Antioxidant Properties of Artemisia annua Extracts in Model Food Emulsions
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Extraction
2.3. Total Phenol and Flavonoid Content
2.4. Antioxidant Capacity Determination
2.5. Liquid Chromatography-Mass Spectrometry
2.6. Oil-in-Water Emulsion System
2.6.1. Removal of Tocopherols from Sunflower oil
2.6.2. Preparation of Emulsions and Storage Conditions
2.6.3. Measurement of Primary Oxidation by Peroxide Value (PV) and pH
2.6.4. Measurement of Secondary Oxidation by TBARs Method
2.7. Statistical Analysis
3. Results and Discussion
3.1.Phenolic Content and in-Vitro Antioxidant Activity of Extract
| Method | Amount detected * |
|---|---|
| Total polyphenol content (mg GAE/g DW) | 23.36 ± 0.92 |
| Total flavonoid content (mg CE/g DW) | 2.68 ± 0.07 |
| ABTS (µM TE/g DW) | 314.99 ± 7.70 |
| ORAC (µM TE/g DW) | 736.26 ± 17.55 |
| FRAP (µM TE/g DW) | 212.18 ± 6.02 |
| Compounds | Rt (min) | Linear regression equation | R2 | Linear range (ppm) | MS (m/z) [M − H] | Content µg/g DW |
|---|---|---|---|---|---|---|
| Rutin | 5.33 | y = 333.54x + 2184.6 | 0.998 | 0.1–1 | 609 | 0.764 |
| Caffeic acid | 5.41 | y = 588.03x + 198.38 | 0.999 | 0.1–1.5 | 179 | 1.353 |
| Apigenin | 7.85 | y = 1028.4x + 37085 | 0.991 | 0.1–0.5 | 269 | 0.135 |
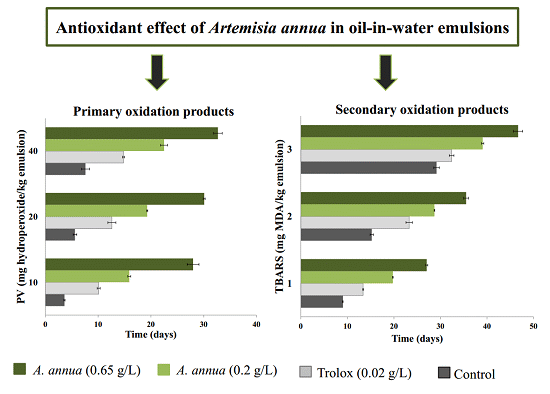
3.2. Antioxidant Activity of Extracts in Model Emulsion System



4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Alamed, J.; Chaiyasit, W.; McClements, D.J.; Decker, E.A. Relationships between free radical scavenging and antioxidant activity in foods. J. Agric. Food Chem. 2009, 57, 2969–2976. [Google Scholar] [CrossRef]
- Conde, E.; Gordon, M.H.; Moure, A.; Dominguez, H. Effects of caffeic acid and bovine serum albumin in reducing the rate of development of rancidity in oil-in-water and water-in-oil emulsions. Food Chem. 2011, 129, 1652–1659. [Google Scholar] [CrossRef]
- Waraho, T.; Cardenia, V.; Nishino, Y.; Seneviratne, K.N.; Rodriguez-Estrada, M.T.; McClements, D.J.; Decker, E.A. Antioxidant effects of mono- and diacylglycerols in non-stripped and stripped soybean oil-in-water emulsions. Food Res. Int. 2012, 48, 353–358. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; El Gendy, A.E.-N.G.S.; Sendra, E.; Fernández-López, J.; Abd El Razik, K.; Omer, E.; Pérez-Alvarez, J. Chemical composition and antioxidant and anti-Listeria activities of essential oils obtained from some Egyptian plants. J. Agric. Food Chem. 2010, 58, 9063–9070. [Google Scholar] [CrossRef]
- Ferreira, J.F.S.; Luthria, D.L.; Sasaki, T.; Heyerick, A. Flavonoids from Artemisia annua L. as antioxidants and their potential synergism with artemisinin against malaria and cancer. Molecules 2010, 15, 3135–3170. [Google Scholar] [CrossRef]
- Radulović, N.S.; Randjelović, P.J.; Stojanović, N.M.; Blagojević, P.D.; Stojanović-Radić, Z.Z.; Ilić, I.R.; Djordjević, V.B. Toxic essential oils. Part II: Chemical, toxicological, pharmacological and microbiological profiles of Artemisia annua L. volatiles. Food Chem. Toxicol. 2013, 58, 37–49. [Google Scholar] [CrossRef]
- Ćavar, S.; Maksimović, M.; Vidic, D.; Parić, A. Chemical composition and antioxidant and antimicrobial activity of essential oil of Artemisia annua L. from Bosnia. Ind. Crops Prod. 2012, 37, 479–485. [Google Scholar] [CrossRef]
- Huang, L. Antipyretic and anti-inflammatory effects of Artemisia annua L. Zhongguo Zhong Yao Za Zhi 1993, 18, 44–48, 63–64. (in Chinese). [Google Scholar] [PubMed]
- Kim, E.-K.; Lee, S.-J.; Moon, S.-H.; Jeon, S.-T.; Ahn, C.-B.; Kim, B.; Lim, B.-O.; Park, P.-J. Free radical scavenging activity and comparative proteomic analysis of antioxidative protein against H2O2-induced oxidative stress in neuronal cells. Food Chem. 2009, 117, 232–240. [Google Scholar] [CrossRef]
- Chan, H.W.; Singh, N.P.; Lai, H.C. Cytotoxicity of dihydroartemisinin toward Molt-4 cells attenuated by N-tert-butyl-alpha-phenylnitrone and deferoxamine. Anticancer Res. 2013, 33, 4389–4393. [Google Scholar] [PubMed]
- Singh, N.P.; Ferreira, J.F.; Park, J.S.; Lai, H.C. Cytotoxicity of ethanolic extracts of Artemisia annua to Molt-4 human leukemia cells. Planta Med. 2011, 77, 1788–1793. [Google Scholar] [CrossRef]
- Nibret, E.; Wink, M. Volatile components of four Ethiopian Artemisia species extracts and their in vitro antitrypanosomal and cytotoxic activities. Phytomedicine 2010, 17, 369–374. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Almajano, M.P.; Carbo, R.; Jimenez, J.A.L.; Gordon, M.H. Antioxidant and antimicrobial activities of tea infusions. Food Chem. 2008, 108, 55–63. [Google Scholar] [CrossRef]
- Skowyra, M.; Falguera, V.; Gallego, G.; Peiró, S.; Almajano, M.P. Antioxidant properties of aqueous and ethanolic extracts of tara (Caesalpinia spinosa) pods in vitro and in model food emulsions. J. Sci. Food Agric. 2013. [Google Scholar] [CrossRef]
- Gallego, M.G.; Gordon, M.H.; Segovia, F.J.; Skowyra, M.; Almajano, M.P. Antioxidant properties of three aromatic herbs (rosemary, thyme and lavender) in oil-in-water emulsions. J. Am. Oil Chem. Soc. 2013, 90, 1559–1568. [Google Scholar] [CrossRef]
- Yoshida, H.; Kajimoto, G.; Emura, S. Antioxidant effects of d-tocopherols at different concentrations in oils during microwave heating. J. Am. Oil Chem. Soc. 1993, 70, 989–995. [Google Scholar] [CrossRef]
- Frankel, E.N. Lipid Oxidation; The Oily Press: Dundee, UK, 1998; pp. 79–97. [Google Scholar]
- American Oil Chemists’ Society. AOCS Official Method Cd 8-53; Firestone, D., Ed.; Official Methods and Recommended Practices of the American Oil Chemists’ Society: Champaign, IL, USA, 1997. [Google Scholar]
- Maqsood, S.; Benjakul, S. Comparative studies of four different phenolic compounds on in vitro antioxidative activity and the preventive effect on lipid oxidation of fish oil emulsion and fish mince. Food Chem. 2010, 119, 123–132. [Google Scholar] [CrossRef]
- Gouveia, S.C.; Castilho, P.C. Artemisia annua L.: Essential oil and acetone extract composition and antioxidant capacity. Ind. Crops Prod. 2013, 45, 170–181. [Google Scholar] [CrossRef]
- Iqbal, S.; Younas, U.; Chan, K.W.; Zia-Ul-Haq, M.; Ismail, M. Chemical composition of Artemisia annua L. leaves and antioxidant potential of extracts as a function of extraction solvents. Molecules 2012, 17, 6020–6032. [Google Scholar] [CrossRef]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef]
- Carvalho, I.S.; Cavaco, T.; Brodelius, M. Phenolic composition and antioxidant capacity of six artemissia species. Ind. Crops Prod. 2011, 33, 382–388. [Google Scholar] [CrossRef]
- Carbonara, T.; Pascale, R.; Argentieri, M.P.; Papadia, P.; Fanizzi, F.P.; Villanova, L.; Avato, P. Phytochemical analysis of a herbal tea from Artemissia annua L. J. Pharm. Biomed. Anal. 2012, 62, 79–86. [Google Scholar] [CrossRef]
- Ivanescu, B.; Vlase, L.; Corciova, A.; Lazar, M.I. HPLC-DAD_MS study of polyphenols from Artemisia abstinthium, A. annua and A. vulgaris. Chem. Nat. Compd. 2010, 46, 468–470. [Google Scholar] [CrossRef]
- Kiokias, S.; Dimakou, C.; Oreopoulou, V. Activity of natural carotenoid preparations against the autoxidative deterioration of sunflower oil-in-water emulsions. Food Chem. 2009, 114, 1278–1284. [Google Scholar] [CrossRef]
- Ramful Aumjaud, D.B.; Neergheen, V.S.; Soobrattee, M.; Googoolye, K.; Aruoma, O.I.; Bahorun, T. Polyphenolic content and antioxidant activity of Eugenia pollicina leaf extract in vitro and in model emulsion systems. Food Res. Int. 2011, 44, 1190–1196. [Google Scholar] [CrossRef]
- Roedig-Penman, A.; Gordon, M. Antioxidant properties of catechins and green tea extracts in model food emulsions. J. Agric. Food Chem. 1997, 8561, 4267–4270. [Google Scholar]
- Decker, E.A.; Warner, K.; Richards, M.P.; Shahidi, F. Measuring antioxidant effectiveness in food. J. Agric. Food Chem. 2005, 53, 4303–4310. [Google Scholar] [CrossRef]
- Sun, Y.-E.; Wang, W.-D.; Chen, H.-W.; Li, C. Autoxidation of unsaturated lipids in food emulsion. Crit. Rev. Food Sci. Nutr. 2011, 51, 453–466. [Google Scholar] [CrossRef]
- Sørensen, A.-D.M.; Haahr, A.-M.; Becker, E.M.; Skibsted, L.H.; Bergenståhl, B.; Nilsson, L.; Jacobsen, C. Interactions between iron, phenolic compounds, emulsifiers, and pH in omega-3-enriched oil-in-water emulsions. J. Agric. Food Chem. 2008, 56, 1740–1750. [Google Scholar] [CrossRef]
- García-Iñiguez de Ciriano, M.; Rehecho, S.; Calvo, M.I.; Cavero, R.Y.; Navarro, I.; Astiasarán, I.; Ansorena, D. Effect of lyophilized water extracts of Melissa officinalis on the stability of algae and linseed oil-in-water emulsion to be used as a functional ingredient in meat products. Meat Sci. 2010, 85, 373–377. [Google Scholar] [CrossRef] [PubMed]
- Dimakou, C.; Oreopoulou, V. Antioxidant activity of carotenoids against the oxidative destabilization of sunflower oil-in-water emulsions. LWT-Food Sci. Technol. 2012, 46, 393–400. [Google Scholar] [CrossRef]
- Medina, I.; Undeland, I.; Larsson, K.; Storrø, I.; Rustad, T.; Jacobsen, C.; Gallardo, J.M. Activity of caffeic acid in different fish lipid matrices: A review. Food Chem. 2012, 131, 730–740. [Google Scholar] [CrossRef]
- Almajano, M.P.; Carbo, R.; Delgado, M.E.; Gordon, M.H. Effect of pH on the antimicrobial activity and oxidative stability of oil-in-water emulsions containing caffeic acid. J. Food Sci. 2007, 72, 258–263. [Google Scholar] [CrossRef]
- Zhou, L.; Elias, R.J. Antioxidant and pro-oxidant activity of (−)-epigallocatechin-3-gallate in food emulsions: Influence of pH and phenolic concentration. Food Chem. 2013, 138, 1503–1509. [Google Scholar] [CrossRef]
- Conde, E.; Moure, A.; Domínguez, H.; Gordon, M.H.; Parajó, J.C. Purified phenolics from hydrothermal treatments of biomass: Ability to protect sunflower bulk oil and model food emulsions from oxidation. J. Agric. Food Chem. 2011, 59, 9158–9165. [Google Scholar] [CrossRef]
- Poyato, C.; Navarro-Blasco, I.; Calvo, M.I.; Cavero, R.Y.; Astiasaran, I.; Ansorena, D. Oxidative stability of O/W and W/O/W emulsions: Effect of lipid composition and antioxidant polarity. Food Res. Int. 2013, 51, 132–140. [Google Scholar] [CrossRef]
- Alamed, J.; Chaiyasit, W.; McClements, D.J.; Decker, E.A. Relationships between free radical scavenging and antioxidant activity in foods. J. Agric. Food Chem. 2009, 57, 2969–2976. [Google Scholar] [CrossRef]
- Gordon, M.H. Effects of Food Structure and Ingredient Interactions on Antioxidant Capacity. In Oxidation in Foods and Beverages and Antioxidant Applications: Understanding Mechanisms of Oxidation and Antioxidant Activity, 1st ed.; Decker, E.A., Elias, R.J., McClements, D.J., Eds.; Woodhead Publishing: Cambridge, UK, 2010; Volume 1, pp. 321–331. [Google Scholar]
- Iglesias, J.; Pazos, M.; Andersen, M.L.; Skibsted, L.H.; Medina, J. Caffeic acid as antioxidant in fish muscle: Mechanism of synergism with endogenous ascorbic acid and α-tocopherol. J. Agric. Food Chem. 2009, 57, 675–681. [Google Scholar] [CrossRef]
- Becker, E.M.; Ntouma, G.; Skibsted, L.H. Synergism and antagonism between quercetin and other chain-breaking antioxidants in lipid systems of increasing structural organisation. Food Chem. 2007, 103, 1288–1296. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Skowyra, M.; Gallego, M.G.; Segovia, F.; Almajano, M.P. Antioxidant Properties of Artemisia annua Extracts in Model Food Emulsions. Antioxidants 2014, 3, 116-128. https://doi.org/10.3390/antiox3010116
Skowyra M, Gallego MG, Segovia F, Almajano MP. Antioxidant Properties of Artemisia annua Extracts in Model Food Emulsions. Antioxidants. 2014; 3(1):116-128. https://doi.org/10.3390/antiox3010116
Chicago/Turabian StyleSkowyra, Monika, Maria Gabriela Gallego, Francisco Segovia, and Maria Pilar Almajano. 2014. "Antioxidant Properties of Artemisia annua Extracts in Model Food Emulsions" Antioxidants 3, no. 1: 116-128. https://doi.org/10.3390/antiox3010116
APA StyleSkowyra, M., Gallego, M. G., Segovia, F., & Almajano, M. P. (2014). Antioxidant Properties of Artemisia annua Extracts in Model Food Emulsions. Antioxidants, 3(1), 116-128. https://doi.org/10.3390/antiox3010116