Fractioning of Proanthocyanidins of Uncaria tomentosa. Composition and Structure-Bioactivity Relationship
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material, Chemicals and Reagents
2.2. Extraction and Fractioning of Phenolic Compounds
2.3. Analysis of Phenolic Compounds by UPLC-DAD-ESI-TQ MS
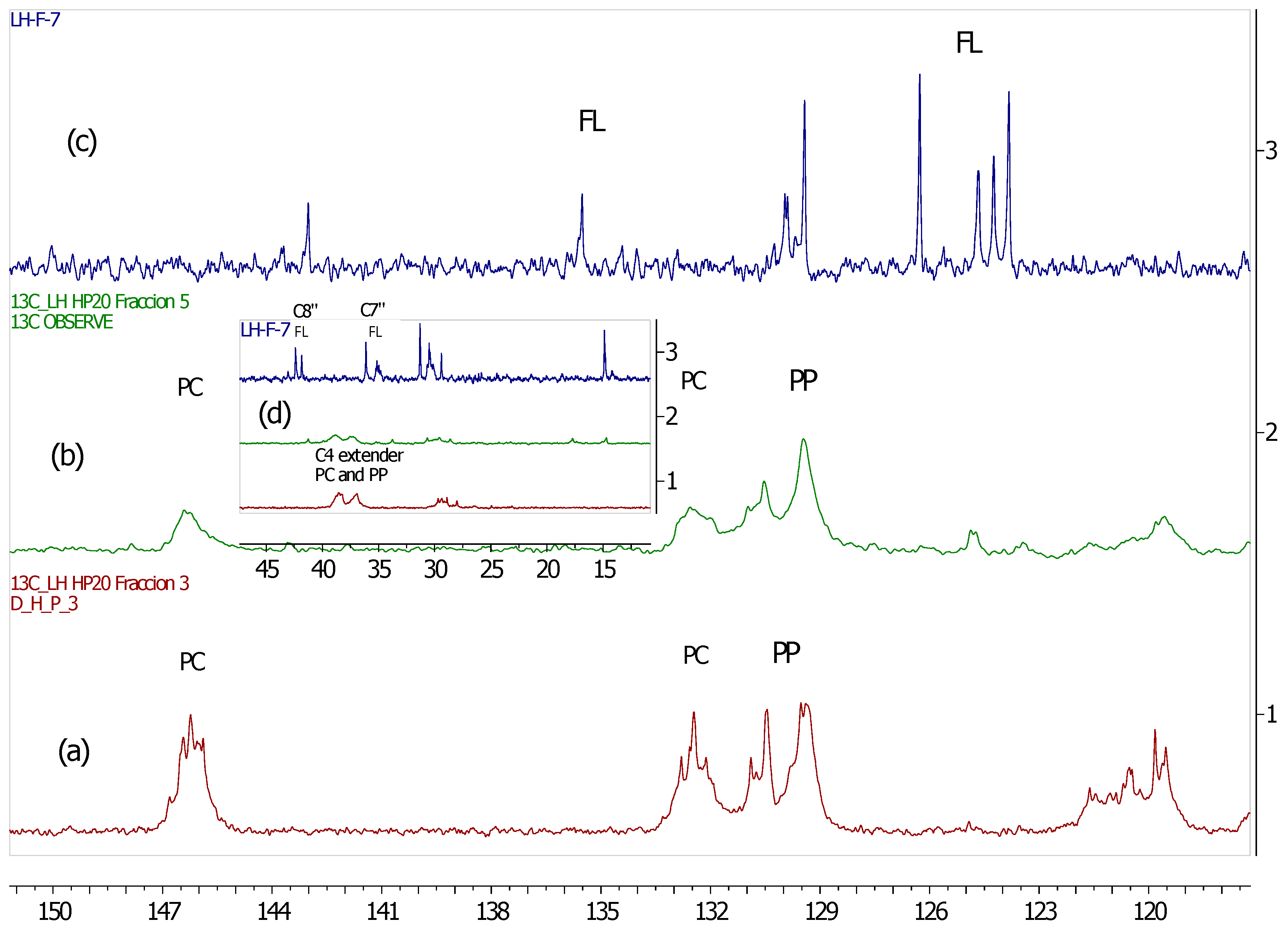
2.4. Analysis by 13C-NMR
2.5. In Vitro Antioxidant Activity
2.6. Assessment of Cytotoxicity
3. Results
3.1. Polyphenolic Composition by UPLC/TQ-ESI-MS
3.2. 13C-NMR Analysis of U. tomentosa Fractions
3.3. Antioxidant Activity
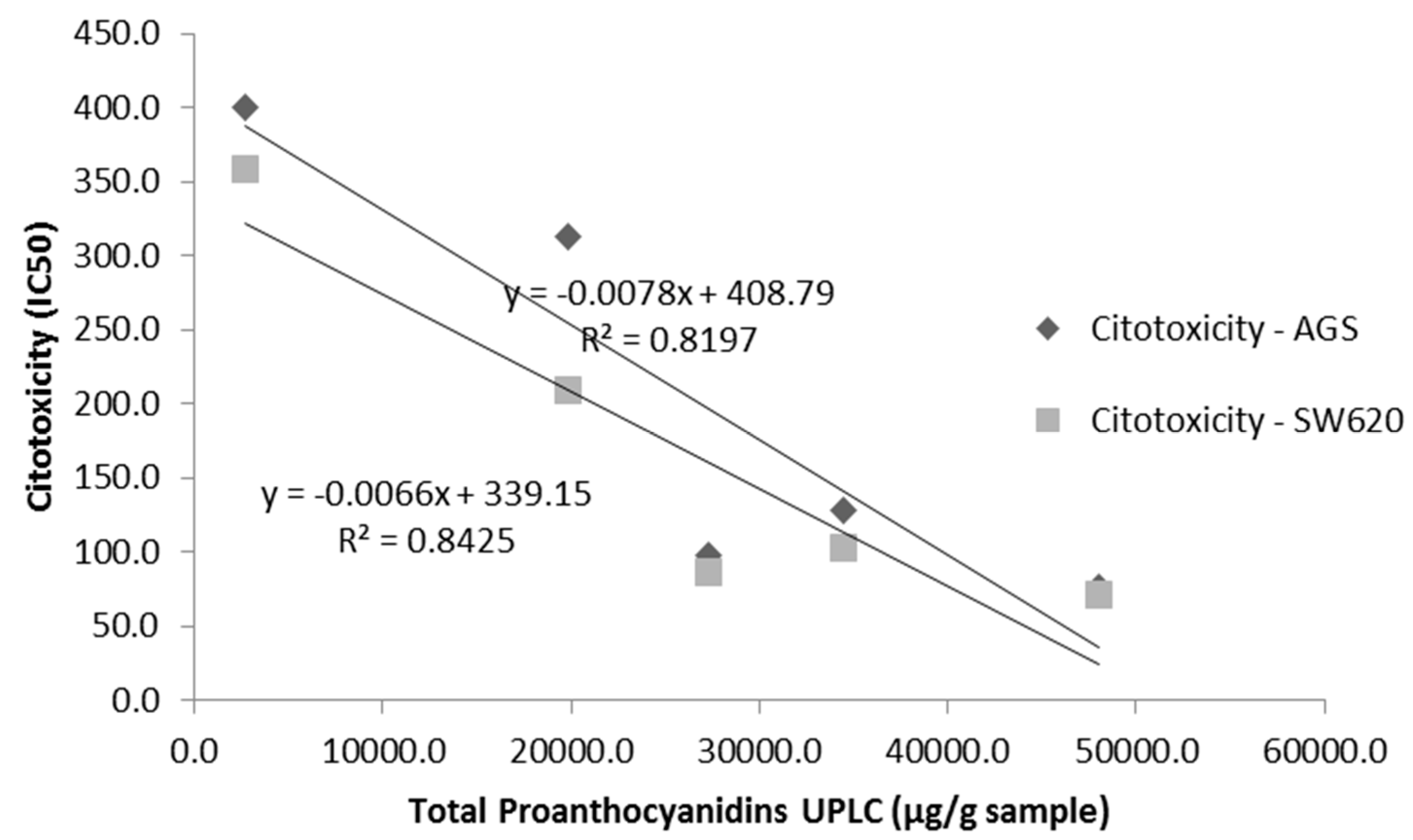
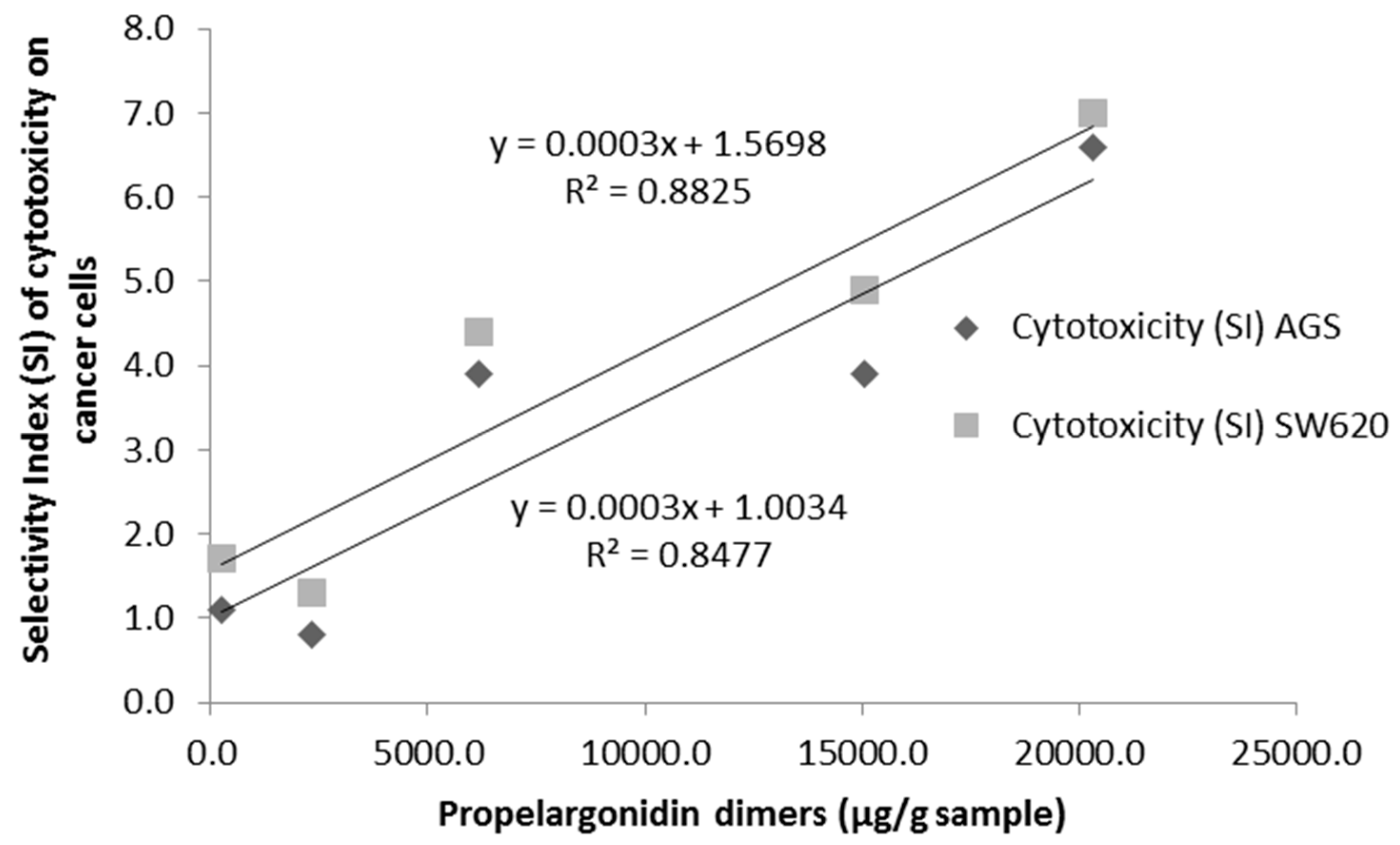
3.4. Cytotoxicity of U. tomentosa Fractions
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cos, P.; De Bruyne, T.; Hermans, S.; Apers, S.; Berghe, D.V.; Vlietinck, A.J. Proanthocyanidins in health care: Current and new trends. Curr. Med. Chem. 2003, 10, 1345–1359. [Google Scholar] [CrossRef]
- Aron, P.M.; Kennedy, J.A. Flavan-3-ols: Nature, occurrence and biological activity. Mol. Nutr. Food Res. 2008, 52, 79–104. [Google Scholar] [CrossRef] [PubMed]
- Barrajon-Catalan, E.; Herranz-López, M.; Joven, J.; Segura-Carretero, A.; Alonso-Villaverde, C.; Menéndez, J.A.; Micol, V. Oxidative Stress and Inflammation in Non-Communicable Diseases-Molecular Mechanisms and Perspectives. In Experimental Medicine and Biology, 1st ed.; Camps, J., Ed.; Springer International Publishing: Cham, Switzerland, 2014; Volume 824, pp. 141–159. ISBN 978-3-319-55857-8. [Google Scholar]
- Loizzo, M.; Said, A.; Tundis, R.; Hawas, U.; Rashed, K.; Menichini, F.; Frega, N.; Menichini, F. Antioxidant and antiproliferative activity of Diospyros lotus L. extract and isolated compounds. Plant Foods Hum. Nutr. 2009, 64, 264–270. [Google Scholar] [CrossRef] [PubMed]
- Ramljak, D.; Romanczyk, L.J.; Metheny-Barlow, L.J.; Thompson, N.; Knezevic, V.; Galperin, M.; Ramesh, A.; Dickso, R.B. Pentameric procyanidin from Theobroma cacao selectively inhibits growth of human breast cancer cells. Mol. Cancer Ther. 2005, 4, 537–546. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.H.; Sun, J. Antiproliferative activity of apples is not due to phenolic induced hydrogen peroxide formation. J. Agric. Food Chem. 2003, 51, 1718–1723. [Google Scholar] [CrossRef] [PubMed]
- Weaver, J.; Briscoe, T.; Hou, M.; Goodman, C.; Kata, S.; Ross, H.; McDougall, G.; Stewart, D.; Riches, A. Strawberry polyphenols are equally cytotoxic to tumourigenic and normal human breast and prostate cell lines. Int. J. Oncol. 2009, 34, 777–786. [Google Scholar] [CrossRef] [PubMed]
- Heitzman, M.E.; Neto, C.C.; Winiarz, E.; Vaisberg, A.J.; Hammond, G.B. Ethnobotany, Phytochemistry and Pharmacology of Uncaria (Rubiaceae). Phytochemistry 2005, 66, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, J.J.; Xu, J.; Feng, F.; Qu, W. Medicinal uses, phytochemistry and pharmacology of the genus Uncaria. Review. J. Ethnopharmacol. 2015, 173, 48–80. [Google Scholar] [CrossRef] [PubMed]
- Falkiewicz, B.; Lukasiak, J. Vilcacora [Uncaria tomentosa (Willd.) DC and Uncaria guianensis (Aublet) Gmell.]—A review of published scientific literature. Case Rep. Clin. Pract. Rev. 2001, 2, 305–316. [Google Scholar]
- Choi, H.R.; Choi, J.S.; Han, Y.N.; Bae, S.J.; Chung, H.Y. Peroxynitrite Scavenging Activity of Herb Extracts. Phytother. Res. 2002, 16, 364–367. [Google Scholar] [CrossRef] [PubMed]
- Sandoval, M.; Okuhama, N.N.; Zhang, X.J.; Condezo, L.A.; Lao, J.; Angeles, F.M.; Musah, R.A.; Bobrowski, P.; Miller, M.J.S. Anti-inflammatory and antioxidant activities of cat’s claw (Uncaria tomentosa and Uncaria guianensis) are independent of their alkaloid content. Phytomedicine 2002, 9, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Hoyos, M.; Sanchez, F.; Murillo, R.; Martín, P.; Zamora, W.; Monagas, M.; Bartolomé, B. Phenolic assesment of Uncaria tomentosa L. (Cat’s Claw): Leaves, stem, bark and wood extracts. Molecules 2015, 20, 22703–22717. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Hoyos, M.; Lebrón-Aguilar, R.; Quintanilla-López, J.E.; Cueva, C.; Hevia, D.; Quesada, S.; Gabriela Azofeifa, G.; Moreno-Arribas, M.V.; María, M.; Bartolomé, B. Proanthocyanidin Characterization and Bioactivity of Extracts from Different Parts of Uncaria tomentosa L. (Cat’s Claw). Antioxidants 2017, 6, 12. [Google Scholar] [CrossRef] [PubMed]
- Monagas, M.; Urpi-Sarda, M.; Sanchez-Patán, F.; Llorach, R.; Garrido, I.; Gómez-Cordoves, C.; Andres-Lacueva, C.; Bartolome, B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Funct. 2010, 1, 233–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Patán, F.; Monagas, M.; Moreno-Arribas, M.V.; Bartolome, B. Determination of microbial phenolic acids in human faeces by UPLC-ESI-TQ MS. J. Agric. Food Chem. 2011, 59, 2241–2247. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Patan, F.; Cueva, C.; Monagas, M.; Walton, G.E.; Gibson, G.R.; Martin-Alvarez, P.J.; Moreno-Arribas, M.V.; Bartolome, B. Gut microbial catabolism of grape seed flavan-3-ols by human faecal microbiota. Targeted analysis of precursor compounds, intermediate metabolites and end products. Food Chem. 2011, 131, 337–347. [Google Scholar] [CrossRef] [Green Version]
- Davalos, A.; Gomez-Cordoves, C.; Bartolome, B. Extending applicability of the oxygen radical absorbance capacity (ORAC-Fluorescein) assay. J. Agric. Food Chem. 2004, 52, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Mahavorasirikul, W.; Viyanant, V.; Chaijaroenkul, W.; Itharat, A.; Na-Bangchang, K. Cytotoxic activity of Thai medicinal plants against human cholangiocarcinoma, laryngeal and hepatocarcinoma cells in vitro. BMC. Complement. Altern. Med. 2010, 10, 55. [Google Scholar] [CrossRef] [PubMed]
- Czochanska, Z.; Foo, L.Y.; Newman, R.H.; Porter, L.J. Polymeric proanthocyanidins, Stereochemistry, structural units and molecular weight. J. Chem. Soc. Perkin Trans. 1980, 1, 2278–2286. [Google Scholar] [CrossRef]
- Fu, C.; Wang, H.; Ling, W.; Song, L.; Huang, D. Antioxidant Activity and Proanthocyanidin Profile of Selliguea feei Rhizomes. Molecules 2013, 18, 4282–4292. [Google Scholar] [CrossRef] [PubMed]
- Adams, L.S.; Phung, S.; Yee, N.; Seeram, N.P.; Li, L.; Chen, S. Blueberry Phytochemicals inhibit growth and metastatic potential of MDA-MB-231 Breast cancer cells through modulation of the phosphatidylinositol 3-kinase pathway. Cancer Res. 2010, 70, 3594–3605. [Google Scholar] [CrossRef] [PubMed]
- Pizzolatti, M.G.; Venson, A.F.; Smânia, A.J.; Smânia Ede, F.; Braz-Filho, R. Two epimeric flavalignans from Trichilia catigua (Meliaceae) with antimicrobial activity. Z. Naturforsch. C 2002, 57, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Lizcano, L.J.; Bakkali, F.; Ruiz-Larrea, B.; Ruiz-Sanz, J.I. Antioxidant activity and polyphenol content of aqueous extracts from Colombia Amazonian plants with medicinal use. Food Chem. 2010, 119, 1566–1570. [Google Scholar] [CrossRef]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Duthie, S.J. Berry phytochemicals, genomic stability and cancer: Evidence for chemoprotection at several stages in the carcinogenic process. Mol. Nutr. Food Res. 2007, 51, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.; Adams, L.; Zhang, Y.; Lee, R.; Sand, D.; Scheuller, H.; Heber, D. Blackberry, black raspberry, blueberry, cranberry, red raspberry and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J. Agric. Food Chem. 2006, 54, 9329–9339. [Google Scholar] [CrossRef] [PubMed]
- Stoner, G.; Wang, L.; Casto, B. Laboratory and clinical studies of cancer chemoprevention by antioxidants in berries. Carcinogenesis 2008, 29, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Seeram, N.; Lee, R.; Heber, D. Bioavailability of ellagic acid in human plasma after consumption of ellagitannins from pomegranate (Punica granatum L.) juice. Clin. Chim. Acta 2004, 348, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Liu, X.F.; Zheng, P.S. Grape seed proanthocyanidins (GSPs) inhibit the growth of cervical cancer by inducing apoptosis mediated by the mitochondrial pathway. PLoS ONE 2014, 9, e107045. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Krohn, R.L.; Liu, W.; Joshi, S.S.; Kuszynski, C.A.; McGinn, T.R.; Bagchi, M.; Preuss, H.G.; Stohs, S.J.; Bagchi, D. The cytotoxic effects of a novel IH636 grape seed proanthocyanidin extract on cultured human cancer cells. Mol. Cell. Biochem. 1999, 196, 99–108. [Google Scholar] [CrossRef] [PubMed]
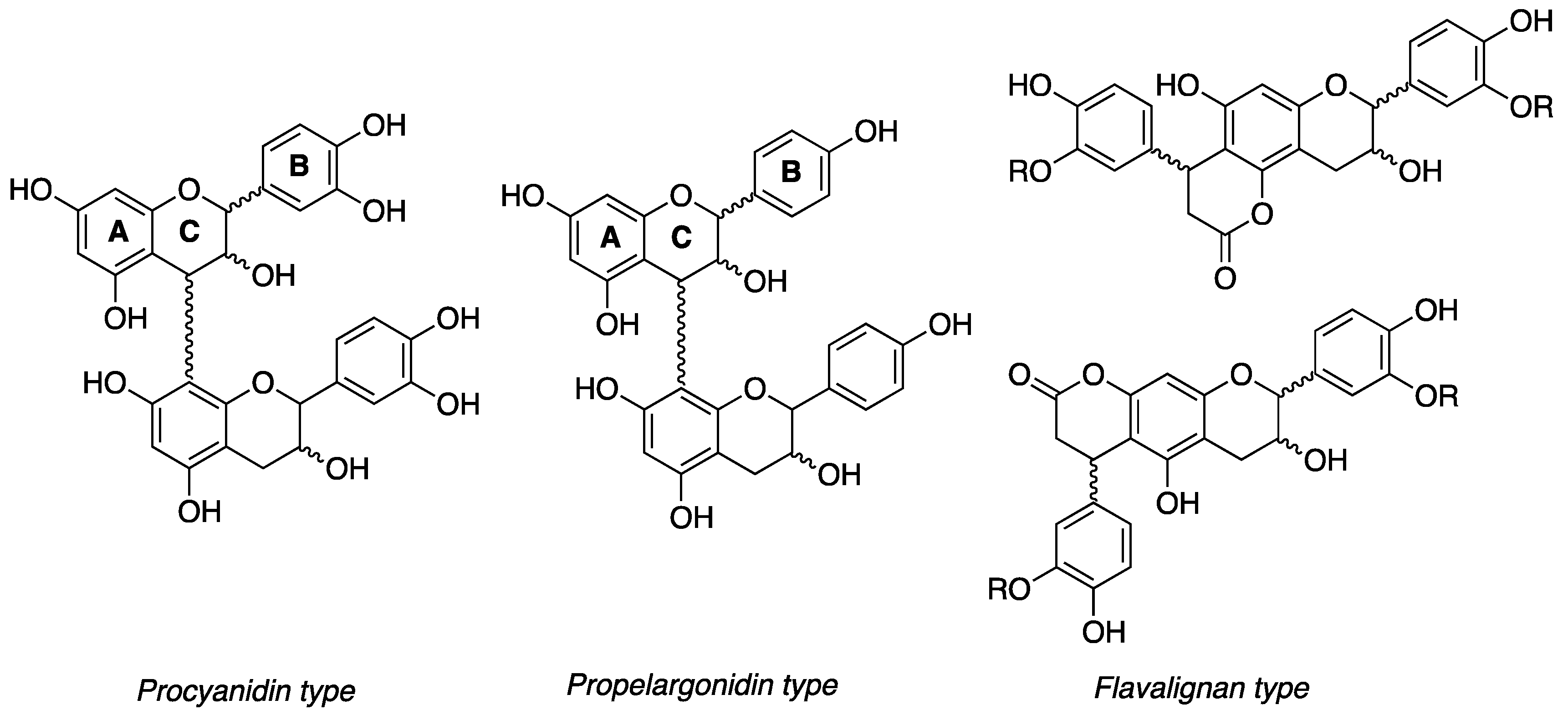
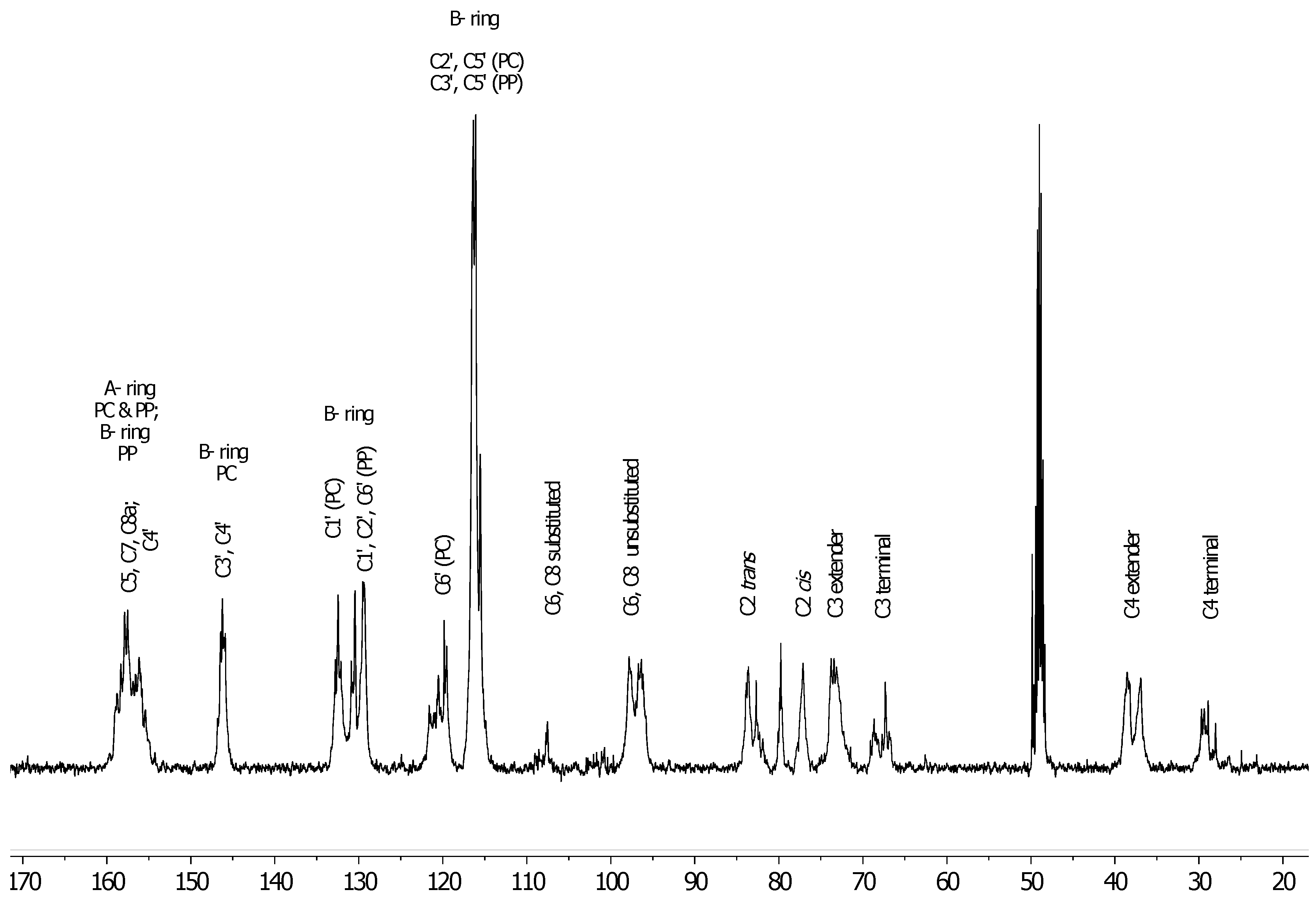
| COMPOUNDS | LH-F3 | LH-F4 | LH-F5 | LH-F6 | LH-F7 |
|---|---|---|---|---|---|
| Concentration (µg/g Extract) | |||||
| Hydroxybenzoic acids | |||||
| Benzoic acid | 24.7 ± 1.2 | 57.2 ± 3.0 | 401.0 ± 23 | 1857.1 ± 59 | 223.1 ± 11 |
| Salicylic acid | 9.2 ± 0.6 | 81.05 ± 4.0 | 23.7 ± 0.8 | 1.6 ± 0.0 | nd |
| 4-Hydroxybenzoic acid | 83.9 ± 3.1 | 254.35 ± 9 | 78.1 ± 4.5 | 20.8 ± 1.9 | 5.8 ± 0.5 |
| Protocatechuic acid | 147.9 ± 6 | 117.5 ± 2 | 28.0 ± 2.1 | 15.9 ± 0.7 | 4.5 ± 0.2 |
| Gallic acid | 23.2 ± 2.4 | 11.5 ± 1.1 | 1.6 ± 0.2 | 1.2 ± 0.1 | nd |
| Vanillic acid | 29.2 ± 0.6 | 63.6 ± 1.7 | 63.8 ± 4.3 | 11.5 ± 0.8 | nd |
| Syringic acid | 2.2 ± 0.1 | 31.4 ± 0.8 | 49.2 ± 0.8 | 11.9 ± 0.8 | nd |
| ∑ Hydroxybenzoic acids | 320.3 | 616.6 | 645.4 | 1920.0 | 233.4 |
| Hydroxycinnamic acids | |||||
| p-Coumaric acid | 4.9 ± 0.3 | 52.2 ± 2.1 | 178.7 ± 11 | 213.6 ± 8 | 11.0 ± 0.7 |
| Caffeic acid | 12.7 ± 0.6 | 32.7 ± 1.4 | 12.0 ± 0.9 | 4.6 ± 0.1 | 2.1 ± 0.1 |
| Ferulic acid | nd | 5.5 ± 0.1 | 146.0 ± 4 | 528.3 ± 13 | 49.5 ± 2.3 |
| Isoferulic acid | nd | 9.0 ± 0.1 | 35.2 ± 0.8 | 151.0 ± 3.3 | 46.8 ± 3.5 |
| ∑ Hydroxycinnamic acids | 17.6 | 99.4 | 371.9 | 897.5 | 109.4 |
| Flavan-3-ols: monomers | |||||
| (+)-Catechin | 2285.7 ± 165.3 | 676.6 ± 24.4 | 140.3 ± 6.8 | 50.2 ± 0.2 | 9.9 ± 0.4 |
| (−)-Epicatechin | 3854.9 ± 287.1 | 2597.0 ± 191.8 | 641.9 ± 56.1 | 165.5 ± 4.9 | 63.0 ± 2.4 |
| ∑ Monomers | 6140.6 | 3273.6 | 782.2 | 215.7 | 72.9 |
| Flavan-3-ols: procyanidin dimers | |||||
| Procyanidin B1 | 1875.4 ± 124.7 | 389.2 ± 15.9 | 83.1 ± 3.6 | 37.1 ± 1.8 | 9.5 ± 0.3 |
| Procyanidin B2 | 3327.4 ± 260.1 | 1137.7 ± 86.7 | 188.7 ± 20.7 | 65.9 ± 6.2 | 13.7 ± 1.4 |
| Procyanidin B3 | 5071.0 ± 273.4 | 992.9 ± 26.3 | 175.9 ± 10.1 | 97.0 ± 8.5 | 28.2 ± 1.5 |
| Procyanidin B4 | 9885.9 ± 490.1 | 3415.9 ± 73.9 | 500.7 ± 31.7 | 203.2 ± 17.7 | 54.4 ± 5.0 |
| Procyanidin B5 | 370.4 ± 15.4 | 801.8 ± 27.6 | 484.1 ± 29.4 | 76.4 ± 6.2 | nd |
| Procyanidin B7 | 760.0 ± 51.9 | 440.5 ± 24.8 | 73.5 ± 1.3 | 19.3 ± 0.5 | nd |
| Procyanidin B (5.40 min) | 616.6 ± 45.9 | 510.0 ± 36.0 | 91.6 ± 3.0 | nd | nd |
| Procyanidin B (9.21 min) | 49.1 ± 0.0 | 156.0 ± 1.5 | 70.8 ± 2.4 | 15.7 ± 1.6 | nd |
| ∑ Procyanidin dimers | 21,955.8 | 7844 | 1668.4 | 514.6 | 105.8 |
| Flavan-3-ols: propelargonidin trimers | |||||
| Propelargonidin dimer (4.36 min) | 9611.4 ± 606.2 | 3470.3 ± 173.6 | 679.4 ± 31.7 | 273.3 ± 16.6 | 76.5 ± 5.1 |
| Propelargonidin dimer (4.97 min) | 5776.7 ± 381.7 | 4734.4 ± 227.0 | 1098.1 ± 81.9 | 279.5 ± 20.4 | 59.3 ± 1.2 |
| Propelargonidin dimer (5.57 min) | 4806.7 ± 284.9 | 6066.8 ± 218.7 | 2410.4 ± 189.5 | 382.3 ± 23.5 | 49.7 ± 2.6 |
| Propelargonidin dimer (9.21 min) | 111.5 ± 11.7 | 793.4 ± 19.6 | 1971.8 ± 73.1 | 1376.3 ± 78.1 | 66.0 ± 5.9 |
| ∑ Properlargonidin dimers | 20,306.3 | 15,064.9 | 6159.7 | 2311.4 | 251.5 |
| Flavan-3-ols: procyanidin trimers | |||||
| Trimer T2 | 291.7 ± 13.1 | nd | nd | nd | nd |
| Procyanidin C1 | 799.0 ± 43.0 | 243.0 ± 23.5 | 57.5 ± 1.0 | nd | nd |
| Trimer B (4.51 min) | 1980.7 ± 58.2 | 327.6 ± 32.5 | 76.1 ± 8.2 | nd | nd |
| ∑ Procyanidin trimers | 3071.4 | 570.6 | 133.6 | nd | nd |
| Flavalignans | |||||
| Cinchonain (7.30 min) | 1156.3 ± 104.2 | 4031.0 ± 231.7 | 2392.7 ± 222.5 | 418.8 ± 21.8 | 19.0 ± 0.1 |
| Cinchonain (9.00 min) | 713.2 ± 58.4 | 3823.0 ± 149.3 | 7342.0 ± 495.8 | 3656.1 ± 192.9 | 188.7 ± 3.7 |
| Cinchonain (9.24 min) | 595.9 ± 52.6 | 2856.4 ± 125.1 | 7488.5 ± 693.7 | 4785.4 ± 302.6 | 264.8 ± 5.9 |
| Cinchonain (12.22 min) | 251.2 ± 21.1 | 230.2 ± 12.0 | 2154.1 ± 165.6 | 8171.1 ± 417.5 | 1908.0 ± 7.9 |
| ∑ Flavalignans | 2716.6 | 10,940.6 | 19,377.3 | 17,031.4 | 2380.5 |
| Fractions | Total Proanthocyanidins 1 (µg/g Extract) | ORAC Value (mmol TE/g Extract) |
|---|---|---|
| LH-F3 | 48,049.9 | 11.28 ± 0.06 |
| LH-F4 | 34,420.1 | 8.28 ± 0.09 |
| LH-F5 | 27,339.1 | 7.38 ± 0.06 |
| LH-F6 | 19,857.2 | 7.06 ± 0.02 |
| LH-F7 | 2738.0 | 3.37 ± 0.10 |
| Sample | AGS Cells IC50 1 (SI) 2 | SW620 Cells IC50 1 (SI) 2 | Vero Cells IC50 1 |
|---|---|---|---|
| LH-F3 | 75.6 ± 4.2 (6.6) | 71.4 ± 4.6 (7.0) | >500 |
| LH-F4 | 128 ± 4 (3.9) | 103 ± 8 (4.9) | >500 |
| LH-F5 | 97.6 ± 0.9 (3.9) | 86.2 ± 0.9 (4.4) | 377 ± 23 |
| LH-F6 | 313± 6 (0.8) | 209 ± 5 (1.3) | 261 ± 29 |
| LH-F7 | 358 ± 24 (1.1) | 232 ± 1 (1.7) | 400 ± 31 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro, M.; Zamora, W.; Quesada, S.; Azofeifa, G.; Alvarado, D.; Monagas, M. Fractioning of Proanthocyanidins of Uncaria tomentosa. Composition and Structure-Bioactivity Relationship. Antioxidants 2017, 6, 60. https://doi.org/10.3390/antiox6030060
Navarro M, Zamora W, Quesada S, Azofeifa G, Alvarado D, Monagas M. Fractioning of Proanthocyanidins of Uncaria tomentosa. Composition and Structure-Bioactivity Relationship. Antioxidants. 2017; 6(3):60. https://doi.org/10.3390/antiox6030060
Chicago/Turabian StyleNavarro, Mirtha, William Zamora, Silvia Quesada, Gabriela Azofeifa, Diego Alvarado, and Maria Monagas. 2017. "Fractioning of Proanthocyanidins of Uncaria tomentosa. Composition and Structure-Bioactivity Relationship" Antioxidants 6, no. 3: 60. https://doi.org/10.3390/antiox6030060






