Characterization of Chemical Compounds with Antioxidant and Cytotoxic Activities in Bougainvillea x buttiana Holttum and Standl, (var. Rose) Extracts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Plant Material
2.3. Extraction, Preparation, Extraction Solvent, Temperature, and Cycles
2.4. Phytochemical Screening
2.4.1. Total Phenolic Content (TPC)
2.4.2. GC-MS Analysis
2.5. Evaluation of Cytotoxicity
2.6. DPPH Free Radical Scavenging Activity
2.7. Statistics
3. Results
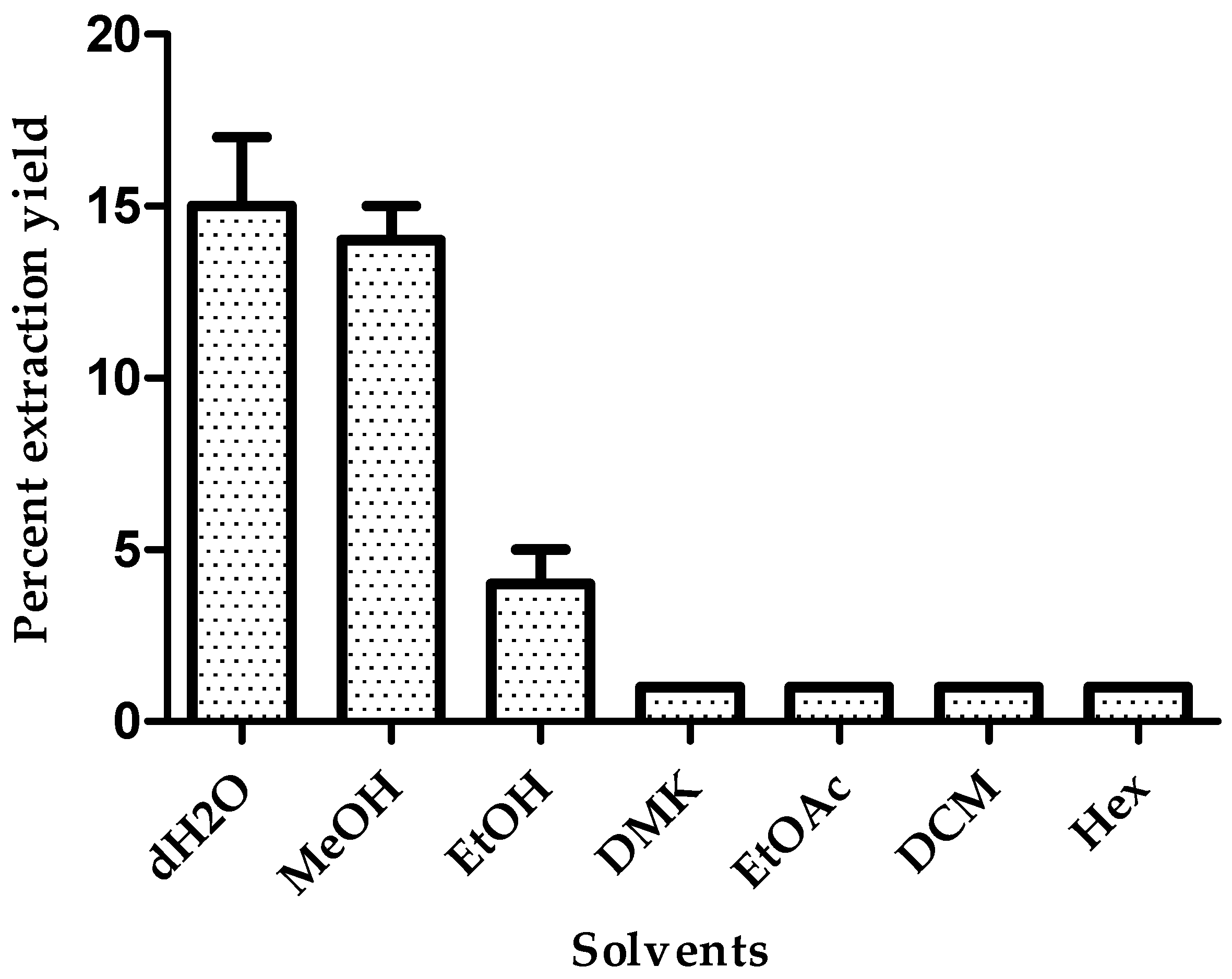
3.1. Effect of Solvent on Extraction Yield
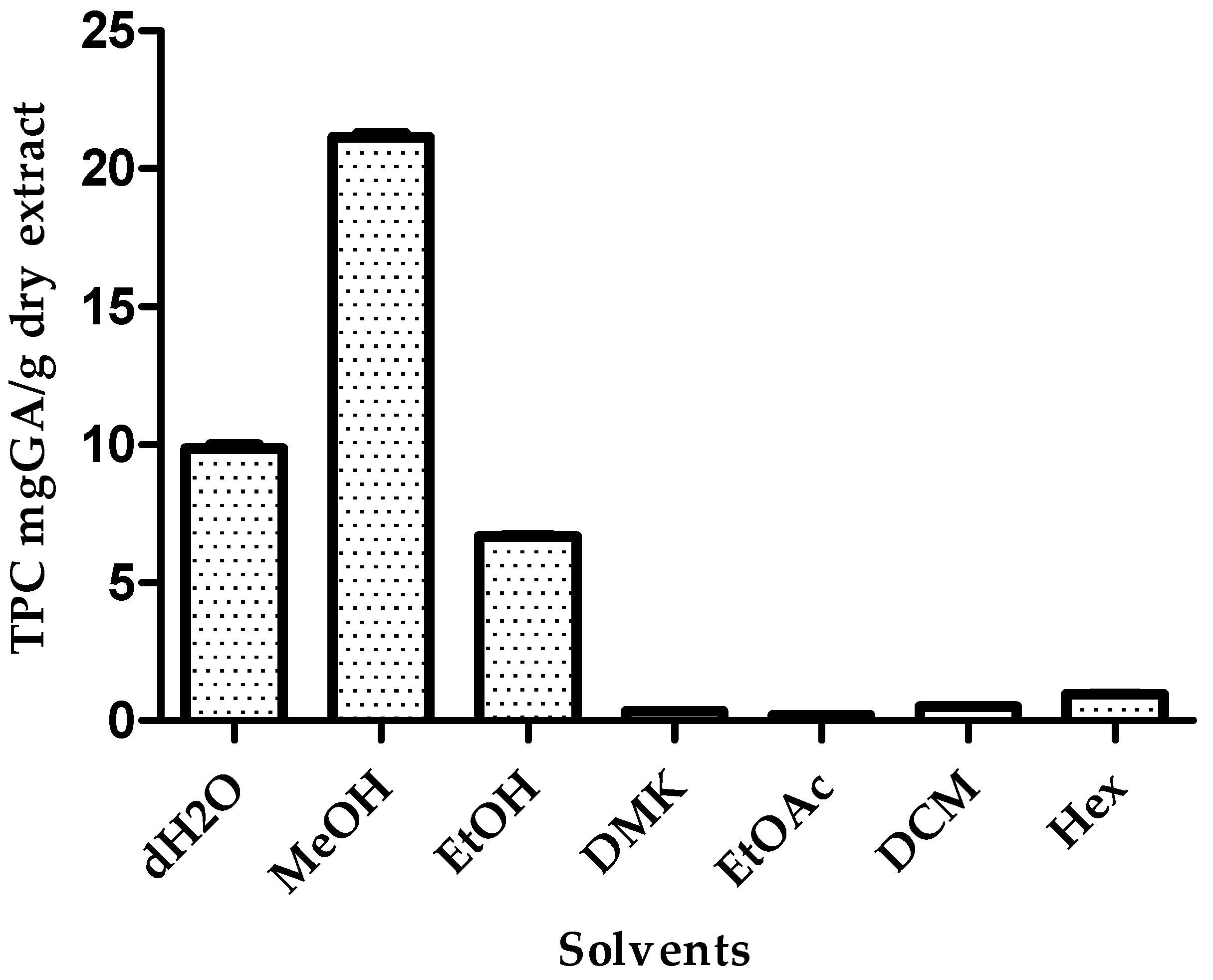
3.2. Effect of Solvent Extraction on TPC Levels
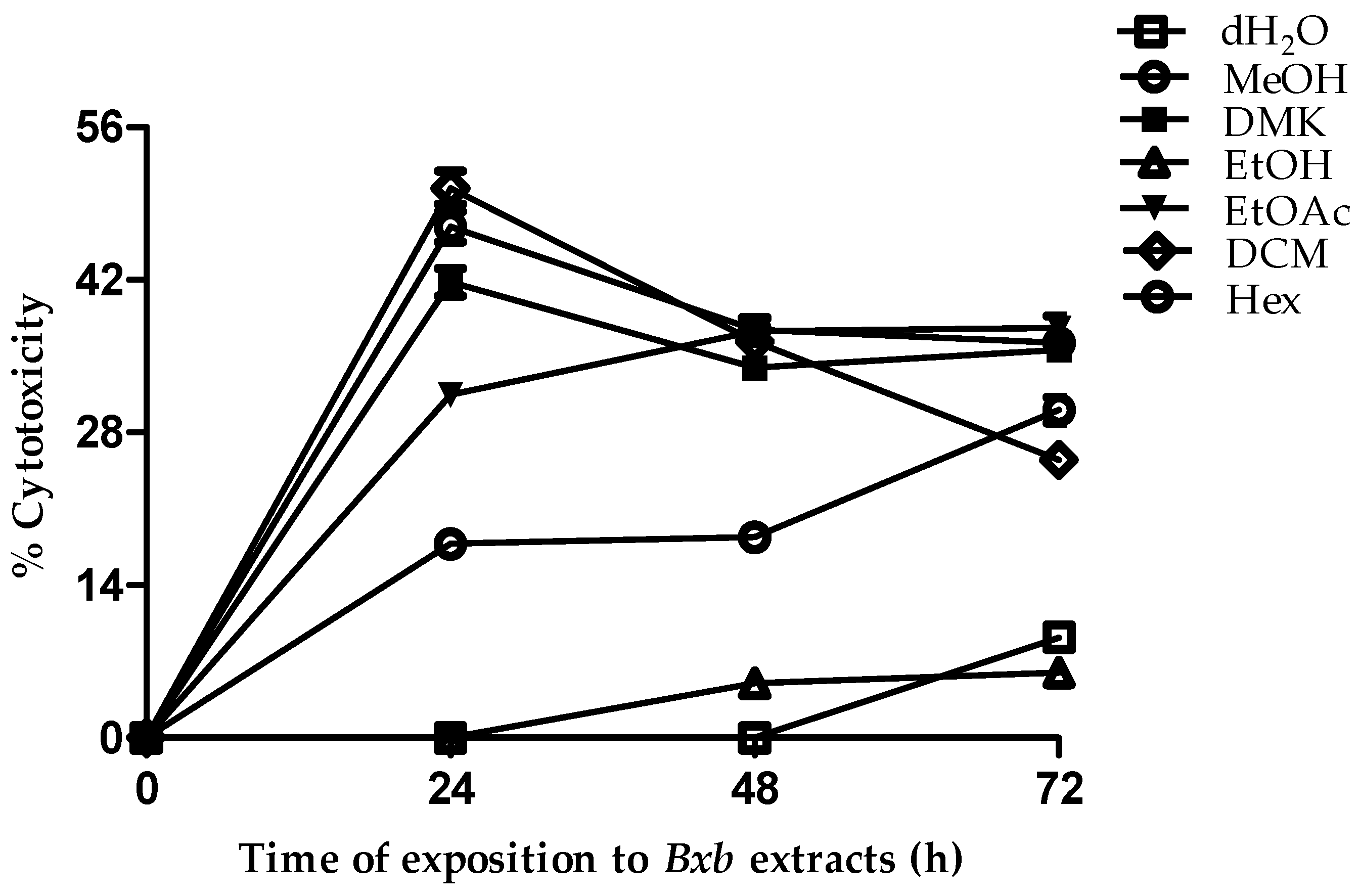
3.3. Effect of Solvent Extraction on Cellular Cytotoxicity
3.4. Effect of Solvent Extraction on Antioxidant Activity
3.5. GC-MS Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Alvarez Perez Gil, A.L.; Barbosa Navarro, L.; Patipo Vera, M.; Petricevich, V.L. Anti-inflammatory and aninocieptive activites of the ethanolic extract of Bougainvillea xbuttiana. J. Ethnopharmacol. 2012, 144, 712–719. [Google Scholar] [CrossRef] [PubMed]
- Arteaga Figueroa, L.; Barbosa Navarro, L.; Patipo Vera, M.; Petricevich, V.L. Antioxidant activity, total phenolic and flavonoid contents, and cytotoxicity evaluation of Bougainvillea xbuttiana. Int. J. Pharmacy Pharm. Sci. 2015, 6, 498–502. [Google Scholar]
- Frydoonfar, H.R.; McGrath, D.R.; Spigelman, A.D. The variable effect on proliferation of a colon cancer cell line by the citurs fruit flavonoi Naringenin. Colorectal Dis. 2003, 5, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Hakimuddin, F.; Paliyath, G.; Meckling, K. Selective cytotoxicity of a red grape wine flavonoid fraction against MCF-7 cells. Breast Cancer Res. Treat. 2004, 85, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.S.; Katan, M.B. Diet, nutrition and the prevention of hypertension and cardiovascular diseases. Public Health Nutr. 2004, 7, 167–186. [Google Scholar] [CrossRef]
- Harbone, J.B.; Mabry, T.J.; Mabry, H. The Flavonoids, 1st ed.; Springer SV Media: Austin, TX, USA, 1975. [Google Scholar]
- Wollenweber, E.; Harborne, J.B.; Mabry, T.J. The Flavonoids: Advances in Research; Harbone, J.B., Mabry, T.J., Eds.; Chapman & Hall: London, UK, 1982; p. 240. [Google Scholar]
- Cottelle, N.; Bernier, J.L.; Catteau, J.P.; Pommery, J.C.; Gydou, E.M. Antioxidant properties of hydroxyl-flavones. Free Radic. Biol. Med. 1996, 20, 35–43. [Google Scholar] [CrossRef]
- Cos, P.; Vlietinck, A.J.; Berghe, D.V.; Maes, L. Anti-infective potential of natural products: How to develop a stronger in vitro “proof-of-concept”. J. Ethnopharmacol. 2006, 106, 290–302. [Google Scholar] [CrossRef] [PubMed]
- Aziz, R.A.; Sarmidi, M.R.; Kumaresan, S.; Taher, Z.M.; Foo, D.C.Y. Phytochemical Processing: The Next Emerging Field in Chemical Engineering–Aspects and Opportunities. J. Kejuruter. Kimia Malays. 2003, 3, 45–60. [Google Scholar]
- Pawliszyn, J. Sample preparation: Quo vadis? Anal. Chem. 2003, 75, 2543–2558. [Google Scholar] [CrossRef] [PubMed]
- Waszkowiak, K.; Gliszczyńska-Świgło, A.; Barthet, V.; Skręty, J. Effect of extraction method on the phenolic and cyanogenic glucoside profile of flaxseed extracts and their antioxidant capacity. J. Am. Oil Chem. Soc. 2015, 92, 1609–1619. [Google Scholar] [CrossRef] [PubMed]
- Anwar, F.; Przybylski, R. Effect of solvents extraction on total phenolics and antioxidant activity of extracts from flaxseed (Linum usitatissimum L.). Acta Sci. Pol. Technol. Aliment. 2012, 11, 293–301. [Google Scholar] [PubMed]
- Quezada, N.; Cherian, G. Lipid characterization and antioxidant status of the seeds and meals of Camelina sativa and flax. Eur. J. Lipid Sci. Technol. 2012, 114, 974–982. [Google Scholar] [CrossRef]
- Shi, J.; Yu, J.; Pohorly, J.; Young, J.C.; Bryan, M.; Wu, Y. Optimization of the extraction of polyphenols from grape seed meal by aqueous ethanol solution. J. Food Agric. Environ. 2003, 1, 42–47. [Google Scholar]
- Thorngate, J.H.; Singleton, V.L. Localization of procyanidins in grape seeds. Am. J. Enol. Vitic. 1994, 45, 259–262. [Google Scholar]
- Sacchi, K.L.; Bisson, L.F.; Adams, D.O. A review of the effect of winemaking techniques on phenolic extraction in red wines. Am. J. Enol. Vitic. 2005, 56, 197–206. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lmuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Hamilton, M.A. Trimmed spearmam-karber method for estimating median lethal concentrations in toxicity bioassays. Environ. Sci. Technol. 1978, 12, 417. [Google Scholar] [CrossRef]
- Miliauskas, G.; Venskutonis, P.R.; Van Beek, T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Latha, L.Y. Extraction, isolation and characterization of bioactive compounds from plants extracts. Afr. J. Tradit. Complement. Altern. Med. 2011, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Cacace, J.E.; Mazza, G. Optimization of extraction of anthocyanins from black currants with aqueous ethanol. J. Food Sci. 2003, 72, 240–248. [Google Scholar] [CrossRef]
- Bucić-Kojić, A.; Planinić, M.; Tomas, S.; Bilić, M.; Velic, D. Study of solid-liquid extraction kinetics of total polyphenols from grape seeds. J. Food Eng. 2007, 81, 236–242. [Google Scholar] [CrossRef]
- Humaira, F.; Khan, K.; Zia, M.; Ur-Rehman, T.; Mirza, B.; Haq, I.U. Extraction optimization of medicinally important metabolites from Datura innoxia Mill.: An in vitro biological and phytochemical investigation. BMC Complement. Altern. Med. 2015, 15, 376. [Google Scholar]
- Ishida, T.; Rossky, P.J. Solvent effects on solute electronic structure and properties: Theoretical study of a betaine dye molecule in polar solvents. J. Phys. Chem. A 2001, 105, 558–565. [Google Scholar] [CrossRef]
- Blumenthal, M. Interaction between herbs and conventional drugs. Introductory considerations. Herbalgram. 2000, 49, 52–63. [Google Scholar]
- Snyder, L.R. Classification of the solvent properties of common liquids. J. Chromatogr. 1974, 92, 233–240. [Google Scholar] [CrossRef]
- Simon, A.; Tóth, G.; Duddeck, H.; Soliman, H.S.; Mahmoud, I.I.; Samir, H. Glycosides from Bougainvillea glabra. Nat. Prod. Res. 2006, 20, 63–67. [Google Scholar] [CrossRef] [PubMed]
- Naczk, M.; Shahidi, F. Phenolics in cereals, fruits and vegetables: Occurrence, extraction and analysis. J. Pharm. Biomed. Anal. 2006, 41, 1523–1542. [Google Scholar] [CrossRef] [PubMed]
- Naczk, M.; Shahidi, F. Extraction and analysis of phenolics in food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar] [CrossRef]
- Fazly Bazzaz, B.S.; Hassanzadeh Khayat, M.; Ahmad Emami, S.; Asili, J.; Sahebkar, A.; Neishabory, J. Antioxidant and antimicrobial activity of metanol, dichloromethane, and ethyl acetate extracts of Scutellaria litwinowii. Sci. ASI 2011, 37, 327–334. [Google Scholar] [CrossRef]
- Plaza, M.; Santoyo, S.; Jaime, L.; Reina, G.G.B.; Herrero, M.; Señoráns, F.J.; Ibáñez, E. Screening for bioactive compounds from algae. J. Pharm. Biomed. Anal. 2010, 51, 450–455. [Google Scholar] [CrossRef] [PubMed]
- Starman, D.A.J.; Nijhuis, H.H. Extraction of Secondary Metabolites from Plant Material: A Review. Trends Food Sci. Technol. 1996, 7, 191–197. [Google Scholar] [CrossRef]
- Li, B.B.; Smith, B.; Hossain, M.M. Extraction of phenolics from citrus peels I. Solvent extraction method. Sep. Purif. Technol. 2006, 48, 182–188. [Google Scholar] [CrossRef]
- Stanojević, L.; Stanković, M.; Nikolić, V.; Nikolić, L.; Ristić, D.; Čanadanovic-Brunet, J.; Tumbas, V. Antioxidant activity and total phenolic and flavonoid contents of Hieracium pilosella L. extracts. Sensors 2009, 9, 5702–5714. [Google Scholar] [CrossRef] [PubMed]
- Marvin was used to draw, display, and characterize chemical structures. (Marvin 15.11.23), 2015, ChemAxon (http://www.cheaxon.com).
| Solvent | Polarity Index (PI) |
|---|---|
| Water (dH2O) | 9.0 |
| Methanol (MeOH) | 6.6 |
| Ethanol (EtOH) | 5.2 |
| Acetone (DMK) | 5.1 |
| Ethyl acetate (EtOAc) | 4.4 |
| Dichloromethane (DCM) | 3.7 |
| Hexane (Hex) | 0 |
| Solvents | IC50 (µg/mL) |
|---|---|
| dH2O | 286.80 ± 0.01 |
| MeOH | 223.10 ± 0.02 |
| EtOH | 232.10 ± 0.02 |
| DMK | 300.90 ± 0.05 |
| EtOAc | 353.80 ± 0.01 |
| DCM | 1455.68 ± 0.19 |
| Hex | 373.70 ± 0.03 |
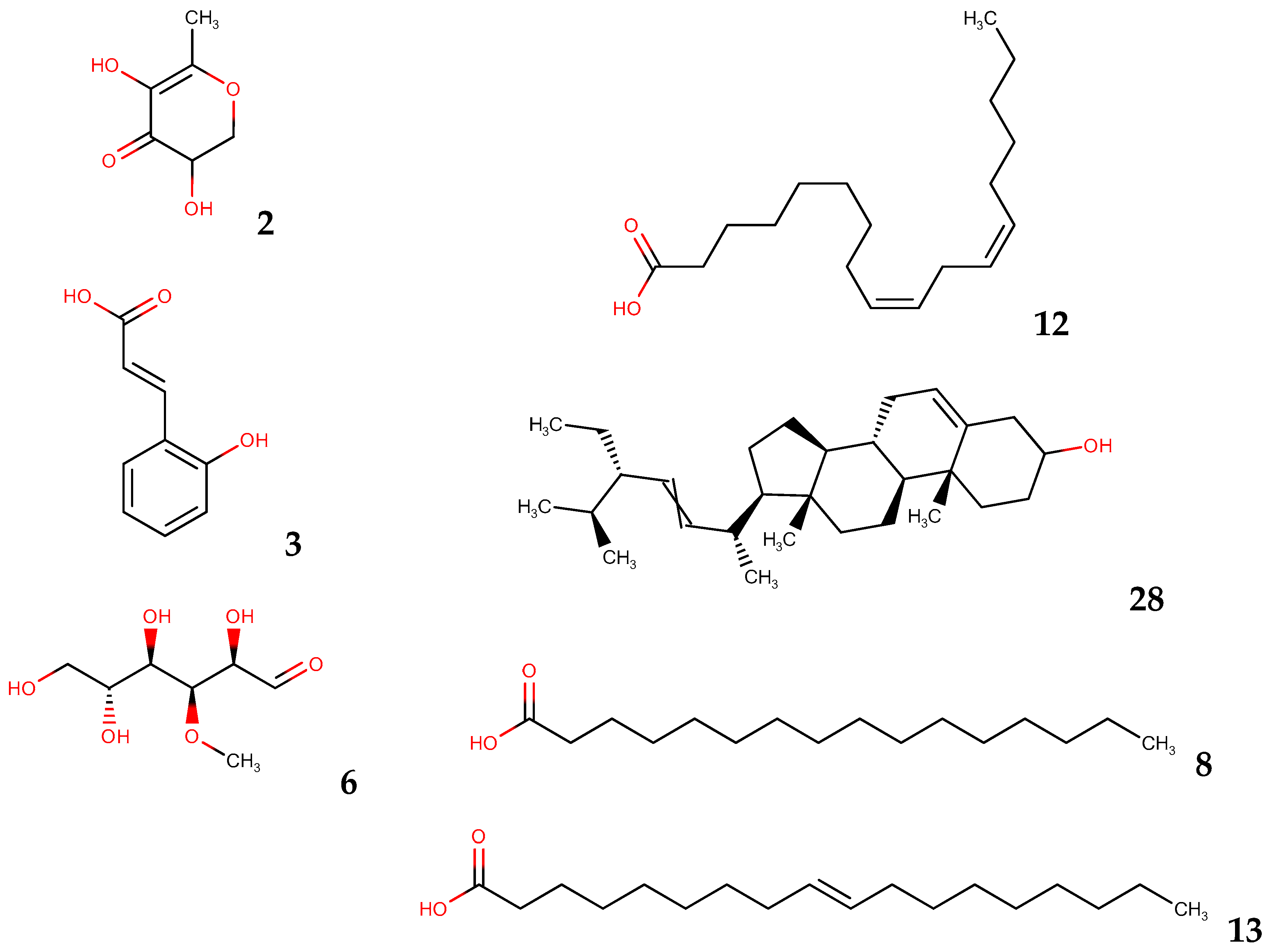
| N° | Compounds | RT (min) | Solvent‘s | ||||||
|---|---|---|---|---|---|---|---|---|---|
| dH2O | MeOH | EtOH | DMK | EtOAc | DCM | Hex | |||
| GC Area % | |||||||||
| 1 | 2,5-Dimethyl-4-hydroxy-3(2H)-furanone | 8.20 | 0.87 | ||||||
| 2 | 4H-Pyran-4-one, 2,3-dihydro-3,5-dihydroxy-6-methyl- | 9.55 | 0.48 | 0.62 | |||||
| 3 | 2-Propenoic acid, 3-(2-hydrophenyl)-(E)- | 10.61 | 2.57 | 2.75 | 2.11 | ||||
| 4 | Ethanone, 1-(2-hydroxy-5-methylphenyl)- | 12.04 | 0.51 | ||||||
| 5 | Phenol, 2,4-bis(1,1-dimethylethyl)- | 14.57 | 9.88 | ||||||
| 6 | 3-O-Methyl-d-glucose | 17.19 | 96.01 | 94.17 | 51.44 | 16.80 | |||
| 7 | Tetradecanoic acid | 17.38 | 1.92 | ||||||
| 8 | n-Hexadecanoic acid | 19.49 | 10.76 | 23.10 | 12.40 | 4.14 | 26.50 | ||
| 9 | Hexadecanoic acid, ethyl ester | 19.8 | 1.96 | ||||||
| 10 | Naphthalene, 3,4-dihydro-1,8-bis (trimethylsilyloxy)- | 19.99 | 8.94 | ||||||
| 11 | 2-(Phenyl-piperidin-1-yl-methyl)-cyclohexanol | 21.16 | 0.86 | ||||||
| 12 | 9,12-Octadecadienoic acid (Z,Z)- | 21.16 | 6.35 | 19.90 | 14.05 | 3.37 | |||
| 13 | 9-Octadecenoic acid (E)- | 21.21 | 10.40 | 10.64 | |||||
| 14 | Octadecanoic acid | 21.35 | 1.35 | 1.32 | 5.29 | ||||
| 15 | 9,12-Octadecadienoic acid, ethyl ester | 21.39 | 0.90 | 2.95 | |||||
| 16 | 9,12,15-Octadecatrienoic acid, ethyl ester, (Z,Z,Z)- | 21.46 | 2.93 | ||||||
| 17 | Pentacosane | 24.88 | 1.49 | ||||||
| 18 | Heptacosane | 28.16 | 2.56 | 2.50 | |||||
| 19 | Oxirane, heptadecyl- | 30.04 | 2.93 | ||||||
| 20 | Hexacosane, 9-octyl- | 30.33 | 2.42 | 1.86 | |||||
| 21 | Nonacosane | 30.76 | 5.33 | 7.61 | |||||
| 22 | 1,30-Triacontanediol | 32.56 | 4.45 | 11.30 | 14.47 | ||||
| 23 | 1-Dotriacontanol | 33.46 | 19.90 | 16.07 | 18.07 | ||||
| 24 | 1-Hexacosene | 33.59 | 29.87 | ||||||
| 25 | Vitamin E | 34.18 | 2.93 | ||||||
| 26 | Cyclooctacosane | 35.34 | 4.36 | ||||||
| 27 | 1-Triacontanol | 37.34 | 10.39 | 22.21 | |||||
| 28 | Stigmasta-5,22-dien-3-ol | 37.34 | 7.72 | 10.60 | 4.63 | 2.53 | 25.81 | ||
| 29 | Chondrillasterol | 37.55 | 1.02 | 8.98 | |||||
| 30 | Stigmast-7-en-3-ol, (3β,5α) | 38.86 | 9.62 | ||||||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abarca-Vargas, R.; Peña Malacara, C.F.; Petricevich, V.L. Characterization of Chemical Compounds with Antioxidant and Cytotoxic Activities in Bougainvillea x buttiana Holttum and Standl, (var. Rose) Extracts. Antioxidants 2016, 5, 45. https://doi.org/10.3390/antiox5040045
Abarca-Vargas R, Peña Malacara CF, Petricevich VL. Characterization of Chemical Compounds with Antioxidant and Cytotoxic Activities in Bougainvillea x buttiana Holttum and Standl, (var. Rose) Extracts. Antioxidants. 2016; 5(4):45. https://doi.org/10.3390/antiox5040045
Chicago/Turabian StyleAbarca-Vargas, Rodolfo, Carlos F. Peña Malacara, and Vera L. Petricevich. 2016. "Characterization of Chemical Compounds with Antioxidant and Cytotoxic Activities in Bougainvillea x buttiana Holttum and Standl, (var. Rose) Extracts" Antioxidants 5, no. 4: 45. https://doi.org/10.3390/antiox5040045
APA StyleAbarca-Vargas, R., Peña Malacara, C. F., & Petricevich, V. L. (2016). Characterization of Chemical Compounds with Antioxidant and Cytotoxic Activities in Bougainvillea x buttiana Holttum and Standl, (var. Rose) Extracts. Antioxidants, 5(4), 45. https://doi.org/10.3390/antiox5040045