The Effect of Gender and Menstrual Phase on Serum Creatine Kinase Activity and Muscle Soreness Following Downhill Running
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Menstrual Cycle Characterisation
2.3. Downhill Running Experimental Testing
2.4. Statistical Analyses
3. Results
3.1. Anthropometry and Ovarian Hormone Concentrations
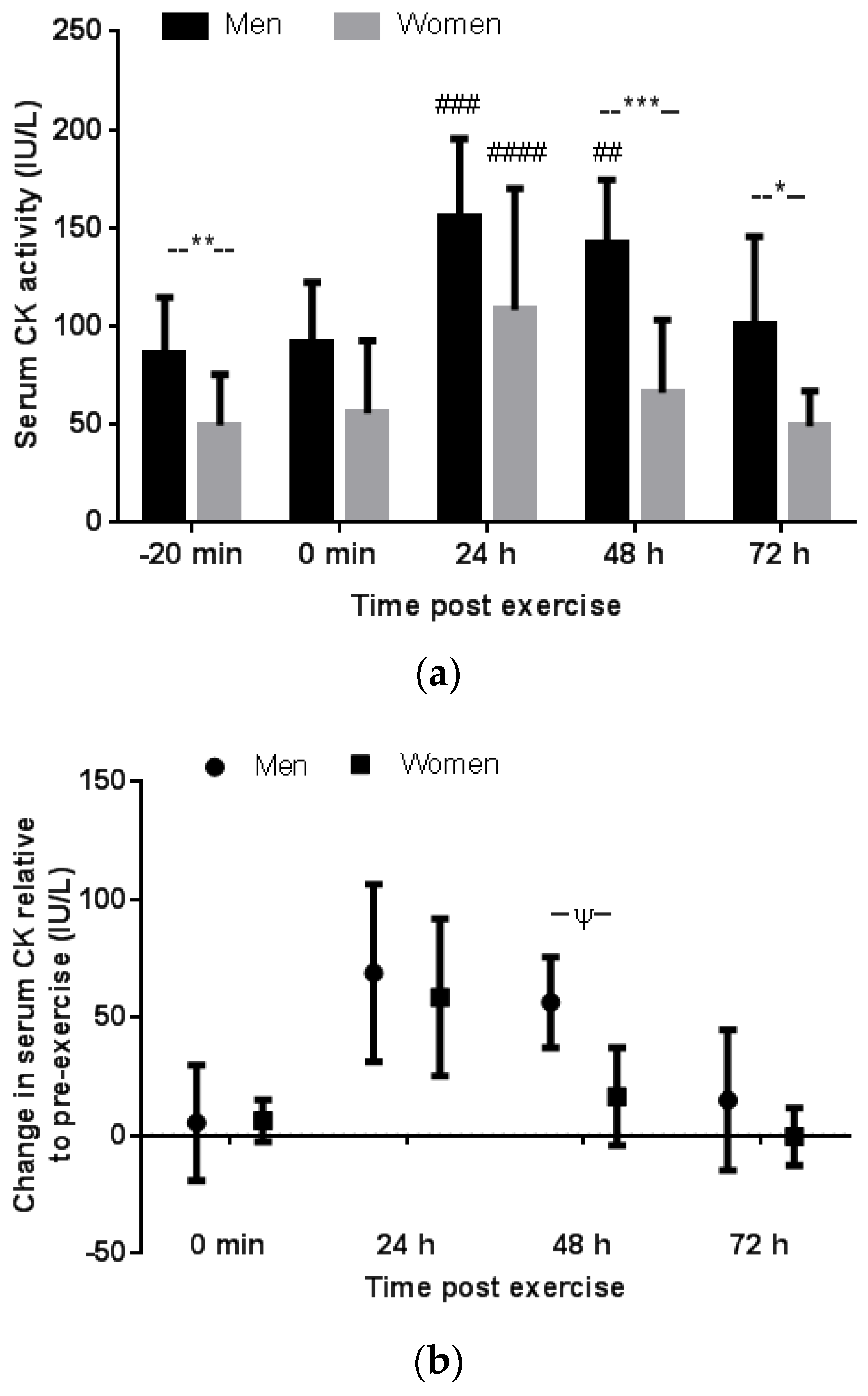
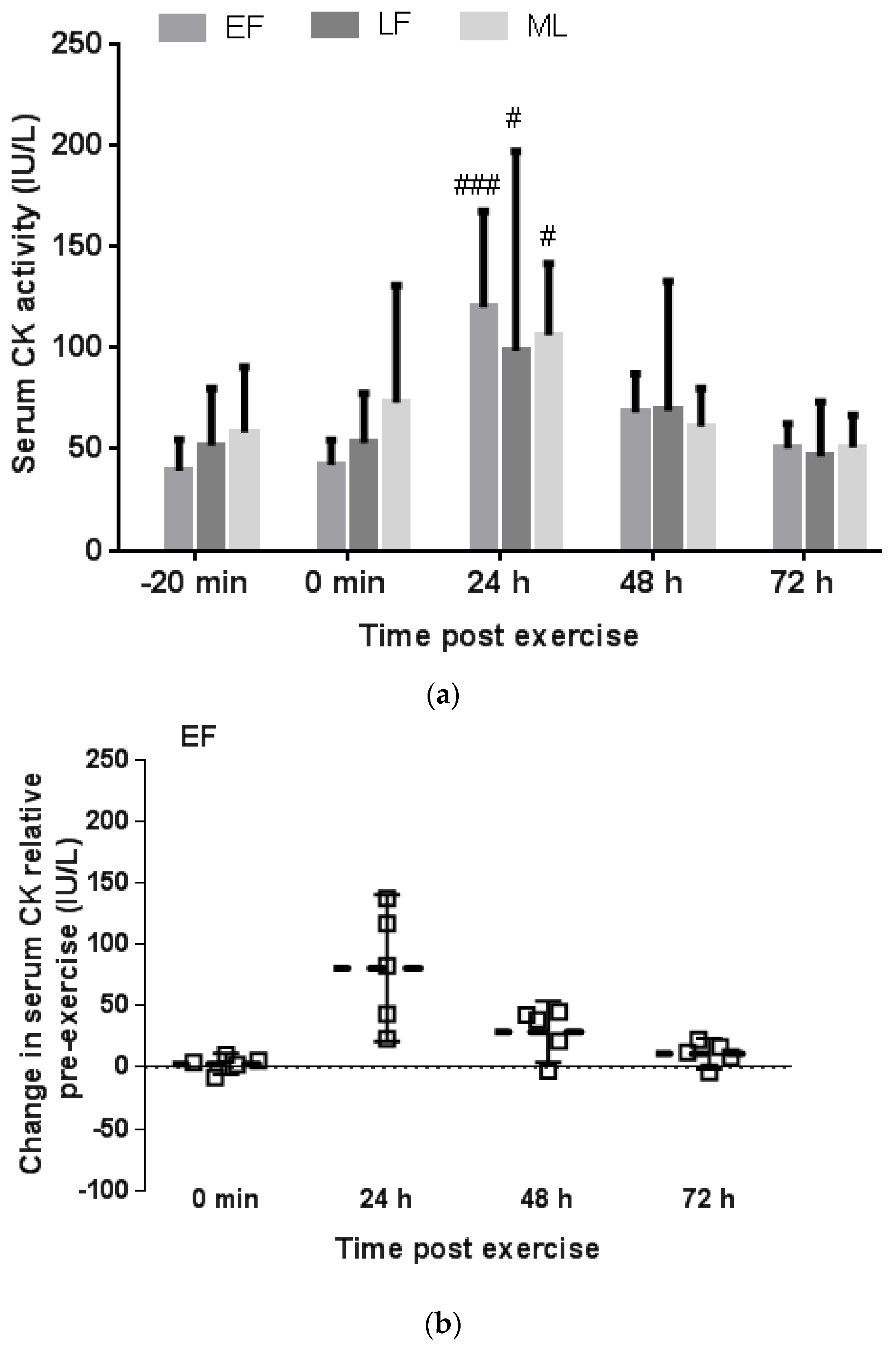
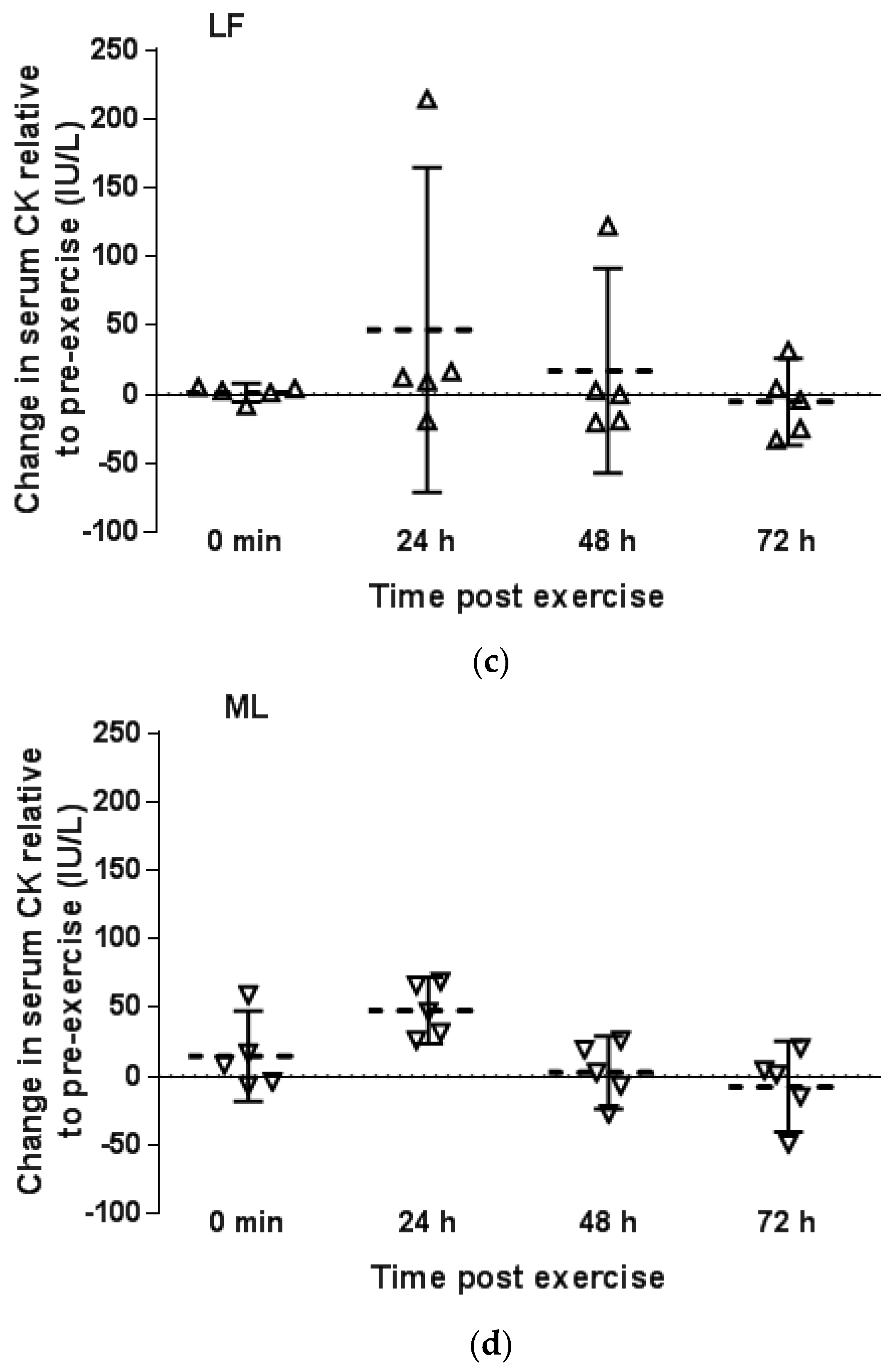
3.2. Serum CK Response to Downhill Running
3.2.1. Gender Comparison
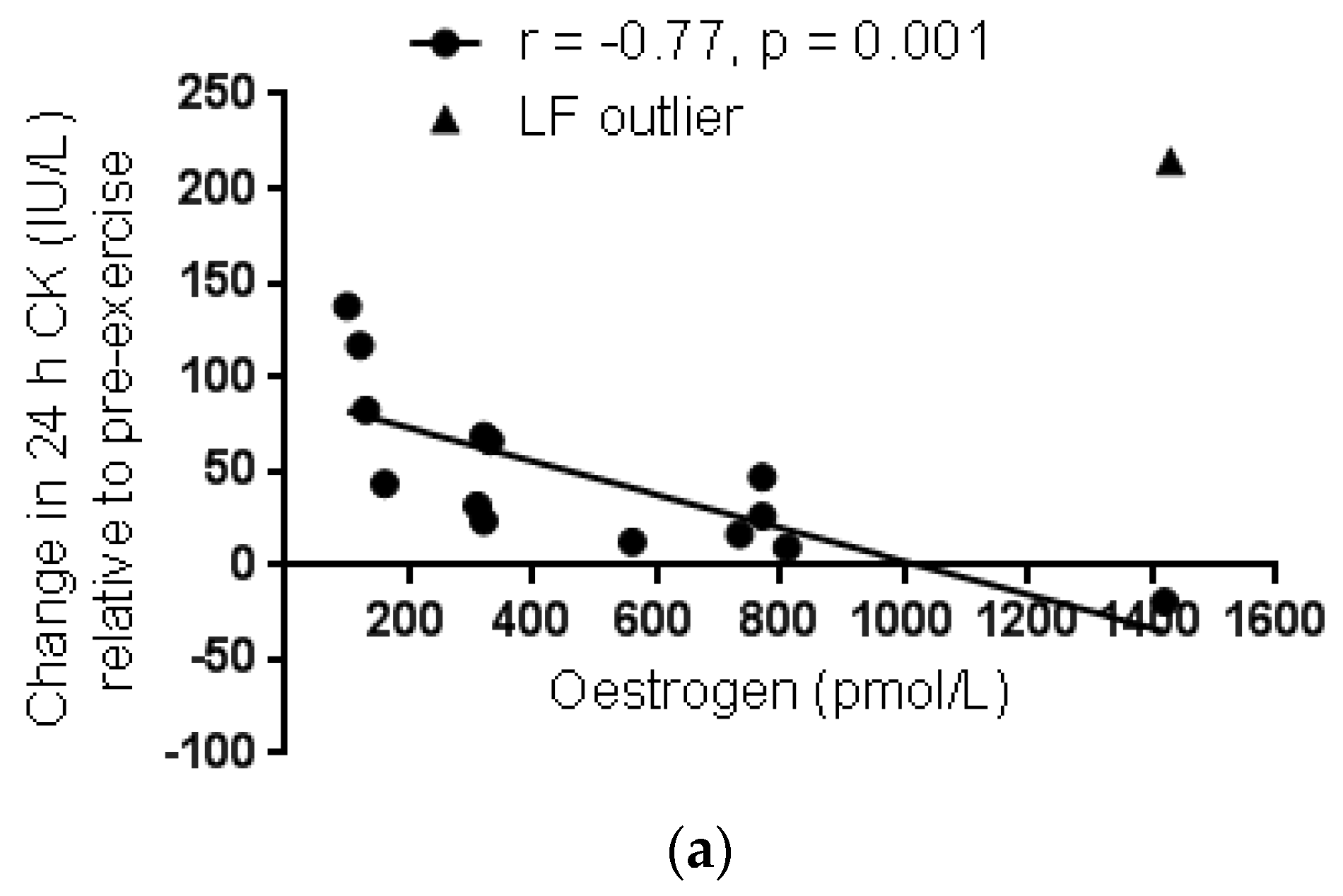
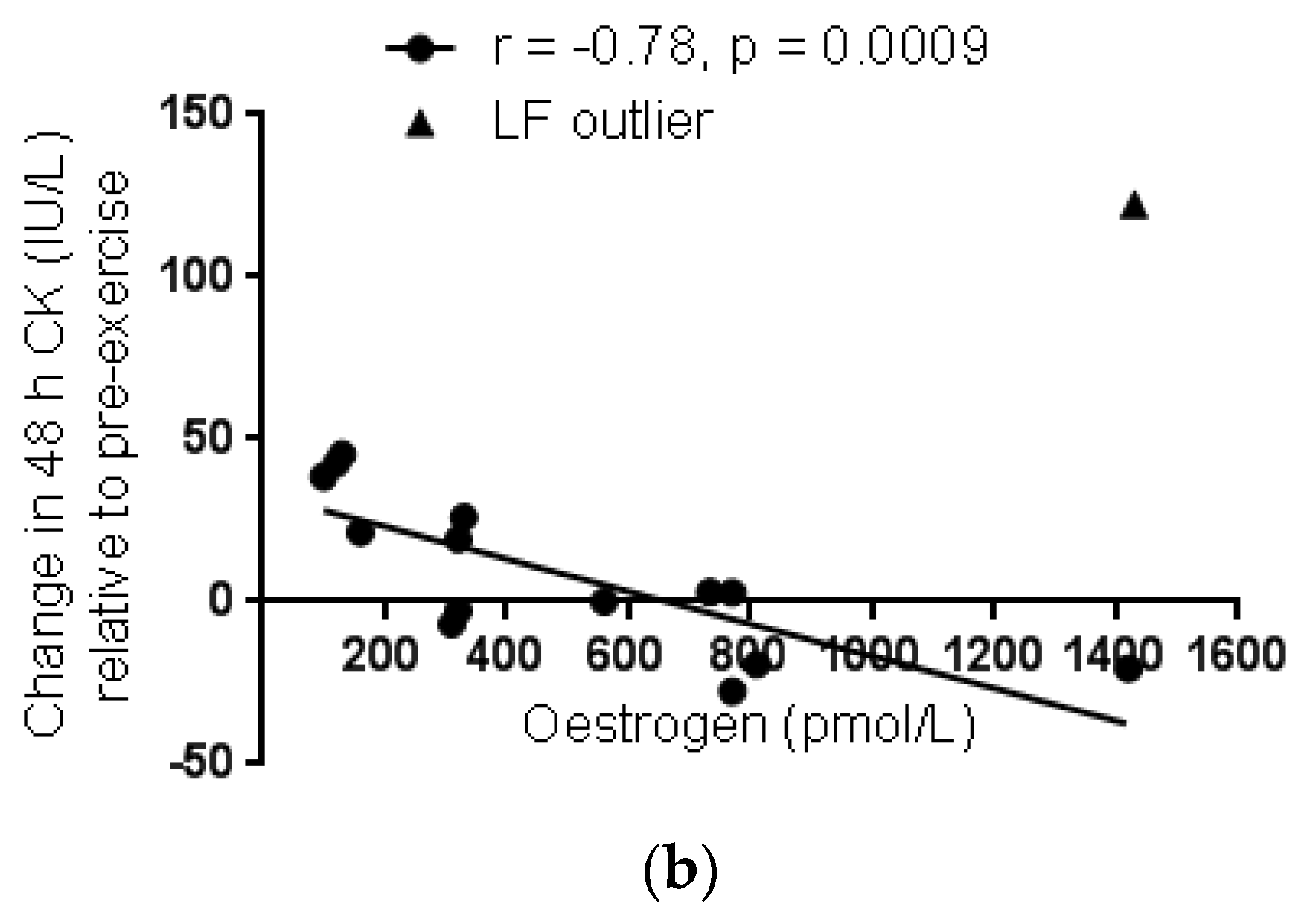
3.2.2. Menstrual Phase Comparison
3.3. Perceived Muscle Soreness
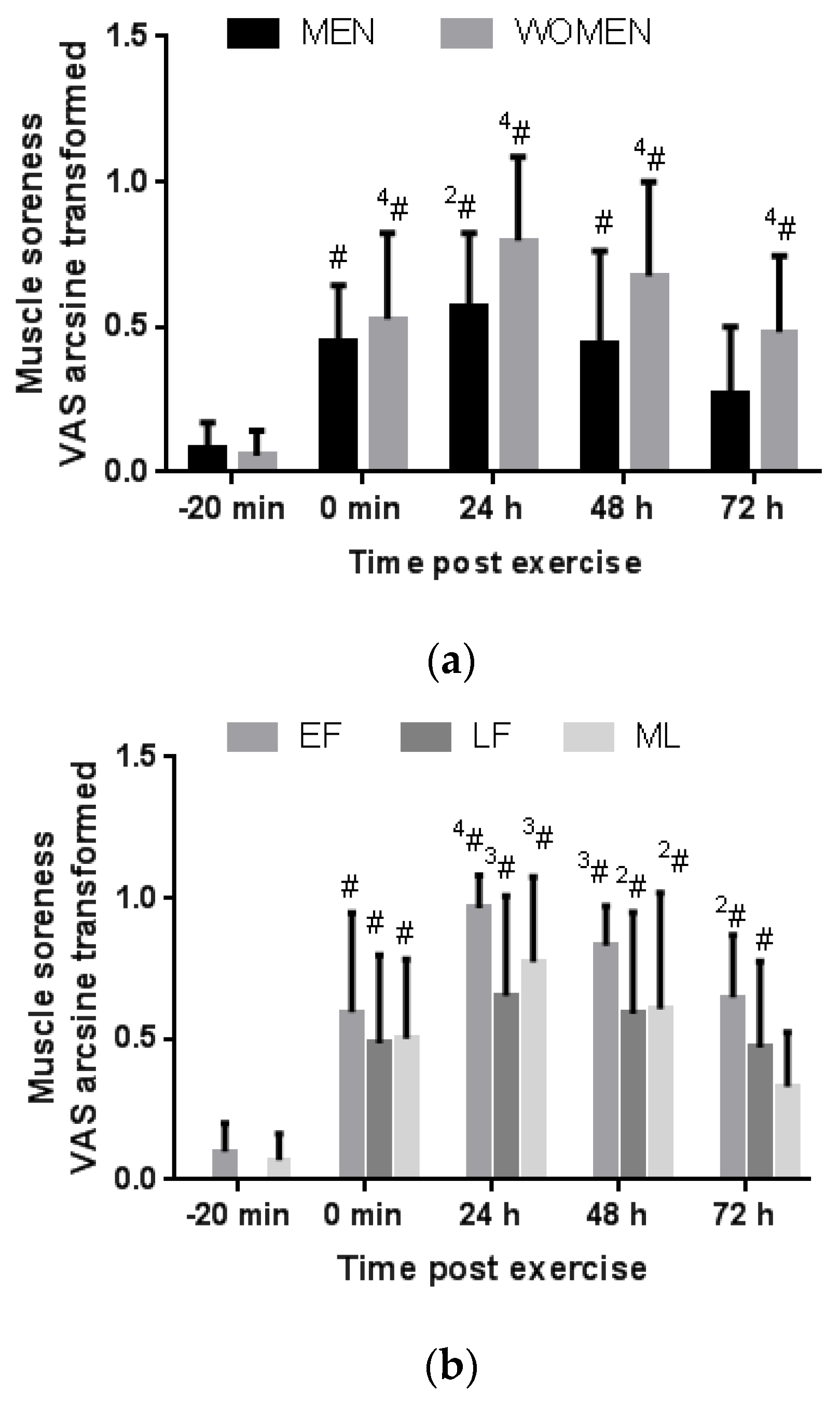
3.3.1. Gender Comparison
3.3.2. Menstrual Phase Comparison
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Brancaccio, P.; Maffulli, N.; Limongelli, F.M. Creatine kinase monitoring in sport medicine. Br. Med. Bull. 2007, 81–82, 209–230. [Google Scholar] [CrossRef] [PubMed]
- Enns, D.L.; Tiidus, P.M. The influence of estrogen on skeletal muscle: Sex matters. Sports Med. 2010, 40, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, J. The relationship of creatine kinase variability with body composition and muscle damage markers following eccentric muscle contractions. J. Exerc. Nutr. Biochem. 2015, 19, 123–129. [Google Scholar] [CrossRef] [PubMed]
- Sewright, K.A.; Hubal, M.J.; Kearns, A.; Holbrook, M.T.; Clarkson, P.M. Sex differences in response to maximal eccentric exercise. Med. Sci. Sports Exerc. 2008, 40, 242–251. [Google Scholar] [CrossRef] [PubMed]
- Mougois, V. Reference intervals for serum creatine kinase in athletes. Br. J. Sports Med. 2007, 41, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Stachenfeld, N.S.; Taylor, H.S. Challenges and methodology for testing young healthy women in physiological studies. Am. J. Physiol. Endocrinol. Metab. 2014, 306, E849–E853. [Google Scholar] [CrossRef] [PubMed]
- Claassen, H.; Schünke, M.; Kurz, B. Estradiol protects cultured articular chondrocytes from oxygen-radical-induced damage. Cell Tissue Res. 2005, 319, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Amelink, G.J.; Kamp, H.H.; Bär, P.R. Creatine kinase isoenzyme profiles after exercise in the rat: Sex-linked differences in leakage of CK-MM. Pflugers Arch. 1988, 412, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Amelink, G.J.; Koot, R.W.; Erich, W.B.; Van Gijn, J.; Bär, P.R. Sex-linked variation in creatine kinase release, and its dependence on oestradiol, can be demonstrated in an in vitro rat skeletal muscle preparation. Acta Physiol. Scand. 1990, 138, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Bär, P.R.; Amelink, G.J.; Oldenburg, B.; Blankenstein, M.A. Prevention of exercise-induced muscle membrane damage by oestradiol. Life Sci. 1988, 42, 2677–2681. [Google Scholar] [CrossRef]
- Komulainen, J.; Koskinen, S.O.; Kalliokoski, R.; Takala, T.E.; Vihko, V. Gender differences in skeletal muscle fibre damage after eccentrically biased downhill running in rats. Acta Physiol. Scand. 1999, 165, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Tiidus, P.M.; Holden, D.; Bombardier, E.; Zajchowski, S.; Enns, D.; Belcastro, A. Estrogen effect on post-exercise skeletal muscle neutrophil infiltration and calpain activity. Can. J. Physiol. Pharmacol. 2001, 79, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Tiidus, P.M.; Enns, D.L. Point:Counterpoint: Estrogen and sex do/do not influence post-exercise indexes of muscle damage, inflammation, and repair. J. Appl. Physiol. 2009, 106. [Google Scholar] [CrossRef]
- Hubal, M.J.; Clarkson, P.M. Counterpoint: Estrogen and sex do not significantly influence post-exercise indexes of muscle damage, inflammation, and repair. J. Appl. Physiol. 2009, 106, 1012–1014. [Google Scholar] [CrossRef] [PubMed]
- Pizza, F.X.; Clark, B.C.; De Meersman, R.E.; Philips, S.M.; Stupka, N.; Sipila, S.; Kovanen, V.; Suominen, H.; Sipila, S.; Warren, G.L.; Lowe, D.A.; et al. Comments on Point:Counterpoint: Estrogen and sex do/do not influence post-exercise indexes of muscle damage, inflammation, and repair. J. Appl. Physiol. 2009, 106, 1016–1020. [Google Scholar] [CrossRef] [PubMed]
- Minahan, C.; Joyce, S.; Bulmer, A.C.; Cronin, N.; Sabapathy, S. The influence of estradiol on muscle damage and leg strength after intense eccentric exercise. Eur. J. Appl. Physiol. 2015, 115, 1493–1500. [Google Scholar] [CrossRef] [PubMed]
- Stupka, N.; Lowther, S.; Chorneyko, K.; Bourgeois, J.M.; Hogben, C.; Tarnopolsky, M.A. Gender differences in muscle inflammation after eccentric exercise. J. Appl. Physiol. 2000, 89, 2325–2332. [Google Scholar] [PubMed]
- Sorichter, S.; Mair, J.; Koller, A.; Calzolari, C.; Huonker, M.; Pau, B.; Puschendorf, B. Release of muscle proteins after downhill running in male and female subjects. Scand. J. Med. Sci. Sports 2001, 11, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Thompson, H.S.; Scordilis, S.P.; De Souza, M.J. Serum creatine kinase activity varies with ovulatory status in regularly exercising, premenopausal women. Horm. Res. 2006, 65, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.; Dobridge, J.; Hackney, A.C. Influence of estrogen on markers of muscle tissue damage following eccentric exercise. Fizol. Cheloveka 2001, 27, 133–137. [Google Scholar]
- Dieli-Conwright, C.M.; Spektor, T.M.; Rice, J.C.; Sattler, F.R.; Schroeder, E.T. Hormone therapy attenuates exercise-induced skeletal muscle damage in postmenopausal women. J. Appl. Physiol. 2009, 107, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Markofski, M.M.; Braun, W.A. Influence of menstrual cycle on indices of contraction-induced muscle damage. J. Strength Cond. Res. 2014, 28, 2649–2656. [Google Scholar] [CrossRef] [PubMed]
- Cauci, S.; Francescato, M.P.; Curcio, F. Combined oral contraceptives increase high-sensitivity C-reactive protein but not haptoglobin in female athletes. Sports Med. 2016, in press. [Google Scholar] [CrossRef] [PubMed]
- Cauci, S.; Buligan, C.; Marangone, M.; Francescato, M.P. Oxidative stress in female athletes using combined oral contraceptives. Sports Med. 2016, 2, 40. [Google Scholar] [CrossRef] [PubMed]
- Casazza, G.A.; Jacobs, K.A.; Suh, S.-H.; Miller, B.F.; Horning, M.A.; Brooks, G.A. Menstrual cycle phase and oral contraceptive effects on triglyceride mobilization during exercise. J. Appl. Physiol. 2004, 97, 302–309. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M.; Neubauer, O.; Della Gatta, P.A.; Nosaka, K. Muscle damage and inflammation during recovery from exercise. J. Appl. Physiol. 2016, in press. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, D.L.; Reid, W.D.; McKenzie, D.C. Delayed muscle soreness. The inflammatory response to muscle injury and its clinical implications. Sports Med. 1995, 20, 24–40. [Google Scholar]
- Dannecker, E.A.; Liu, Y.; Rector, R.S.; Thomas, T.R.; Filingim, R.B.; Robinson, M.E. Sex differences in exercise-induced muscle pain and muscle damage. J. Pain 2012, 13, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Kerksick, C.; Taylor, L.; Harvey, A.; Willoughby, D. Gender-related differences in muscle injury, oxidative stress, and apoptosis. Med. Sci. Sports Exerc. 2008, 40, 1772–1780. [Google Scholar] [CrossRef] [PubMed]
- Thompson, H.S.; Hyatt, J.-P.; De Souza, M.J.; Clarkson, P.M. The ffects of oral contraceptives on delayed onset muscle soreness following exercise. Contraception 1997, 56, 59–65. [Google Scholar] [CrossRef]
- Oosthuyse, T.; Bosch, A.N.; Jackson, S. Cycling time trial performance during different phases of the menstrual cycle. Eur. J. Appl. Physiol. 2005, 94, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Eston, R.G.; Mickleborough, J.; Baltzopoulos, V. Eccentric activation and muscle damage: Biomechanical and physiological considerations during downhill running. Br. J. Sports Med. 1995, 29, 89–94. [Google Scholar] [CrossRef] [PubMed]
- Maciejczyk, M.; Więcek, M.; Szymura, J.; Szyguła, Z. Comparison of physiological and acid-base balance response during uphill, level and downhill running performed at constant velocity. Acta Physiol. Hung. 2013, 100, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Robergs, R.A.; Wagner, D.R.; Skemp, K.M. Oxygen consumption and energy expenditure of level versus downhill running. J. Sports Med. Phys. Fit. 1997, 37, 168–174. [Google Scholar]
- Eston, R.G.; Lemmey, A.B.; McHugh, P.; Byrne, C.; Walsh, S.E. Effect of stride length on symptoms of exercise-induced muscle damage during a repeated bout of downhill running. Scand J. Med. Sci. Sports 2000, 10, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Rowlands, A.V.; Eston, R.G.; Tilzey, C. Effect of stride length manipulation on symptoms of exercise-induced muscle damage and the repeated bout effect. J. Sports Sci. 2001, 19, 333–340. [Google Scholar] [CrossRef] [PubMed]
- Van de Vyver, M.; Engelbrecht, L.; Smith, C.; Myburgh, K.H. Neutrophil and monocyte responses to downhill running: Intracellular contents of MPO, IL-6, IL-10, pstat3, and SOCS3. Scand. J. Med. Sci. Sports 2016, 26, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Ferreira-Valente, M.A.; Pais-Ribeiro, J.L.; Jensen, M.P. Validity of four pain intensity rating scales. Pain 2011, 152, 2399–2404. [Google Scholar] [CrossRef] [PubMed]
- Canalias, F.; Saco, Y.; Gella, F.-J. Comparison of kits for the determination of creatine kinase activity in serum. Eur. J. Clin. Chem. Clin. Biochem. 1995, 33, 535–539. [Google Scholar] [PubMed]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive statistics for studies in sports medicine and exercise science. Med. Sci. Sports Exerc. 2009, 41, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Tiidus, P.M.; Bombardier, E. Oestrogen attenuates post-exercise myeloperoxidase activity in skeletal muscle of male rats. Acta Physiol. Scand. 1999, 166, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Enns, D.L.; Tiidus, P.M. Estrogen influences satellite cell activation and proliferation following downhill running in rats. J. Appl. Physiol. 2008, 104, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Cadwell Hooper, A.E.; Bryan, A.D.; Eaton, M. Menstrual cycle effects on perceived exertion and pain during exercise among sedentary women. J. Women’s Health 2011, 20, 439–446. [Google Scholar] [CrossRef] [PubMed]
- Iacovides, S.; Avidon, I.; Baker, F.C. Dose pain vary across the menstrual cycle? A review. Eur. J. Pain 2015, 19, 1389–1405. [Google Scholar] [CrossRef] [PubMed]
| Parameter | Men (n = 6) | Women (n = 15) | EF (n = 5) | LF (n = 5) | ML (n = 5) |
|---|---|---|---|---|---|
| Age (years) | 21.8 ± 0.9 | 21.7 ± 2.4 | 20.7 ± 0.5 | 23.3 ± 2.4 | 21.2 ± 4.4 |
| Body mass (kg) | 71.4 ± 10.5 | 58.0 ± 10.2 a | 55.6 ± 7.8 | 57.6 ± 4.9 | 60.8 ± 16.2 |
| Height (cm) | 179.2 ± 8.5 | 160.6 ± 5.5 a | 158.1 ± 5.6 a | 161.6 ± 3.1 a | 162.1 ± 7.2 a |
| BMI (kg/m2) | 22.1 ± 1.8 | 22.4 ± 3.4 | 22.5 ± 4.7 | 22.0 ± 1.2 | 22.8 ± 4.0 |
| 17β-Oestradiol (pmol/L) | 156.7 ± 51.6 | 552.3 ± 435.7 a | 166.0 ±88.8 | 990.8 ± 406.6 a,b,c | 500.0 ± 246.6 a,b |
| Progesterone (nmol/L) | 2.7 ± 0.5 | 10.8 ± 12.0 | 2.3 ± 0.9 | 4.1 ± 1.5 | 25.9 ± 8.2 a,b,d |
| Length of MC (days) | 29.4 ± 4.5 | 29.2 ± 5.3 | 29.2 ± 5.8 | 29.8 ± 2.5 | |
| MC day of running | 3.6 ± 1.1 | 14.4 ± 2.7 | 20.4 ± 2.2 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oosthuyse, T.; Bosch, A.N. The Effect of Gender and Menstrual Phase on Serum Creatine Kinase Activity and Muscle Soreness Following Downhill Running. Antioxidants 2017, 6, 16. https://doi.org/10.3390/antiox6010016
Oosthuyse T, Bosch AN. The Effect of Gender and Menstrual Phase on Serum Creatine Kinase Activity and Muscle Soreness Following Downhill Running. Antioxidants. 2017; 6(1):16. https://doi.org/10.3390/antiox6010016
Chicago/Turabian StyleOosthuyse, Tanja, and Andrew N. Bosch. 2017. "The Effect of Gender and Menstrual Phase on Serum Creatine Kinase Activity and Muscle Soreness Following Downhill Running" Antioxidants 6, no. 1: 16. https://doi.org/10.3390/antiox6010016






