Proanthocyanidin Characterization and Bioactivity of Extracts from Different Parts of Uncaria tomentosa L. (Cat’s Claw)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Chemicals and Reagents
2.2. Extraction of Phenolic Compounds from the Different Parts of U. tomentosa
2.3. Total Proanthocyanidin Determination
2.4. Characterization of the Proanthocyanidin Extracts by DI-ESI-QTOF MS
2.5. In Vitro Antioxidant Activity
2.6. Cell Culture
2.7. Assessment of Cytotoxicity by MTT Assay
2.8. Antimicrobial Activity
3. Results
3.1. Total Proanthocyanidin Content of U. tomentosa Extracts
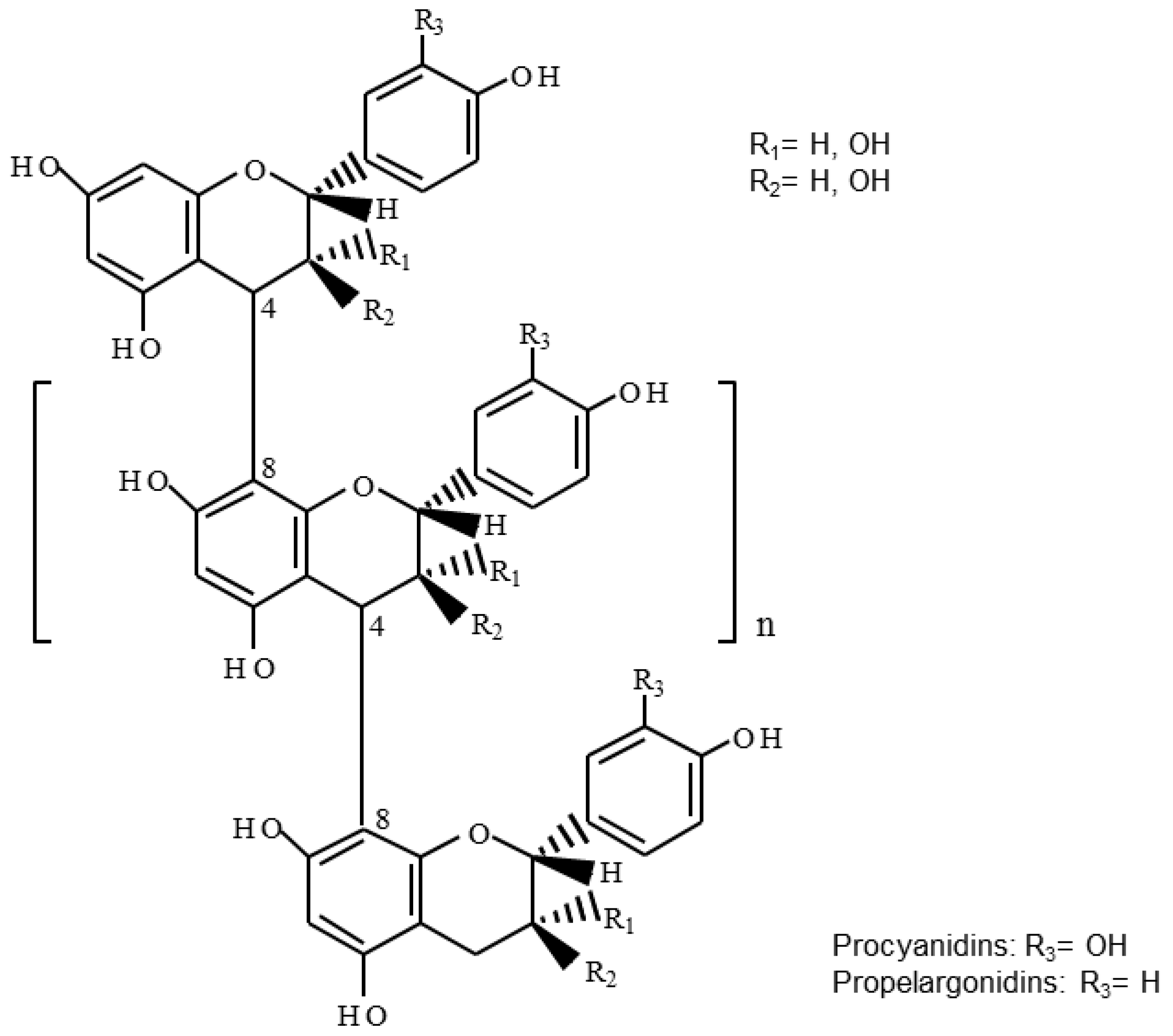
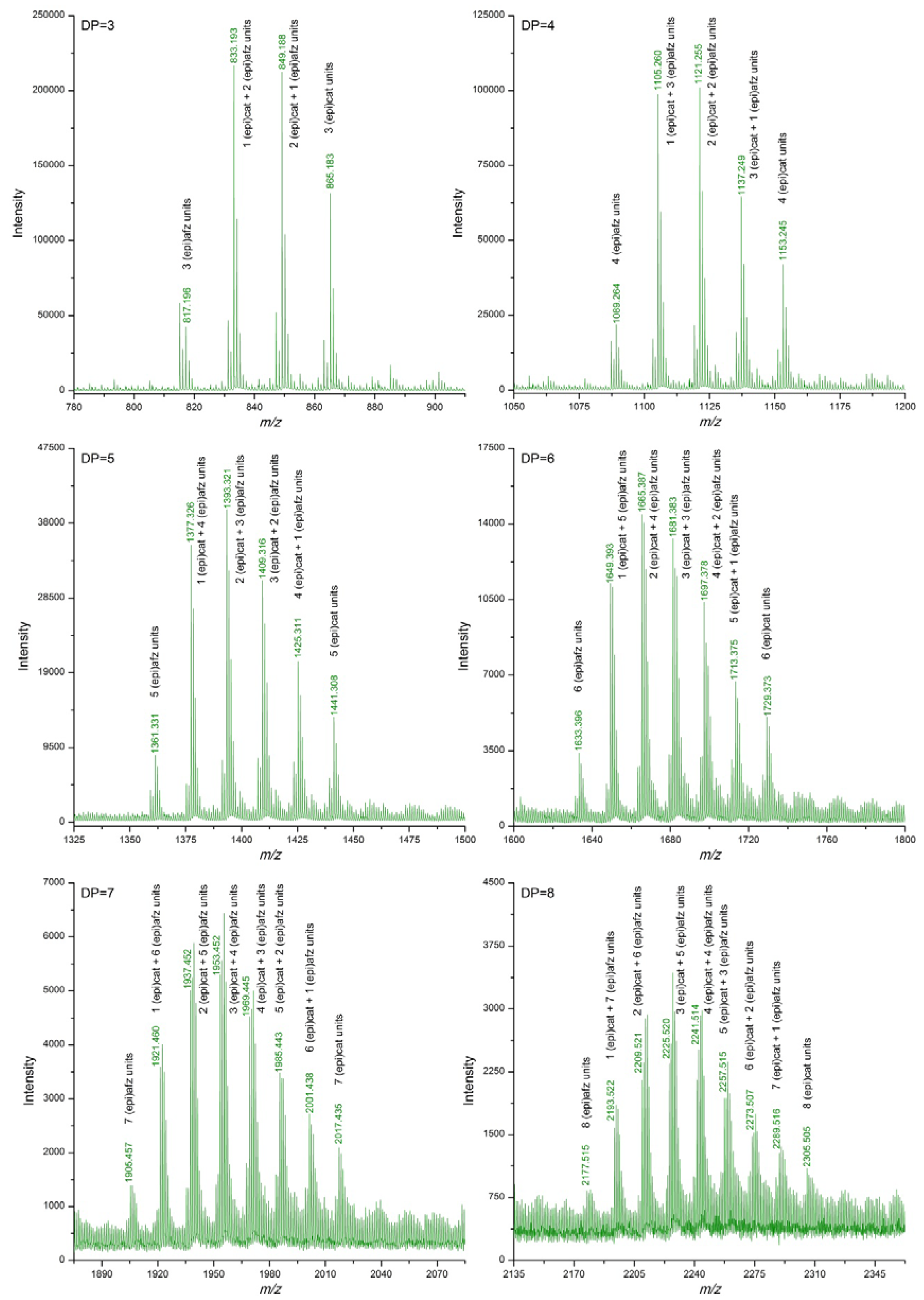
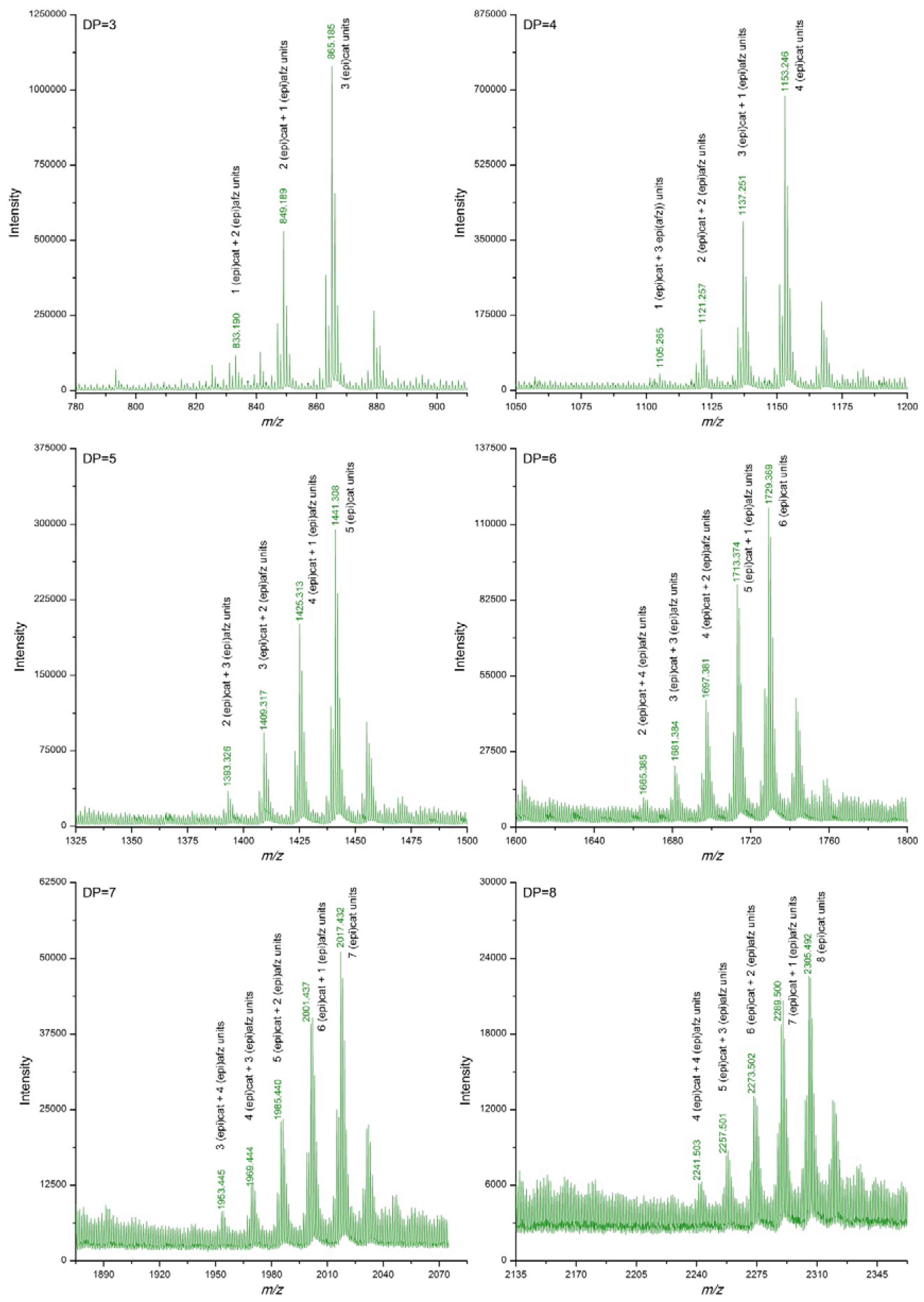
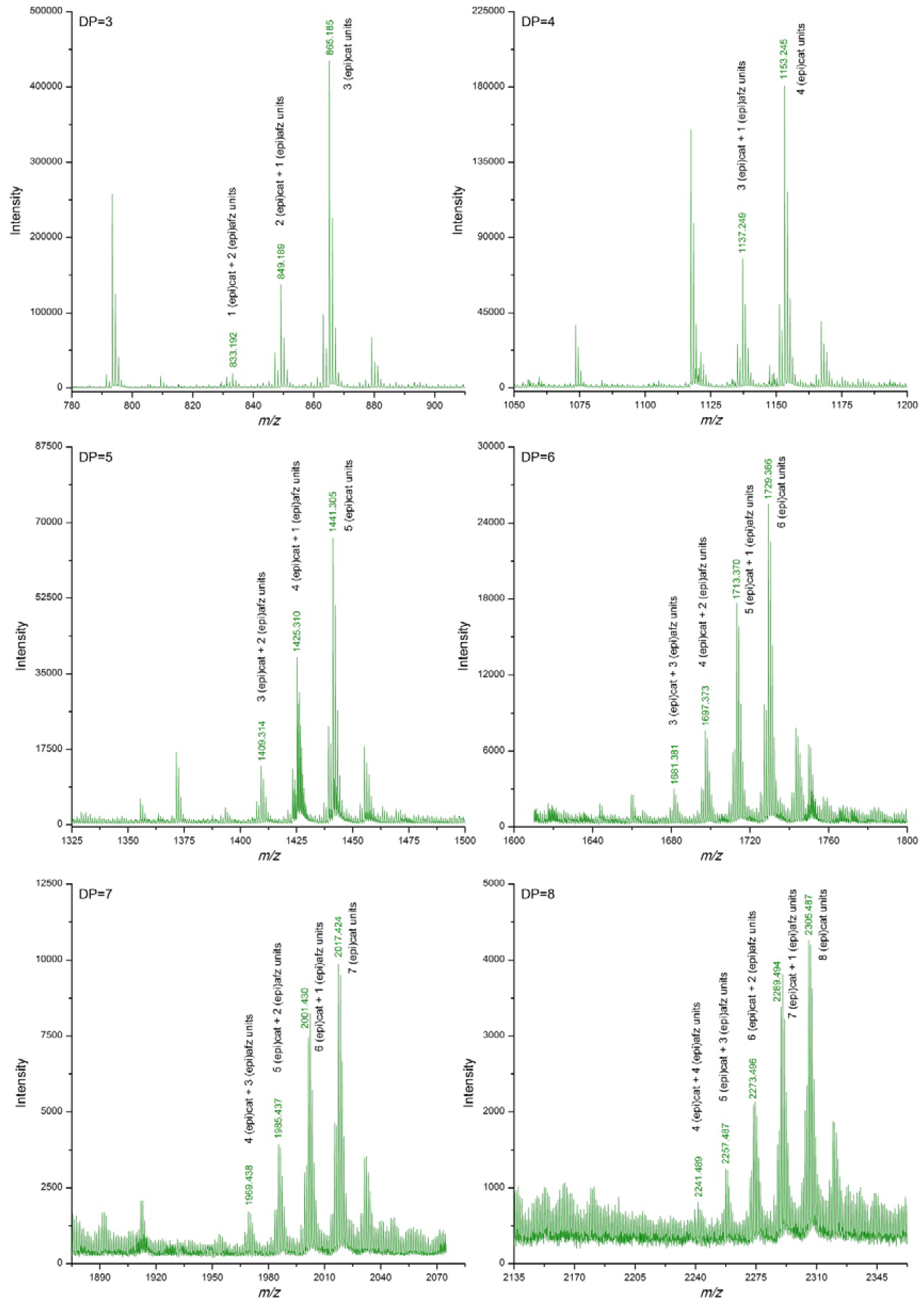
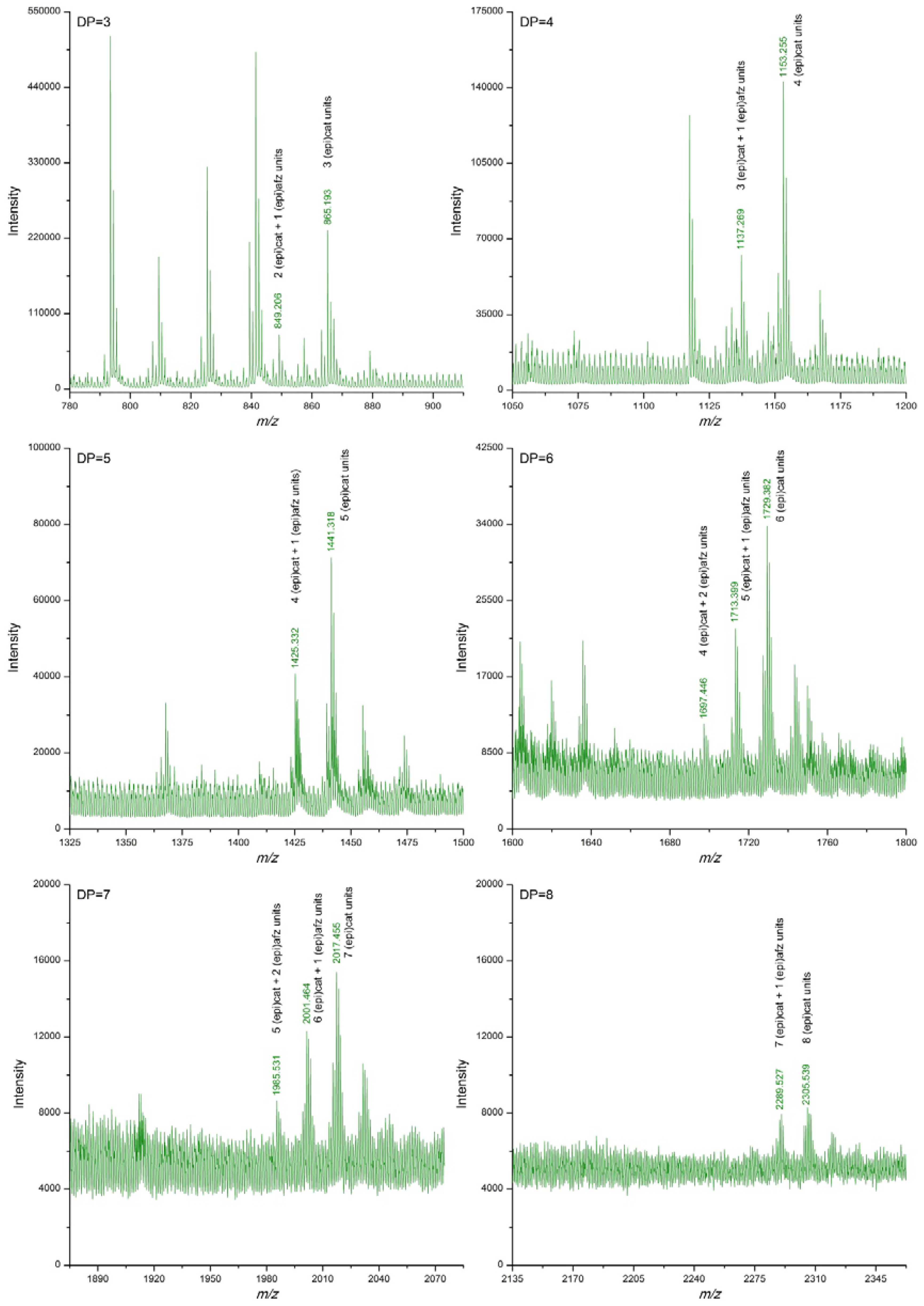
3.2. Proanthocyanidin Profile of U. tomentosa Extracts from Leaves, Stems, Bark and Wood
3.3. Antioxidant Activity of U. tomentosa Extracts
3.4. Citotoxicity of U. tomentosa Extracts
3.5. Antimicrobial Activity of U. tomentosa Extracts
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhou, K.; Raffoul, J.J. Potential anticancer properties of grape antioxidants. J. Oncol. 2012, 803294. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, J.J.; Xu, J.; Feng, F.; Qu, W. Medicinal uses, phytochemistry and pharmacology of the genus Uncaria. Review. J. Ethnopharmol. 2015, 173, 48–80. [Google Scholar] [CrossRef] [PubMed]
- Bertol, G.; Franco, L.; de Oliveira, B.H. HPLC analysis of oxindole alkaloids in Uncaria Tomentosa: Sample preparation and analysis optimisation by factorial design. Phytochem. Anal. 2012, 23, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, M.; Okuhama, N.N.; Zhang, X.J.; Condezo, L.A.; Lao, J.; Angeles, F.M.; Musah, R.A.; Bobrowski, P.; Miller, J.S. Anti-inflammatory and antioxidant activities of cat’s claw (Uncaria tomentosa and Uncaria guianensis) are independent of their alkaloid content. Phytomedicine 2002, 9, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Ccahuana-Vasquez, R.A.; Ferreira dos Santos, S.S.; Koga-Ito, C.Y.; Cardoso, A.O. Antimicrobial activity of Uncaria tomentosa against oral human pathogens. Braz. Oral Res. 2007, 21, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.; Tay, L.; Rezende, E.; Kozlowski, V.; dos Santos, E. In vitro antimicrobial activity of phytoterapic Uncaria tomentosa against endodontic pathogens. J. Oral Sci. 2010, 52, 473–476. [Google Scholar] [CrossRef] [PubMed]
- Alakomi, H.L.; Pimia, R.P.; Aura, A.M.; Helander, I.M.; Hohynek, L.; Osman-Caldente, K.M.; Saarela, M. Weakening of salmonella with selected microbial metabolites of berry-derived phenolic compounds and organic acids. J. Agric. Food Chem. 2007, 55, 3905–3912. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Jenner, A.M.; Low, C.S.; Lee, Y.K. Effect of tea phenolics and their aromatic fecal bacterial metabolites on intestinal microbiota. Res. Microbiol. 2006, 157, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.H.; Mackie, R.I. Effect of condensed tannins on bacterial diversity and metabolic activity in the rat gastrointestinal tract. Appl. Environ. Microbiol. 2004, 70, 1104–1115. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Hoyos, M.; Sanchez, F.; Murillo, R.; Martín, P.; Zamora, W.; Monagas, M.; Bartolomé, B. Phenolic assesment of Uncaria tomentosa L. (Cat’s Claw): Leaves, stem, bark and wood extracts. Molecules 2015, 20, 22703–22717. [Google Scholar] [CrossRef] [PubMed]
- Ribéreau-Gayon, P.; Stonestreet, E. Dósage des tannins du vin rouges et determination du leur structure. Chem. Anal. 1966, 48, 188–196. [Google Scholar]
- Sánchez-Patán, F.; Cueva, C.; Monagas, M.; Walton, G.E.; Gibson, G.R.; Quintanilla-López, J.E.; Lebrón-Aguilar, R.; Martín-Álvarez, P.J.; Moreno-Arribas, M.V.; Bartolomé, B. In vitro fermentation of a red wine extract by human gut microbiota: Changes in microbial groups and formation of phenolic metabolites. J. Agric. Food Chem. 2012, 60, 2136–2147. [Google Scholar] [CrossRef] [PubMed]
- Dávalos, A.; Gomez-Cordoves, C.; Bartolome, B. Extending applicability of the oxygen radical absorbance capacity (ORAC-Fluorescein) assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Cueva, C.; Mingo, S.; Munoz, I.; Bustos, I.; Requena, T.; del Campo, R.; Martın, P.; Bartolome, B.; Moreno, V. Antibacterial activity of wine phenolic compounds and oenological extracts against potential respiratory pathogens. Lett. Appl. Microb. 2012, 54, 557–563. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Sánchez, F.; Quintanilla, J.; Lebrón, R.; Bartolomé, B.; Navarro, M. Phenolic Extracts of Uncaria Tomentosa L. (Cat’s Claw) Containing Procyanidins, Propelargonidins and Flavanolignans. Method for the Production Thereof, and Applications of Same. Patent WO2014/096488 A1, 26 June 2014. [Google Scholar]
- Gu, L.; Kelm, M.; Hammestorne, J.; Beecher, G.; Holden, J.; Haytowitz, D.; Proir, R. Screening of foods containing proanthocyanidins and their structural characterization using LC-MS/MS and thiolytic degradation. J. Agric. Food Chem. 2003, 51, 7513–7521. [Google Scholar] [CrossRef] [PubMed]
- Mateos-Martín, M.L.; Fuguet, E.; Quero, C.; Pérez-Jiménez, J.; Torres, J.L. New identification of proanthocyanidins in cinnamon (Cinnamomum zeylanicum L.) using MALDI-TOF/TOF mass spectrometry. Anal. Bioanal. Chem. 2012, 402, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Chai, M.K.; Wei, M.K.; Wang, R.; Deng, R.G.; Zou, Z.R.; Peng, Y.Y. Avocado proanthocyanidins as source of tyrosinase inhibitors: Structure characterization, inhibitory activity and mechanism. J. Agric. Food Chem. 2015, 63, 7381–7387. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.M.; Shi, Y.; Feng, H.L.; Xu, L.; Xiang, Z.H.; Gao, Y.S.; Chen, Q.Z. Structure characterization and anti-tyrosinase mechanism of polymeric proanthocyanidins fractionated from kiwifruit pericarp. J. Agric. Food Chem. 2014, 62, 6382–6389. [Google Scholar] [CrossRef] [PubMed]
- Hellstrom, J.; Torronen, R.; Mattila, P. Proanthocyanidins in common food products of plant origin. J. Agric. Food Chem. 2009, 57, 7899–7906. [Google Scholar] [CrossRef] [PubMed]
- Kiprovski, B.; Petkovsek, M.; Slatnar, A.; Veberic, R.; Stampar, F.; Malencic, D.; Latkovic, D. Comparison of phenolic profiles and antioxidant properties of European Fagopyrum esculentum cultivars. Food Chem. 2015, 185, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Furuuchi, R.; Yokoyama, T.; Watanabe, Y.; Hirayama, M. Identification and quantification of short oligomeric proanthocyanidins and other polyphenols in boysenberry seeds and juice. J. Agric. Food Chem. 2011, 59, 3738–3746. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.W.; Zhu, S.; Kazuma, K.; Wei, S.L.; Yoshimatsu, K.; Komatsu, K. Molecular ion index assisted comprehensive profiling of B-type oligomeric proanthocyanidins in rhubarb by high performance liquid chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 3555–3570. [Google Scholar] [CrossRef] [PubMed]
- Bartolome, B.; Monagas, M.; Garrido, I.; Gómez, C.; Martín, P.; Lebrón, R.; Urpí-Sardà, M.; Llorach, R.; Andrés, C. Almond (Prunus dulcis (Mill.) D.A. Webb) polyphenols: From chemical characterization to targeted analysis of phenolic metabolites in humans. Arch. Biochem. Biophys. 2010, 501, 124–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behrens, A.; Nagamitsu, M.; Knicker, H.; Kogel, I. MALDI-TOF mass spectrometry and PSD fragmentation as means for the analysis of condensed tannins in plant leaves and needles. Phytochemistry 2003, 62, 1159–1170. [Google Scholar] [CrossRef]
- Ramsay, A.; Mueller-Harvey, I. Senna alata leaves are a good source of propelargonidins. Nat. Prod. Res. 2015, 13, 1–4. [Google Scholar]
- Bicker, J.; Petereit, F.; Hensel, A. Proanthocyanidins and a phloroglucinol derivative from Rumex acetosa L. Fitoterapia 2009, 80, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Chai, W.M.; Shi, Y.; Feng, H.L.; Qiu, L.; Zhou, H.C.; Deng, Z.W.; Yan, C.L.; Chen, Q.X. NMR, HPLC-ESI-MS, and MALDI-TOF MS analysis of condensed tannins from Delonix regia (Bojer ex Hook.) Raf. and their bioactivities. J. Agric. Food Chem. 2012, 60, 5013–5022. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.D.; Zhou, H.C.; Lin, Y.M.; Liao, M.M.; Chai, W.M. MALDI-TOF MS analysis of condensed tannins with potent antioxidant activity from the leaf, stem bark and root bark of Acacia confuse. Molecules 2010, 15, 4369–4381. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.J.; Lin, Y.M.; Zhou, H.C.; Wei, S.D.; Lin, G.H.; Ye, G.F. Antioxidant tannins from stem bark and fine root of Casuarina equisetifolia. Molecules 2010, 15, 5658–5670. [Google Scholar] [CrossRef] [PubMed]
- Pilarski, R.; Zielinski, H.; Ciesiołka, D.; Gulewicz, K. Antioxidant activity of ethanolic and aqueous extracts of Uncaria tomentosa (Willd.) DC. J. Ethnopharm. 2006, 104, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Amaral, S.; Mira, L.; Nogueira, J.M.; da Silva, A.P.; Florêncio, H.M. Plant extracts with anti-inflammatory properties. A new approach for characterization of their bioactive compounds and establishment of structure-antioxidant activity relationships. Biorg. Med. Chem. 2009, 17, 1876–1883. [Google Scholar] [CrossRef] [PubMed]
- Dreifuss, A.A.; Bastos-Pereira, A.L.; Avila, T.V.; Soley, B.S.; Rivero, A.J.; Aguilar, J.L.; Acco, A. Antitumoral and antioxidant effects of a hydroalcoholic extract of cat’s claw (Uncaria tomentosa) (Willd. Ex Roem. & Schult) in an in vivo carcinosarcoma model. J. Ethnopharm. 2010, 130, 127–133. [Google Scholar]
- Monagas, M.; Hernandez, B.; Garrido, I.; Martin-Álvarez, P.J.; Gomez, C.; Bartolomé, B. Quality assessment of commercial dietary antioxidant products from Vitis vinifera L. grape seeds. Nutr. Cancer 2005, 53, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Heitzman, M.E.; Neto, C.C.; Winiarz, E.; Vaisberg, A.J.; Hammond, G.B. Ethnobotany, phytochemistry and pharmacology of Uncaria (Rubiaceae). Phytochemistry 2005, 66, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Pilarski, R.; Filip, B.; Wietrzykb, J.; Kura´s, M.; Gulewicz, K. Anticancer activity of the Uncaria tomentosa (Willd.) DC. preparations with different oxindole alkaloid composition. Phytomedicine 2010, 17, 1133–1139. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.L.; Wang, Z.Y.; Liu, J.R. Cold-field fruit extracts exert different antioxidant and antiproliferative activities in vitro. Food Chem. 2011, 129, 402–407. [Google Scholar] [CrossRef]
- Loizzo, M.; Said, A.; Tundis, R.; Hawas, U.; Rashed, K.; Menichini, F.; Frega, N.; Menichini, F. Antioxidant and antiproliferative activity of Diospyros lotus L. extract and isolated compounds. Plant Foods Hum. Nutr. 2009, 64, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H.; Sun, J. Antiproliferative activity of apples is not due to phenolic induced hydrogen peroxide formation. J. Agric. Food Chem. 2003, 51, 1718–1723. [Google Scholar] [CrossRef] [PubMed]
- Herrera, D.R.; Durand-Ramirez, J.E.; Falcao, A.; da Silva, E.J.L.N.; dos Santos, E.B.; de Gomes, B.P.F.A. Antimicrobial activity and substantivity of Uncaria tomentosa in infected root canal dentin. Braz. Oral Res. 2016, 30, e61. [Google Scholar] [CrossRef] [PubMed]
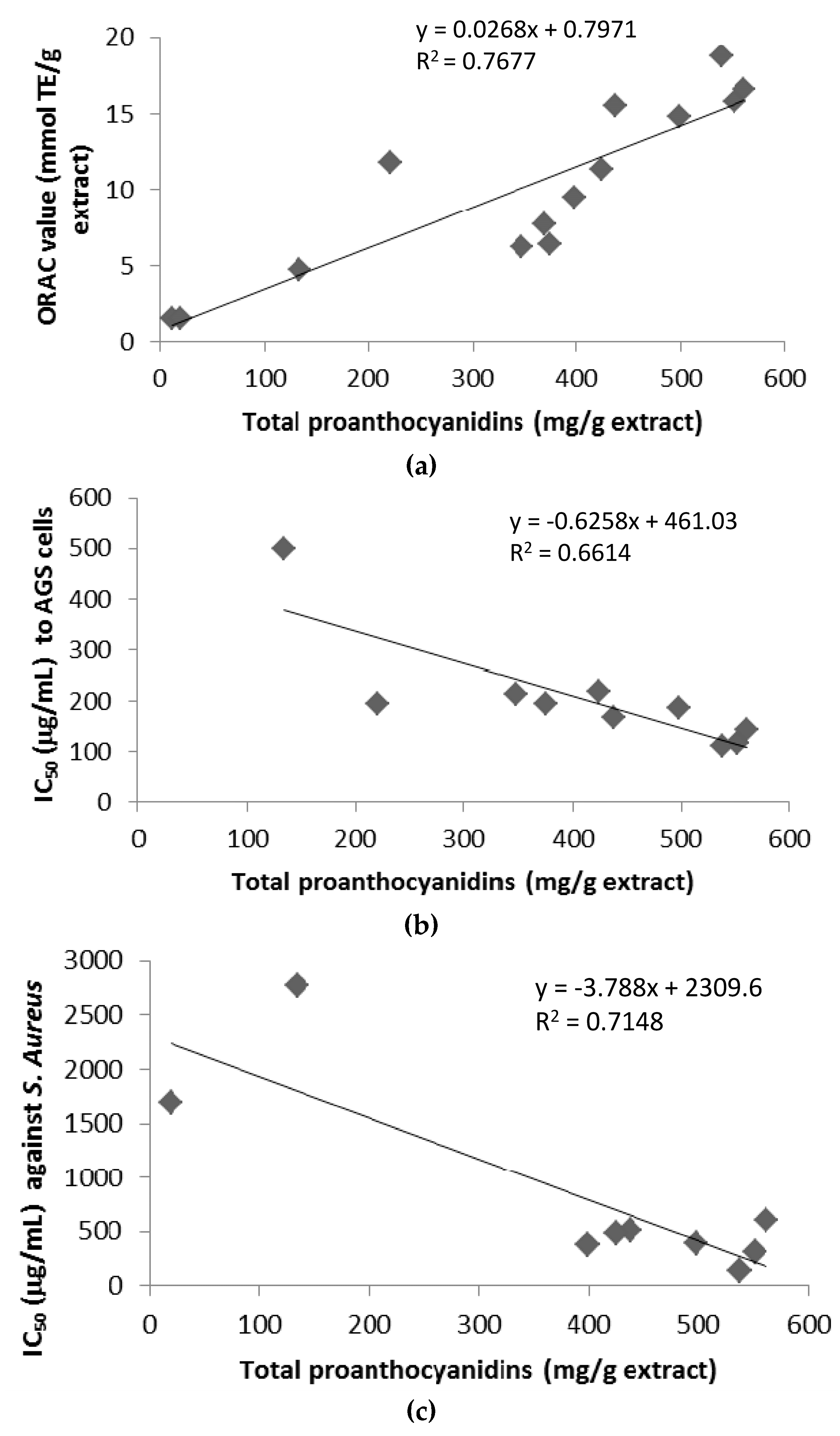
| Sample Location | Total Proanthocyanidins (mg/g Extract) 1 | ORAC Value (mmol TE/g Extract) 2 |
|---|---|---|
| Leaves | ||
| Asomat | 220.9 ± 0.5 | 11.8 ± 0.2 |
| Los Chiles | 551.5 ± 18.3 | 15.8 ± 0.2 |
| Palacios | 437.2 ± 36.9 | 15.5 ± 0.6 |
| Sarapiquí | 561.3 ± 13.5 | 16.6 ± 1.2 |
| Stems | ||
| Asomat | 368.9 ± 3.3 | 7.7 ± 0.1 |
| Palacios | 398.7 ± 13.3 | 9.5 ± 0.3 |
| Sarapiquí | 497.8 ± 27.8 | 14.8 ± 0.1 |
| Bark | ||
| Asomat | 347.0 ± 18.8 | 6.2 ± 0.1 |
| Los Chiles | 374.3 ± 12.1 | 6.4 ± 0.2 |
| Palacios | 424.4 ± 0.1 | 11.4 ± 1.1 |
| Sarapiquí | 538.0 ± 29.2 | 18.8 ± 0.2 |
| Wood | ||
| Asomat | 11.3 ± 4.3 | 1.5 ± 0.0 |
| Palacios | 19.4 ± 2.2 | 1.5 ± 0.1 |
| Sarapiquí | 134.0 ± 4.8 | 4.7 ± 0.3 |
| DP | (epi)cat | (epi)afz | m/z Theoretical [M−H]− | m/z Experimental [M−H]− | |||
|---|---|---|---|---|---|---|---|
| Bark (n = 4) | Leaves (n = 4) | Stems (n = 3) | Wood (n = 3) | ||||
| 3 | 3 | 0 | 865.198 | 865.185 | 865.183 | 865.185 | 865.193 |
| 2 | 1 | 849.203 | 849.189 | 849.188 | 849.189 | 849.203 | |
| 1 | 2 | 833.208 | 833.192 | 833.193 | 833.190 | ||
| 0 | 3 | 817.213 | 817.196 | ||||
| 4 | 4 | 0 | 1153.261 | 1153.245 | 1153.245 | 1153.246 | 1153.255 |
| 3 | 1 | 1137.266 | 1137.249 | 1137.249 | 1137.251 | 1137.269 | |
| 2 | 2 | 1121.272 | 1121.255 | 1121.257 | |||
| 1 | 3 | 1105.277 | 1105.260 | 1105.265 | |||
| 0 | 4 | 1089.282 | 1089.264 | ||||
| 5 | 5 | 0 | 1441.325 | 1441.305 | 1441.308 | 1441.308 | 1441.318 |
| 4 | 1 | 1425.330 | 1425.310 | 1425.311 | 1425.313 | 1425.332 | |
| 3 | 2 | 1409.335 | 1409.314 | 1409.316 | 1409.317 | ||
| 2 | 3 | 1393.340 | 1393.321 | 1393.326 | |||
| 1 | 4 | 1377.345 | 1377.326 | ||||
| 0 | 5 | 1361.350 | 1361.331 | ||||
| 6 | 6 | 0 | 1729.388 | 1729.366 | 1729.373 | 1729.369 | 1729.382 |
| 5 | 1 | 1713.393 | 1713.370 | 1713.375 | 1713.374 | 1713.399 | |
| 4 | 2 | 1697.398 | 1697.373 | 1697.378 | 1697.381 | 1697.446 | |
| 3 | 3 | 1681.403 | 1681.381 | 1681.383 | 1681.384 | ||
| 2 | 4 | 1665.409 | 1665.387 | 1665.385 | |||
| 1 | 5 | 1649.414 | 1649.393 | ||||
| 0 | 6 | 1633.419 | 1633.396 | ||||
| 7 | 7 | 0 | 2017.452 | 2017.424 | 2017.435 | 2017.432 | 2017.455 |
| 6 | 1 | 2001.457 | 2001.430 | 2001.438 | 2001.437 | 2001.464 | |
| 5 | 2 | 1985.462 | 1985.437 | 1985.443 | 1985.440 | 1985.513 | |
| 4 | 3 | 1969.467 | 1969.438 | 1969.445 | 1969.444 | ||
| 3 | 4 | 1953.472 | 1953.452 | 1953.445 | |||
| 2 | 5 | 1937.477 | 1937.452 | ||||
| 1 | 6 | 1921.482 | 1921.460 | ||||
| 0 | 7 | 1905.487 | 1905.457 | ||||
| 8 | 8 | 0 | 2305.515 | 2305.487 | 2305.505 | 2305.492 | 2305.539 |
| 7 | 1 | 2289.520 | 2289.494 | 2289.516 | 2289.500 | 2289.527 | |
| 6 | 2 | 2273.525 | 2273.496 | 2273.507 | 2273.502 | ||
| 5 | 3 | 2257.530 | 2257.487 | 2257.515 | 2257.501 | ||
| 4 | 4 | 2241.535 | 2241.489 | 2241.514 | 2241.503 | ||
| 3 | 5 | 2225.540 | 2225.520 | ||||
| 2 | 6 | 2209.545 | 2209.521 | ||||
| 1 | 7 | 2193.551 | 2193.522 | ||||
| 0 | 8 | 2177.556 | 2177.515 | ||||
| 9 | 9 | 0 | 2593.578 | 2593.547 | 2593.596 | 2593.563 | |
| 8 | 1 | 2577.583 | 2577.547 | 2577.579 | 2577.571 | ||
| 7 | 2 | 2561.589 | 2561.553 | 2561.578 | 2561.568 | ||
| 6 | 3 | 2545.594 | 2545.560 | 2545.586 | 2545.563 | ||
| 5 | 4 | 2529.599 | 2529.582 | 2530.574 | |||
| 4 | 5 | 2513.604 | 2513.587 | ||||
| 3 | 6 | 2497.609 | 2497.585 | ||||
| 2 | 7 | 2481.614 | 2481.588 | ||||
| 1 | 8 | 2465.619 | 2465.579 | ||||
| 10 | 10 | 0 | 2881.642 | 2881.618 | 2881.635 | ||
| 9 | 1 | 2865.647 | 2865.606 | 2865.632 | |||
| 8 | 2 | 2849.652 | 2849.642 | 2849.617 | |||
| 7 | 3 | 2833.657 | 2833.641 | 2833.696 | 2833.649 | ||
| 6 | 4 | 2817.662 | 2817.626 | ||||
| 5 | 5 | 2801.667 | 2801.656 | ||||
| 4 | 6 | 2785.672 | 2785.670 | ||||
| 3 | 7 | 2769.677 | 2769.657 | ||||
| 2 | 8 | 2753.682 | 2753.680 | ||||
| 1 | 9 | 2737.687 | 2737.666 | ||||
| 11 | 11 | 0 | 3169.705 | 3169.693 | |||
| 10 | 1 | 3153.710 | 3153.685 | ||||
| 9 | 2 | 3137.715 | 3136.719 | ||||
| Sample Location | AGS Cells IC50 (μg/mL) | SW620 Cells IC50 (μg/mL) | Vero Cells IC50 (μg/mL) |
|---|---|---|---|
| Leaves | |||
| Asomat | 195 ± 14 | 118 ± 12 | >500 |
| Los Chiles | 116 ± 5 | 120 ± 7 | 458 ± 17 |
| Palacios | 167 ± 17 | 160 ± 14 | >500 |
| Sarapiquí | 145 ± 14 | 149 ± 29 | >500 |
| Stems | |||
| Sarapiquí | 188 ± 20 | 178 ± 13 | >500 |
| Bark | |||
| Asomat | 215 ± 23 | 111 ± 4 | >500 |
| Los Chiles | 196 ± 17 | 111 ± 8 | >500 |
| Palacios | 220 ± 13 | 132 ± 5 | >500 |
| Sarapiquí | 111 ± 3 | 142 ± 5 | 468 ± 13 |
| Wood | |||
| Sarapiquí | >500 | >500 | >500 |
| IC50 (μg/mL) | |||
|---|---|---|---|
| Sample Location | S. aureus ATCC 5923 | E. faecalis V583 | P. aeruginosa PSP |
| Leaves | |||
| Los Chiles | 312 | 1164 | 1012 |
| Palacios | 516 | 1444 | 521 |
| Sarapiquí | 603 | 2095 | 475 |
| Stems | |||
| Palacios | 380 | 6831 | n.e |
| Sarapiquí | 396 | 1383 | 417 |
| Bark | |||
| Palacios | 490 | n.e. | n.e. |
| Sarapiquí | 133 | 395 | 152 |
| Wood | |||
| Palacios | 1688 | 1112 | 449 |
| Sarapiquí | 2774 | n.e. | 811 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-Hoyos, M.; Lebrón-Aguilar, R.; Quintanilla-López, J.E.; Cueva, C.; Hevia, D.; Quesada, S.; Azofeifa, G.; Moreno-Arribas, M.V.; Monagas, M.; Bartolomé, B. Proanthocyanidin Characterization and Bioactivity of Extracts from Different Parts of Uncaria tomentosa L. (Cat’s Claw). Antioxidants 2017, 6, 12. https://doi.org/10.3390/antiox6010012
Navarro-Hoyos M, Lebrón-Aguilar R, Quintanilla-López JE, Cueva C, Hevia D, Quesada S, Azofeifa G, Moreno-Arribas MV, Monagas M, Bartolomé B. Proanthocyanidin Characterization and Bioactivity of Extracts from Different Parts of Uncaria tomentosa L. (Cat’s Claw). Antioxidants. 2017; 6(1):12. https://doi.org/10.3390/antiox6010012
Chicago/Turabian StyleNavarro-Hoyos, Mirtha, Rosa Lebrón-Aguilar, Jesús E. Quintanilla-López, Carolina Cueva, David Hevia, Silvia Quesada, Gabriela Azofeifa, M. Victoria Moreno-Arribas, María Monagas, and Begoña Bartolomé. 2017. "Proanthocyanidin Characterization and Bioactivity of Extracts from Different Parts of Uncaria tomentosa L. (Cat’s Claw)" Antioxidants 6, no. 1: 12. https://doi.org/10.3390/antiox6010012






