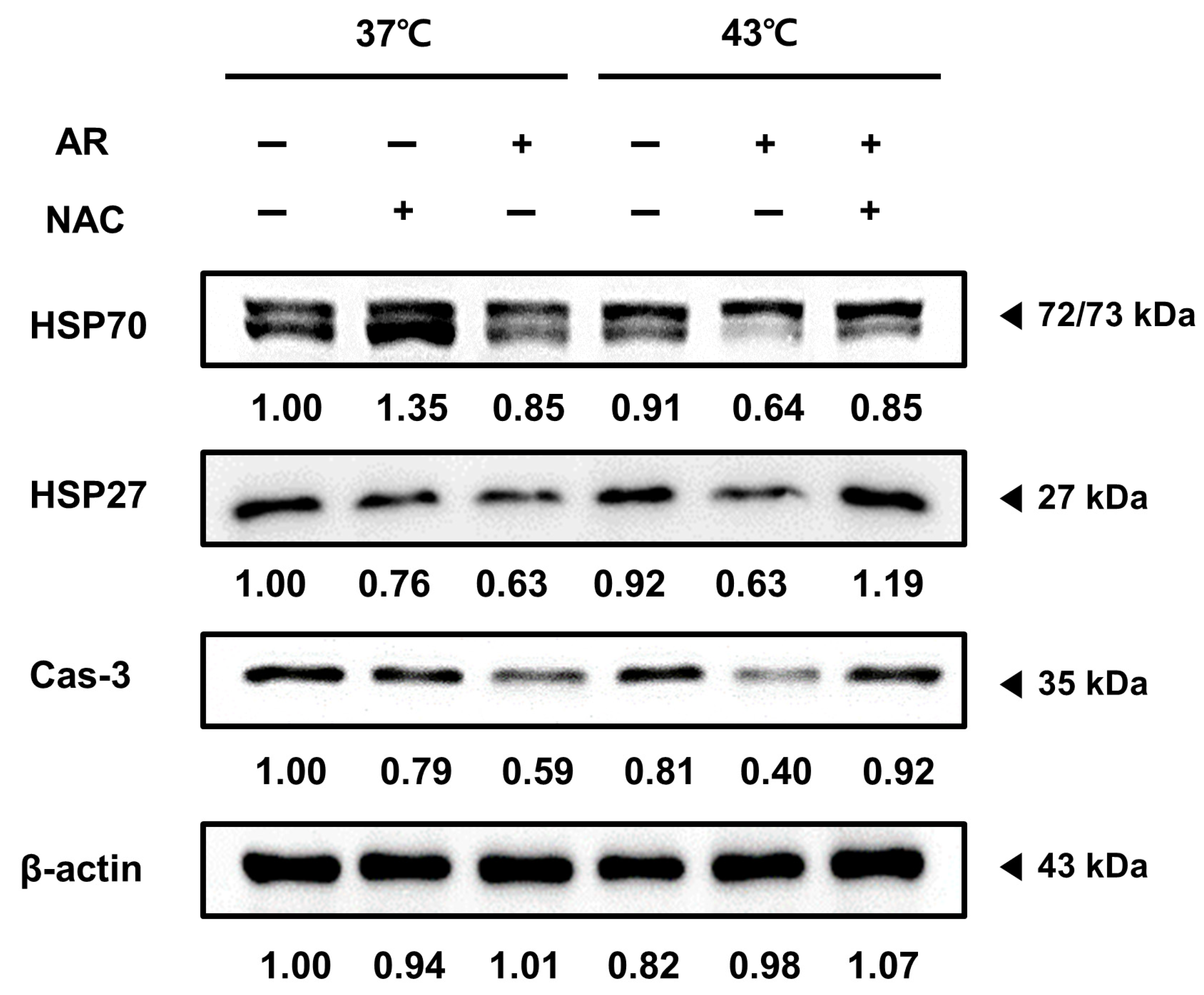
3.6. ROS Scavenging Inhibited the Apoptotic Effect of the Combined AR and HT Treatment and Recovered HSP Expression
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef]
- Mellman, I.; Coukos, G.; Dranoff, G. Cancer immunotherapy comes of age. Nature 2011, 480, 480–489. [Google Scholar] [CrossRef]
- DeVita, V.T., Jr.; Chu, E. A History of cancer chemotherapy. Cancer Res. 2008, 68, 8643–8653. [Google Scholar] [CrossRef]
- Formenti, S.C.; Demaria, S. Combining radiotherapy and cancer immunotherapy: A paradigm shift. JNCI J. Natl. Cancer Inst. 2013, 105, 256–265. [Google Scholar] [CrossRef]
- Kwon, S.; Jung, S.; Baek, S.H. Combination Therapy of Radiation and Hyperthermia, Focusing on the Synergistic Anti-Cancer Effects and Research Trends. Antioxidants 2023, 12, 924. [Google Scholar] [CrossRef]
- Marino, C.; Cividalli, A. Combined radiation and hyperthermia: Effects of the number of heat fractions and their interval on normal and tumour tissues. Int. J. Hyperth. 1992, 8, 771–781. [Google Scholar] [CrossRef]
- Ahn, C.R.; Kim, H.I.; Kim, J.-E.; Ha, I.J.; Ahn, K.S.; Park, J.; Kim, Y.W.; Baek, S.H. Ponciri Fructus Immatarus Sensitizes the Apoptotic Effect of Hyperthermia Treatment in AGS Gastric Cancer Cells through ROS-Dependent HSP Suppression. Biomedicines 2023, 11, 405. [Google Scholar] [CrossRef]
- van der Zee, J. Heating the patient: A promising approach? Ann. Oncol. 2002, 13, 1173–1184. [Google Scholar] [CrossRef]
- Yi, G.Y.; Kim, M.J.; Kim, H.I.; Park, J.; Baek, S.H. Hyperthermia Treatment as a Promising Anti-Cancer Strategy: Therapeutic Targets, Perspective Mechanisms and Synergistic Combinations in Experimental Approaches. Antioxidants 2022, 11, 625. [Google Scholar] [CrossRef]
- Zhuang, K.; Xia, Q.; Zhang, S.; Maharajan, K.; Liu, K.; Zhang, Y. A comprehensive chemical and pharmacological review of three confusable Chinese herbal medicine-Aucklandiae radix, Vladimiriae radix, and Inulae radix. Phytother. Res. 2021, 35, 6655–6689. [Google Scholar] [CrossRef]
- Feng, L.; A, L.; Li, H.; Mu, X.; Ta, N.; Bai, L.; Fu, M.; Chen, Y. Pharmacological Mechanism of Aucklandiae Radix against Gastric Ulcer Based on Network Pharmacology and In Vivo Experiment. Medicina 2023, 59, 666. [Google Scholar] [CrossRef]
- Song, S.; Zhou, J.; Li, Y.; Liu, J.; Li, J.; Shu, P. Network pharmacology and experimental verification based research into the effect and mechanism of Aucklandiae Ra-dix-Amomi Fructus against gastric cancer. Sci. Rep. 2022, 12, 9401. [Google Scholar] [CrossRef]
- Shum, K.; Chen, F.; Li, S.; Wang, J.; But, P.P.; Shaw, P. Authentication of Radix Aucklandiae and its substitutes by GC-MS and hierarchical clustering analysis. J. Sep. Sci. 2007, 30, 3233–3239. [Google Scholar] [CrossRef]
- Cai, X.; Yang, C.; Qin, G.; Zhang, M.; Bi, Y.; Qiu, X.; Lu, L.; Chen, H. Antimicrobial Effects and Active Compounds of the Root of Aucklandia Lappa Decne (Radix Aucklandiae). Front. Chem. 2022, 10, 872480. [Google Scholar] [CrossRef]
- Chen, Z.; Wei, C.; Yu, Z.; Yang, K.; Huang, Z.; Hu, H.; Wang, Z.-G. An effective method for preventing cholestatic liver injury of Aucklandiae Radix and Vladimiriae Radix: Inflammation suppression and regulate the expression of bile acid receptors. J. Ethnopharmacol. 2022, 294, 115330. [Google Scholar] [CrossRef]
- Huang, Z.; Wei, C.; Yang, K.; Yu, Z.; Wang, Z.; Hu, H. Aucklandiae Radix and Vladimiriae Radix: A systematic review in ethnopharmacology, phytochemistry and pharmacology. J. Ethnopharmacol. 2021, 280, 114372. [Google Scholar] [CrossRef]
- Chen, Y.; Miao, Z.; Sheng, X.; Li, X.; Ma, J.; Xu, X.; Li, H.; Kang, A. Sesquiterpene lactones-rich fraction from Aucklandia lappa Decne. alleviates dextran sulfate sodium induced ulcerative colitis through co-regulating MAPK and Nrf2/Hmox-1 signaling pathway. J. Ethnopharmacol. 2022, 295, 115401. [Google Scholar] [CrossRef]
- Lin, X.; Peng, Z.; Su, C. Potential anti-cancer activities and mechanisms of costunolide and dehydrocostuslactone. Int. J. Mol. Sci. 2015, 16, 10888–10906. [Google Scholar] [CrossRef] [PubMed]
- El-Far, A.H.; Godugu, K.; Salaheldin, T.A.; Darwish, N.H.E.; Saddiq, A.A.; Mousa, S.A. Nanonutraceuticals: Anti-Cancer Activity and Improved Safety of Chemotherapy by Costunolide and Its Nanoformulation against Colon and Breast Cancer. Biomedicines 2021, 9, 990. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Choi, Y.K.; Ko, S.-G.; Cheon, C.; Kim, T.Y. Investigation of Molecular Mechanisms Involved in Sensitivity to the Anti-Cancer Activity of Costunolide in Breast Cancer Cells. Int. J. Mol. Sci. 2023, 24, 4009. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Cho, Y.-C.; Lim, J.S. Costunolide, a Sesquiterpene Lactone, Suppresses Skin Cancer via Induction of Apoptosis and Blockage of Cell Proliferation. Int. J. Mol. Sci. 2021, 22, 2075. [Google Scholar] [CrossRef]
- Li, Q.; Wang, Z.; Xie, Y.; Hu, H. Antitumor activity and mechanism of costunolide and dehydrocostus lactone: Two natural sesquiterpene lactones from the Asteraceae family. Biomed. Pharmacother. 2020, 125, 109955. [Google Scholar] [CrossRef] [PubMed]
- Trachootham, D.; Alexandre, J.; Huang, P. Targeting cancer cells by ROS-mediated mechanisms: A radical therapeutic approach? Nat. Rev. Drug Discov. 2009, 8, 579–591. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.-S. The signaling mechanism of ROS in tumor progression. Cancer Metastasis Rev. 2006, 25, 695–705. [Google Scholar] [CrossRef] [PubMed]
- He, M.; Wang, M.; Xu, T.; Zhang, M.; Dai, H.; Wang, C.; Ding, D.; Zhong, Z. Reactive oxygen species-powered cancer immunotherapy: Current status and challenges. J. Control. Release 2023, 356, 623–648. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Lu, T.; Wang, G.-D.; Ma, C.; Zhou, Y.-F. Costunolide, an active sesquiterpene lactone, induced apoptosis via ROS-mediated ER stress and JNK pathway in human U2OS cells. Biomed. Pharmacother. 2016, 80, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Issels, R.D. Hyperthermia adds to chemotherapy. Eur. J. Cancer 2008, 44, 2546–2554. [Google Scholar] [CrossRef]
- Reinhold, H.S.; Endrich, B. Tumour microcirculation as a target for hyperthermia. Int. J. Hyperth. 1986, 2, 111–137. [Google Scholar] [CrossRef]
- Mallory, M.; Gogineni, E.; Jones, G.C.; Greer, L.; Simone, C.B. Therapeutic hyperthermia: The old, the new, and the upcoming. Crit. Rev. Oncol. Hematol. 2016, 97, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Song, C.W.; Shakil, A.; Osborn, J.L.; Iwata, K. Tumour oxygenation is increased by hyperthermia at mild temperatures. Int. J. Hyperth. 2009, 25, 91–95. [Google Scholar] [CrossRef]
- Chatterjee, S.; Burns, T.F. Targeting Heat Shock Proteins in Cancer: A Promising Therapeutic Approach. Int. J. Mol. Sci. 2017, 18, 1978. [Google Scholar] [CrossRef] [PubMed]
- Gümüş, M.; Koca, I.; Sert, Y.; Dişli, A.; Tunoğlu, E.N.Y.; Tutar, L.; Tutar, Y. Triad pyrazole–thiazole–coumarin heterocyclic core effectively inhibit HSP and drive cancer cells to apoptosis. J. Biomol. Struct. Dyn. 2023, 41, 14382–14397. [Google Scholar] [CrossRef]
- Wu, J.; Liu, T.; Rios, Z.; Mei, Q.; Lin, X.; Cao, S. Heat Shock Proteins and Cancer. Trends Pharmacol. Sci. 2017, 38, 226–256. [Google Scholar] [CrossRef]
- Ikwegbue, P.C.; Masamba, P.; Oyinloye, B.E.; Kappo, A.P. Roles of Heat Shock Proteins in Apoptosis, Oxidative Stress, Human Inflammatory Diseases, and Cancer. Pharmaceuticals 2018, 11, 2. [Google Scholar] [CrossRef] [PubMed]
- Rosidi, B.; Priyatno, D.; Putra, T.P.; Yusuf, I. Metformin Induces a Caspase 3-Unrelated Apoptosis in Human Colorectal Cancer Cell Lines HCT116 and SW620. Cancer Manag. Res. 2023, 15, 475–485. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, D.; Wang, X.; Wang, Y.; Ren, F.; Chang, D.; Chang, Z.; Jia, B. Caspase 3 is Activated through caspase 8 instead of caspase 9 during H2O2-induced apoptosis in HeLa cells. Cell. Physiol. Biochem. 2011, 27, 539–546. [Google Scholar] [CrossRef]
- Kale, J.; Osterlund, E.J.; Andrews, D.W. BCL-2 family proteins: Changing partners in the dance towards death. Cell Death Differ. 2018, 25, 65–80. [Google Scholar] [CrossRef]
- Yang, F.; Wang, Y.-H.; Dong, S.-Y.; Chen, C.-Z.; Huang, D.-P. MLF1IP promotes cells proliferation and apoptosis by regulating CyclinD1 in breast cancer. Int. J. Clin. Exp. Pathol. 2017, 10, 11554–11562. [Google Scholar]
- Ferrara, N. VEGF as a therapeutic target in cancer. Oncology 2005, 69 (Suppl. S3), 11–16. [Google Scholar] [CrossRef] [PubMed]
- John, A.; Tuszynski, G. The role of matrix metalloproteinases in tumor angiogenesis and tumor metastasis. Pathol. Oncol. Res. 2001, 7, 14–23. [Google Scholar] [CrossRef]
- Shah, M.A.; Abuzar, S.M.; Ilyas, K.; Qadees, I.; Bilal, M.; Yousaf, R.; Kassim, R.M.T.; Rasul, A.; Saleem, U.; Alves, M.S.; et al. Ginsenosides in cancer: Targeting cell cycle arrest and apoptosis. Chem. Interactions 2023, 382, 110634. [Google Scholar] [CrossRef] [PubMed]
- Shapiro, G.I.; Harper, J.W. Anticancer drug targets: Cell cycle and checkpoint control. J. Clin. Investig. 1999, 104, 1645–1653. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.H.; El-Naggar, A.K.; Priebe, W.; Perez-Soler, R. Cell cycle-dependent cytotoxicity, G2/M phase arrest, and disruption of p34cdc2/cyclin B1 activity induced by dox-orubicin in synchronized P388 cells. Mol. Pharmacol. 1996, 49, 832–841. [Google Scholar]
- Liang, H.-H.; Huang, C.-Y.; Chou, C.-W.; Makondi, P.T.; Huang, M.-T.; Wei, P.-L.; Chang, Y.-J. Heat shock protein 27 influences the anti-cancer effect of curcumin in colon cancer cells through ROS production and autophagy activation. Life Sci. 2018, 209, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.H.; Kim, C.; Lee, J.H.; Nam, D.; Lee, J.; Lee, S.-G.; Chung, W.-S.; Jang, H.-J.; Kim, S.-H.; Ahn, K.S. Cinobufagin exerts anti-proliferative and pro-apoptotic effects through the modulation ROS-mediated MAPKs signaling pathway. Immunopharmacol. Immunotoxicol. 2015, 37, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Wang, Y.; Fan, J.; Lin, X.; Liu, C.; Xu, Y.; Ji, W.; Yan, C.; Su, C. Costunolide and dehydrocostuslactone combination treatment inhibit breast cancer by inducing cell cycle arrest and apoptosis through c-Myc/p53 and AKT/14-3-3 pathway. Sci. Rep. 2017, 7, 41254. [Google Scholar] [CrossRef]
- Ahn; Choi, E.J.; Ahn, W.S. Antiproliferative effects of dehydrocostuslactone through cell cycle arrest and apoptosis in human ovarian cancer SK-OV-3 cells. Int. J. Mol. Med. 2009, 23, 211–216. [Google Scholar] [CrossRef]
- Huang, H.; Yi, J.-K.; Lim, S.-G.; Park, S.; Zhang, H.; Kim, E.; Jang, S.; Lee, M.-H.; Liu, K.; Kim, K.-R.; et al. Costunolide Induces Apoptosis via the Reactive Oxygen Species and Protein Kinase B Pathway in Oral Cancer Cells. Int. J. Mol. Sci. 2021, 22, 7509. [Google Scholar] [CrossRef]
- Butturini, E.; Di Paola, R.; Suzuki, H.; Paterniti, I.; Ahmad, A.; Mariotto, S.; Cuzzocrea, S. Costunolide and Dehydrocostuslactone, two natural sesquiterpene lactones, ameliorate the inflammatory process associated to experimental pleurisy in mice. Eur. J. Pharmacol. 2014, 730, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Li, J.; Qiu, J.; Yan, Y.; Wang, H.; Wu, Z.; Liu, Y.; Shen, X.; Su, C.; Guo, Q.; et al. Costunolide induces apoptosis and inhibits migration and invasion in H1299 lung cancer cells. Oncol. Rep. 2020, 43, 1986–1994. [Google Scholar] [CrossRef] [PubMed]
- Hua, P.; Sun, M.; Zhang, G.; Zhang, Y.; Song, G.; Liu, Z.; Li, X.; Zhang, X.; Li, B. Costunolide Induces Apoptosis through Generation of ROS and Activation of P53 in Human Esophageal Cancer Eca-109 Cells. J. Biochem. Mol. Toxicol. 2016, 30, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.; Chen, Y.; Wang, J.; Chen, Y.; Zhang, M.; Huang, J.; Wang, Y. Costunolide alleviates hyperglycaemia-induced diabetic cardiomyopathy via inhibiting inflammatory responses and oxidative stress. J. Cell. Mol. Med. 2023, 27, 831–845. [Google Scholar] [CrossRef]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of oxidative stress as an anticancer strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Malhotra, K.; Malik, A.; Almalki, W.H.; Kesharwani, P. Reactive oxygen species and its manipulation strategies in cancer treatment. Curr. Med. Chem. 2023. [Google Scholar] [CrossRef] [PubMed]
- Ku, J.M.; Kim, M.J.; Choi, Y.-J.; Lee, S.Y.; Im, J.-Y.; Jo, Y.-K.; Yoon, S.; Kim, J.-H.; Cha, J.W.; Shin, Y.C.; et al. JI017 Induces Cell Autophagy and Apoptosis via Elevated Levels of Reactive Oxygen Species in Human Lung Cancer Cells. Int. J. Mol. Sci. 2023, 24, 7528. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.G.; Jänicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Vishnu, W.K.; Abeesh, P.; Guruvayoorappan, C. Pyrazole (1, 2-diazole) induce apoptosis in lymphoma cells by targeting BCL-2 and BCL-XL genes and mitigate murine solid tumour development by regulating cyclin-D1 and Ki-67 expression. Toxicol. Appl. Pharmacol. 2021, 418, 115491. [Google Scholar] [CrossRef]
- Behelgardi, M.F.; Shahvir, Z.G.; Asghari, S.M. Apoptosis induction in human lung and colon cancer cells via impeding VEGF signaling pathways. Mol. Biol. Rep. 2022, 49, 3637–3647. [Google Scholar] [CrossRef]
- Hosseini, S.; Chamani, J.; Hadipanah, M.R.; Ebadpour, N.; Hojjati, A.S.; Mohammadzadeh, M.H.; Rahimi, H.R. Nano-curcumin’s suppression of breast cancer cells (MCF7) through the inhibition of cyclinD1 expression. Breast Cancer Targets Ther. 2019, 11, 137–142. [Google Scholar] [CrossRef]
- Choi, E.K.; Kim, H.D.; Park, E.J.; Song, S.Y.; Phan, T.T.; Nam, M.; Kim, M.; Kim, D.-U.; Hoe, K.-L. 8-Methoxypsoralen Induces Apoptosis by Upregulating p53 and Inhibits Metastasis by Downregulating MMP-2 and MMP-9 in Human Gastric Cancer Cells. Biomol. Ther. 2023, 31, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Mi, C.; Shi, H.; Ma, J.; Han, L.Z.; Lee, J.J.; Jin, X. Celastrol induces the apoptosis of breast cancer cells and inhibits their invasion via downregulation of MMP-9. Oncol. Rep. 2014, 32, 2527–2532. [Google Scholar] [CrossRef] [PubMed]
- Ahn, C.R.; Park, J.; Kim, J.-E.; Ahn, K.S.; Kim, Y.W.; Jeong, M.; Kim, H.J.; Park, S.H.; Baek, S.H. Cinnamaldehyde and Hyperthermia Co-Treatment Synergistically Induces ROS-Mediated Apoptosis in ACHN Renal Cell Carcinoma Cells. Biomedicines 2020, 8, 357. [Google Scholar] [CrossRef] [PubMed]
- Singhal, S.; Vachani, A.; Antin-Ozerkis, D.; Kaiser, L.R.; Albelda, S.M. Prognostic implications of cell cycle, apoptosis, and angiogenesis biomarkers in non-small cell lung cancer: A review. Clin. Cancer Res. 2005, 11, 3974–3986. [Google Scholar] [CrossRef] [PubMed]
- Rasul, A.; Bao, R.; Malhi, M.; Zhao, B.; Tsuji, I.; Li, J.; Li, X. Induction of apoptosis by costunolide in bladder cancer cells is mediated through ROS generation and mitochondrial dysfunction. Molecules 2013, 18, 1418–1433. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhao, X.; Gong, X. Costunolide induces lung adenocarcinoma cell line A549 cells apoptosis through ROS (reactive oxygen species)-mediated endoplasmic reticulum stress. Cell Biol. Int. 2016, 40, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-I.; Kim, J.-H.; Lee, K.-T.; Choi, J.-H. Costunolide induces apoptosis in platinum-resistant human ovarian cancer cells by generating reactive oxygen species. Gynecol. Oncol. 2011, 123, 588–596. [Google Scholar] [CrossRef] [PubMed]
- Hung, J.-Y.; Hsu, Y.-L.; Ni, W.-C.; Tsai, Y.-M.; Yang, C.-J.; Kuo, P.-L.; Huang, M.-S. Oxidative and endoplasmic reticulum stress signaling are involved in dehydrocostuslactone-mediated apoptosis in human non-small cell lung cancer cells. Lung Cancer 2010, 68, 355–365. [Google Scholar] [CrossRef]
- Peng, Y.; Zhou, T.; Wang, S.; Bahetjan, Y.; Li, X.; Yang, X. Dehydrocostus lactone inhibits the proliferation of esophageal cancer cells in vivo and in vitro through ROS-mediated apoptosis and autophagy. Food Chem. Toxicol. 2022, 170, 113453. [Google Scholar] [CrossRef]
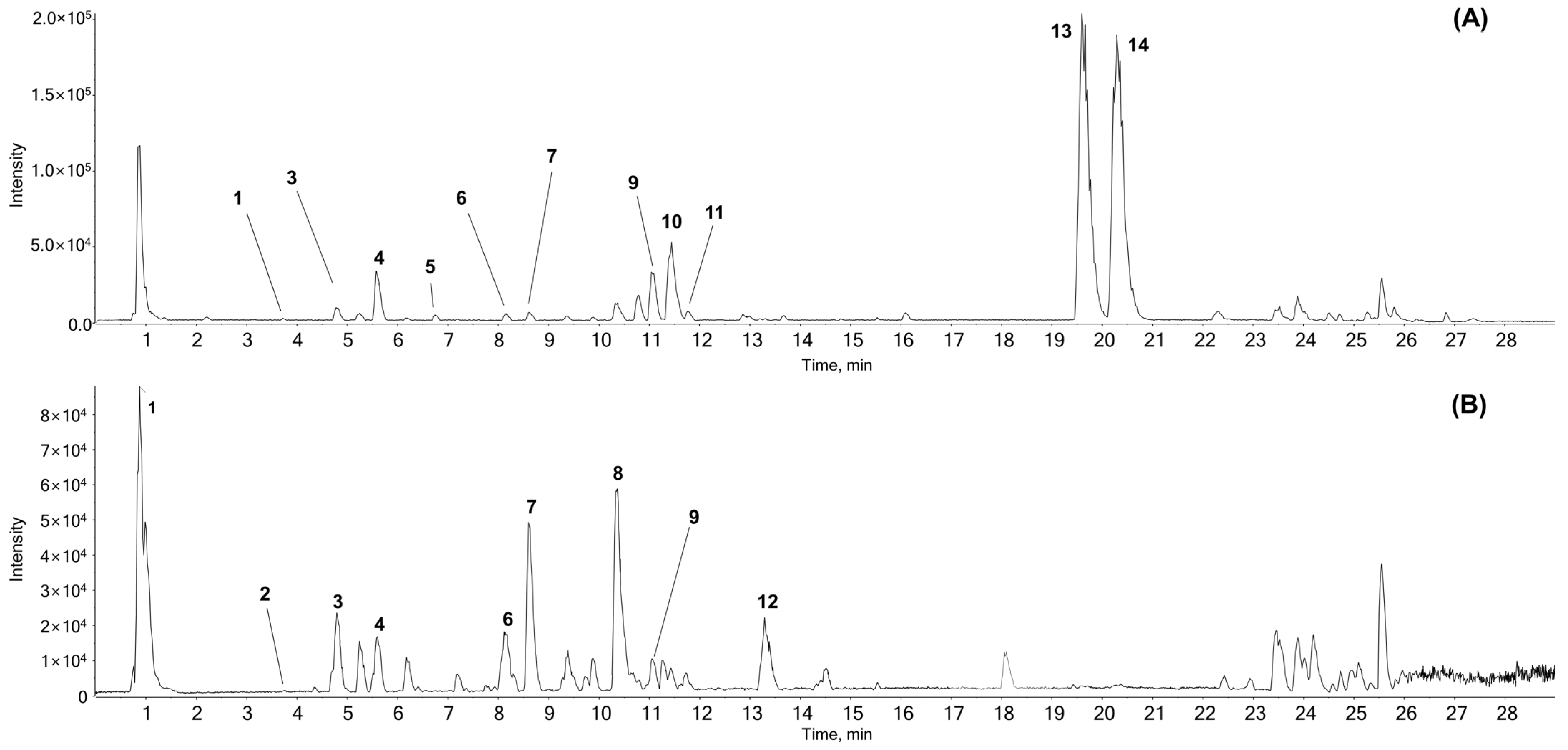



| No. | Name | Formula | Mass (Da) | Expected | Adduct | Found at | Error | MS/MS Product ions | Identified with |
|---|---|---|---|---|---|---|---|---|---|
| RT (min) | Mass (Da) | (ppm) | |||||||
| 1 | Citric acid | C6H8O7 | 192.027 | 1.05 | [M − H]− | 191.0199 | 0.9 | 111.0101, 87.01303, 85.0309 | # |
| 2 | Tryptophan | C11H12N2O2 | 204.08988 | 3.73 | [M + H]+ | 205.09696 | −1 | 146.0593, 118.0656, 188.0686, 143.0728, 144.0801 | # |
| [M − H]− | 203.08269 | 0.5 | 116.0497, 142.0670,74.0270 | ||||||
| 3 | Chlorogenic acid | C16H18O9 | 354.09508 | 4.72 | [M + H]+ | 355.1026 | 0.7 | 163.0384, 145.0276, 135.0435, 117.0332 | # |
| [M − H]− | 353.08777 | −0.1 | 191.0557, 161.0237, 173.0439 | ||||||
| 4 | Unknown | C26H39NO10 | 525.2574 | 5.6 | [M + H]+ | 526.26426 | −0.8 | 364.2122, 128.0708, 346.2013 | * |
| [M − H]− | 524.24949 | −1.2 | 114.0566, 209.1889, 247.1327 | ||||||
| 5 | Syringaldehyde | C9H10O4 | 182.05791 | 6.83 | [M + H]+ | 183.0651 | −0.6 | 77.0402, 95.0497, 140.0465, 123.0442 | # |
| 6 | 1,5-Dicaffeoylquinic acid | C25H24O12 | 516.12678 | 8.15 | [M + H]+ | 517.13348 | −1.1 | 163.0386, 145.0280, 135.0445, 319.0813 | # |
| [M − H]− | 515.11915 | −0.7 | 191.0558, 353.0873, 179.0341, 161.0245 | ||||||
| 7 | 3,4-Dicaffeoylquinic acid | C25H24O12 | 516.12678 | 8.63 | [M + H]+ | 517.13357 | −0.9 | 163.0384, 145.0289, 135.0445, 319.0809 | # |
| [M − H]− | 515.11901 | −0.9 | 353.0876, 173.0451, 179.0346, 191.0551 | ||||||
| 8 | Unknown | C22H32O10 | 456.19955 | 10.35 | [M − H]− | 455.1917 | −1.2 | 247.1333, 409.1877, 203.1431, 135.0809 | * |
| 9 | Unknown | C20H27NO4 | 345.19401 | 11.07 | [M + H]+ | 346.20148 | 0.5 | 300.1963, 128.0708, 100.0766, 88.0668, 70.0671 | * |
| [M − H]− | 344.18673 | 0 | 114.0572, 113.0690 | ||||||
| 10 | Unknown | C21H28N2O | 324.22016 | 11.43 | [M + H]+ | 325.22767 | 0.7 | 91.0560, 86.0983, 233.1653, 84.0825 | * |
| 11 | Parthenolide | C15H20O3 | 248.14124 | 11.77 | [M + H]+ | 249.14857 | 0.6 | 185.1325, 143.0856, 128.0617, 129.0696 | # |
| 12 | Unknown | C18H34O5 | 330.24062 | 13.33 | [M − H]− | 329.23305 | −0.9 | 211.1342, 229.1444, 171.1028, 139.1135 | * |
| 13 | Costunolide† | C15H20O2 | 232.14633 | 19.63 | [M + H]+ | 233.15364 | 0.2 | 187.1482, 145.1017, 131.0854, 105.0708, 91.0554 | * |
| 14 | Dehydrocostuslactone† | C15H18O2 | 230.13068 | 20.30 | [M + H]+ | 231.13814 | 0.8 | 143.0861, 128.0624, 129.0705, 185.1332 | # |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, C.R.; Ha, I.J.; Kim, J.-E.; Ahn, K.S.; Park, J.; Baek, S.H. Inhibiting AGS Cancer Cell Proliferation through the Combined Application of Aucklandiae Radix and Hyperthermia: Investigating the Roles of Heat Shock Proteins and Reactive Oxygen Species. Antioxidants 2024, 13, 564. https://doi.org/10.3390/antiox13050564
Ahn CR, Ha IJ, Kim J-E, Ahn KS, Park J, Baek SH. Inhibiting AGS Cancer Cell Proliferation through the Combined Application of Aucklandiae Radix and Hyperthermia: Investigating the Roles of Heat Shock Proteins and Reactive Oxygen Species. Antioxidants. 2024; 13(5):564. https://doi.org/10.3390/antiox13050564
Chicago/Turabian StyleAhn, Chae Ryeong, In Jin Ha, Jai-Eun Kim, Kwang Seok Ahn, Jinbong Park, and Seung Ho Baek. 2024. "Inhibiting AGS Cancer Cell Proliferation through the Combined Application of Aucklandiae Radix and Hyperthermia: Investigating the Roles of Heat Shock Proteins and Reactive Oxygen Species" Antioxidants 13, no. 5: 564. https://doi.org/10.3390/antiox13050564






