Total Phenolic and Yellow Pigment Contents and Antioxidant Activities of Durum Wheat Milling Fractions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Durum Wheat Samples and Milling
2.2. Total Yellow Pigment Extraction and Measurement
2.3. Analysis of Total Phenolic Content
2.4. Trolox Equivalent Antioxidant Capacity Assay
2.5. UPLC Analysis of Carotenoids
2.6. Statistical Analyses
3. Results and Discussion
3.1. TYP Content of Durum Wheat Milling Fractions
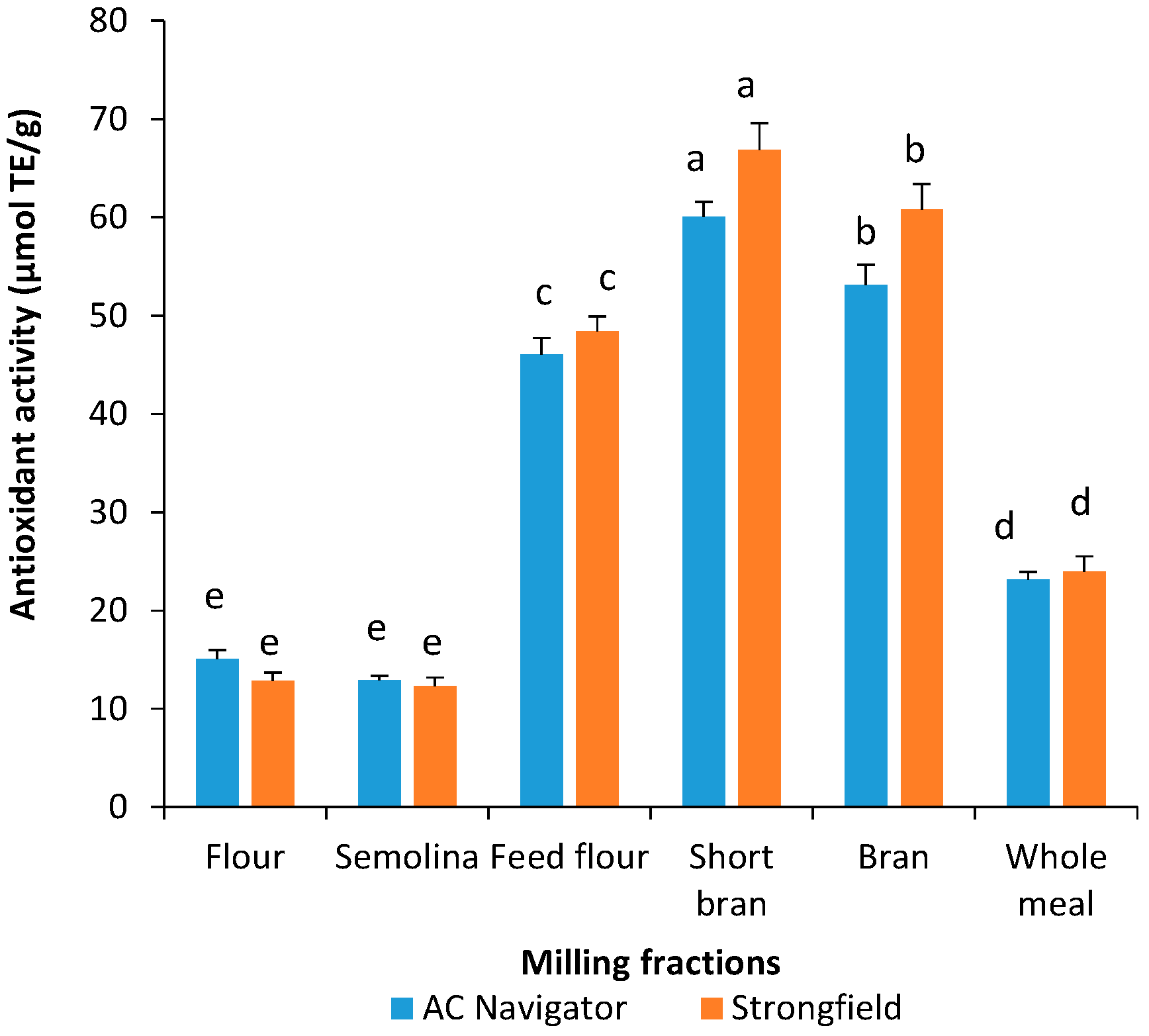
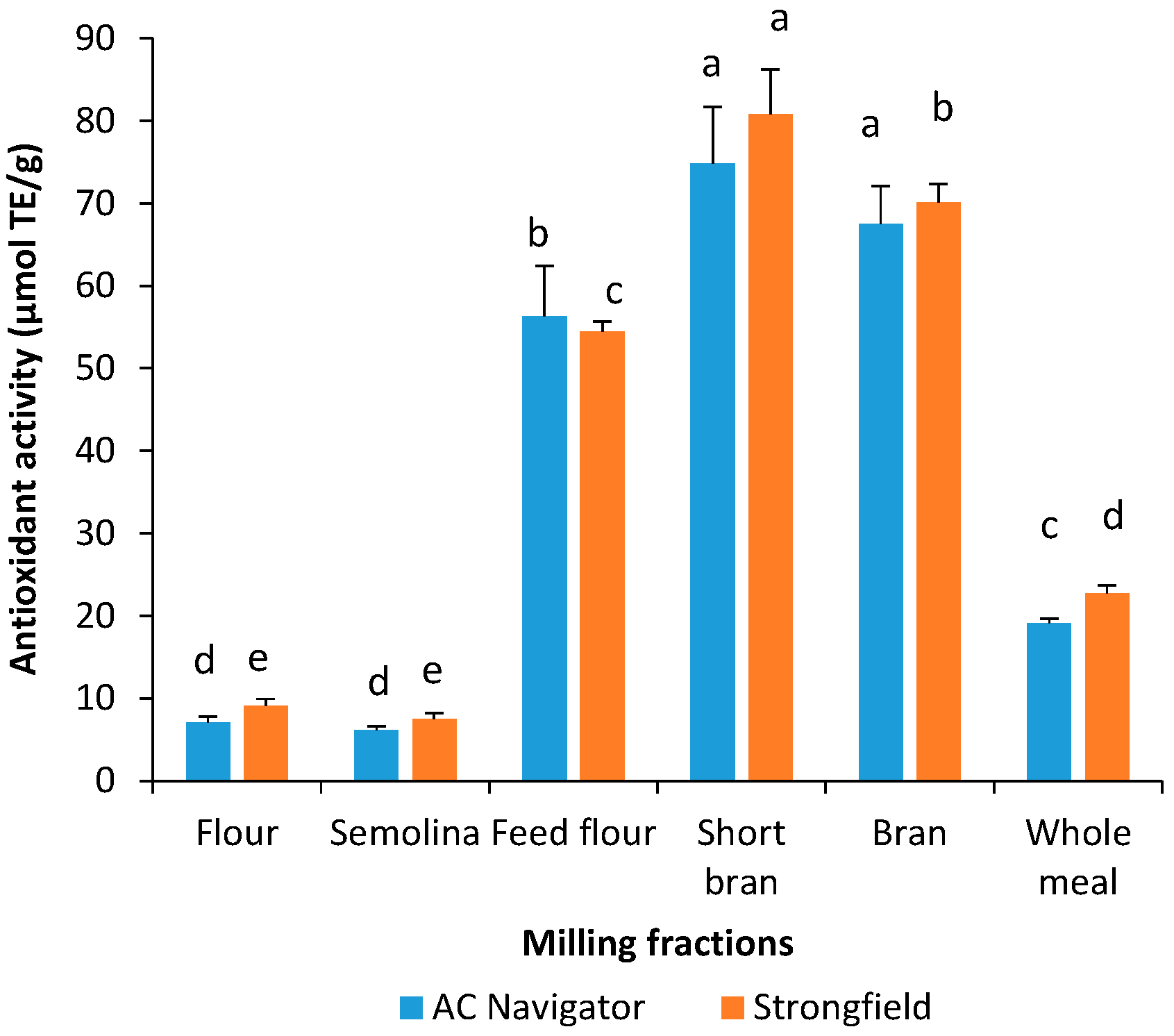
3.2. Total Phenolic Content and Antioxidant Activity of Acidified Methanol Extracts
3.3. Total Phenolic Content and Antioxidant Activity of Water-Saturated Butanol Extracts
3.4. Relationship between, Total Phenolic Compounds, Yellow Pigments and Antioxidant Activity
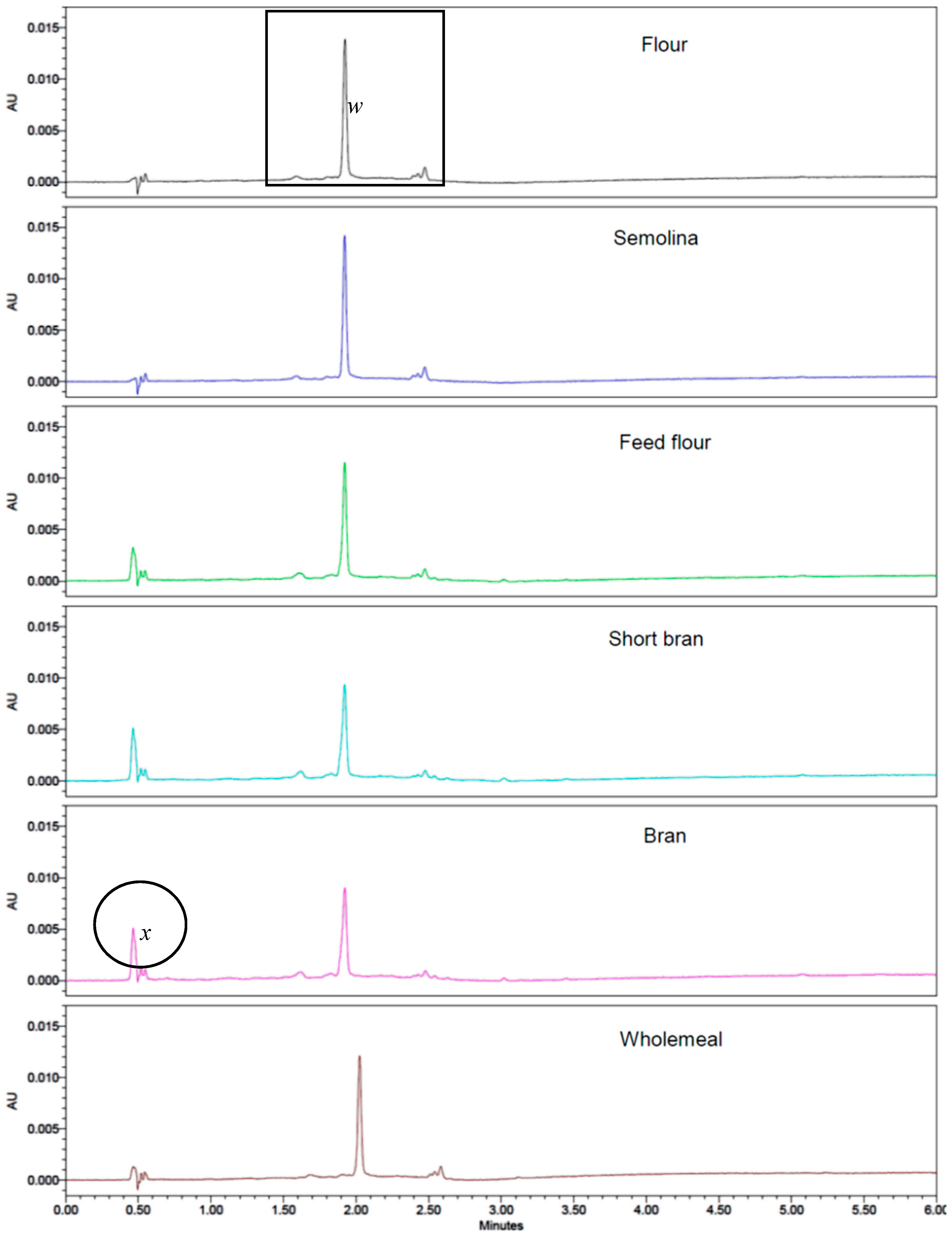
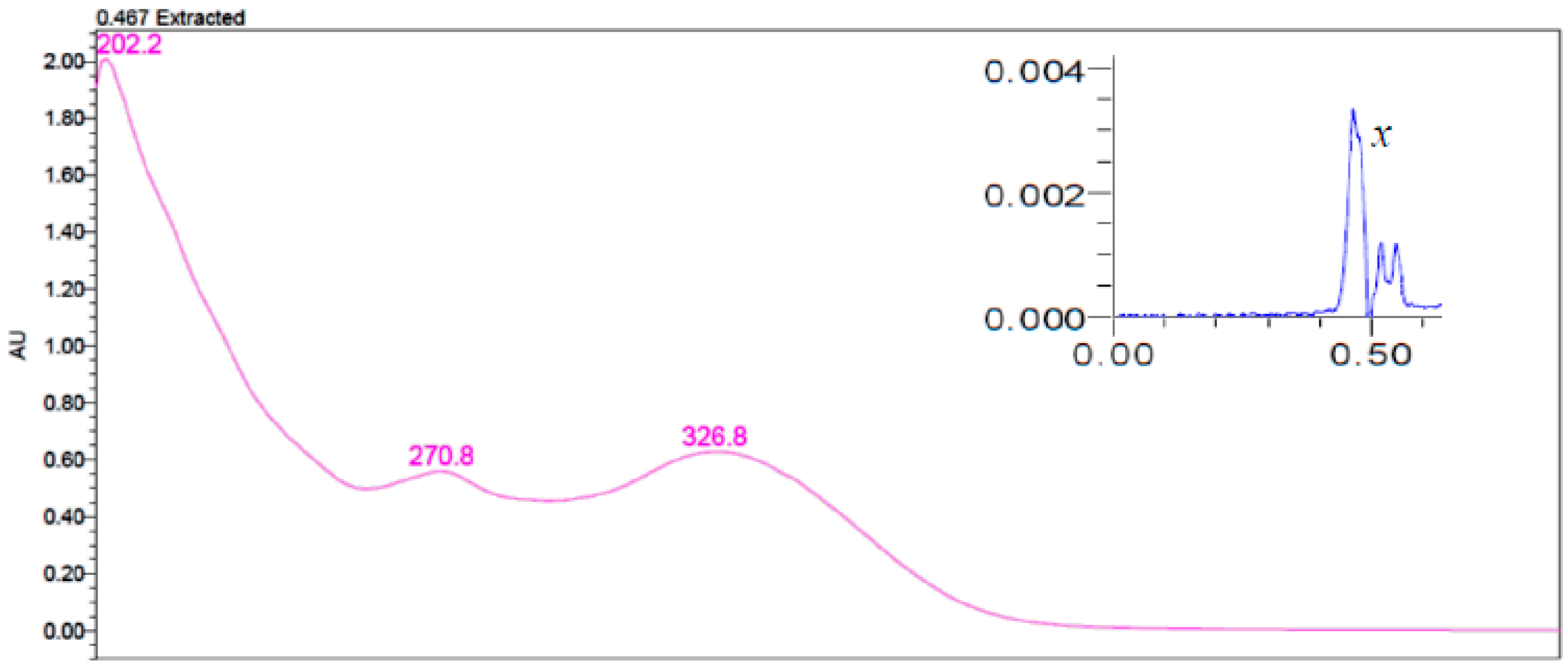
3.5. Separation of Yellow Pigments from Durum Wheat Milling Fraction Using UPLC
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
References
- Liu, R.H. Whole grain phytochemicals and health. J. Cereal Sci. 2007, 46, 207–219. [Google Scholar] [CrossRef]
- Leenhardt, F.; Lyan, B.; Rock, E.; Boussard, A.; Potus, J.; Chanliaud, E.; Remesy, C. Genetic variability of carotenoid concentration, and lipoxygenase and peroxidase activities among cultivated wheat species and bread wheat varieties. Eur. J. Agron. 2006, 25, 170–176. [Google Scholar] [CrossRef]
- Rice-Evans, C.A.; Miller, N.J.; Paganga, G. Structure antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Li, L.; Shewry, P.R.; Ward, J.L. Phenolic acids in wheat varieties in the HEALTHGRAIN diversity screen. J. Agric. Food Chem. 2008, 56, 9732–9739. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Orozco, R.; Li, L.; Harflett, C.; Shewry, P.R.; Ward, J.L. Effects of environment and genotype on phenolic acids in wheat in the HEALTHGRAIN diversity screen. J. Agric. Food Chem. 2010, 58, 9341–9352. [Google Scholar] [CrossRef] [PubMed]
- Miller, N.J.; Sampson, J.; Candeias, L.P.; Bramley, P.M.; Rice-Evans, C.A. Antioxidant activities of carotenes and xanthophylls. FEBS Lett. 1996, 384, 240–242. [Google Scholar] [CrossRef]
- Martini, D.; Taddei, F.; Ciccoritti, R.; Pasquini, M.; Nicoletti, I.; Corradini, D.; D’Egidio, M.G. Variation of total antioxidant activity and of phenolic acid, total phenolics and yellow coloured pigments in durum wheat (Triticum turgidum L. var. durum) as a function of genotype, crop year and growing area. J. Cereal Sci. 2015, 65, 175–185. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.M.; Shahidi, F. Antioxidant and free radical scavenging activities of whole wheat and milling fractions. Food Chem. 2007, 101, 1151–1157. [Google Scholar] [CrossRef]
- Ndolo, V.U.; Beta, T. Distribution of carotenoids in endosperm, germ, and aleurone fractions of cereal grain kernels. Food Chem. 2013, 139, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Žilić, S.; Serpen, A.; Akillioğlu, G.; Janković, M.; Gökmen, V. Distributions of phenolic compounds, yellow pigments and oxidative enzymes in wheat grains and their relation to antioxidant capacity of bran and debranned flour. J. Cereal Sci. 2012, 56, 652–658. [Google Scholar] [CrossRef]
- Levizou, E.; Petropoulou, Y.; Manetas, Y. Total carotenoid amount in crude twig extracts may be overestimated due to interference by high contents of co-extracted phenolics. Photosynthetica 2004, 42, 295–297. [Google Scholar] [CrossRef]
- Fratianni, A.; Irano, M.; Panfill, G.; Acquistucci, R. Estimation of color of durum wheat. Comparison of WSB, HPLC, and reflectance colorimeter measurements. J. Agric. Food Chem. 2005, 53, 2373–2378. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.M.; Young, J.C.; Rabalski, I.; Hucl, P.; Fregeau-Reid, J. Identification and quantification of seed carotenoids in selected wheat species. J. Agric. Food Chem. 2007, 55, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Brandolini, A.; Hidalgo, A.; Moscaritolo, S. Chemical composition and pasting properties of einkorn (Triticum monococcum L. subsp. Monococcum) whole meal flour. J. Cereal Sci. 2008, 47, 599–609. [Google Scholar] [CrossRef]
- Hentschel, V.; Kranl, K.; Hollmann, J.; Lindhauer, M.G.; Böhm, V.; Bitsch, R. Spectrophotometric determination of yellow pigment content and evaluation of carotenoids by high-performance liquid chromatography in durum wheat grain. J. Agric. Food Chem. 2002, 50, 6663–6668. [Google Scholar] [CrossRef] [PubMed]
- Digesù, A.M.; Platani, C.; Cattivelli, L.; Mangini, G.; Blanco, A. Genetic variability in yellow pigment components in cultivated and wild tetraploid wheats. J. Cereal Sci. 2009, 50, 210–218. [Google Scholar] [CrossRef]
- Blanco, A.; Colasuonno, P.; Gadaleta, A.; Mangini, G.; Schiavulli, A.; Simeone, R.; Digesù, A.M.; De Vita, P.; Mastrangelo, A.M.; Cattivelli, L. Quantitative trait loci for yellow pigment concentration and individual carotenoid compounds in durum wheat. J. Cereal Sci. 2011, 54, 255–264. [Google Scholar] [CrossRef]
- Dexter, J.E.; Matsuo, R.R.; Kruger, J.E. The spaghetti making quality of commercial durum wheat samples of variable α-amylase activity. Cereal Chem. 1990, 67, 405–412. [Google Scholar]
- Fu, B.X.; Schlichting, L.; Pozniak, C.J.; Singh, A.K. Pigment loss from semolina to dough: Rapid measurement and relationship with pasta colour. J. Cereal Sci. 2013, 57, 560–566. [Google Scholar] [CrossRef]
- AACC International Method 14.60.0, Total Carotenoid content of Cereal Grains and Flours. In Approved Methods of the AACC International; AACC International: St. Paul, MN, USA, 2012.
- Waterman, P.G.; Mole, S. Analysis of Phenolic Plant Metabolites; Blackwell Scientific Publications: Oxford, UK, 1994. [Google Scholar]
- Awola, J.M.; Rooney, L.W.; Wu, X.; Prior, R.L.; Cisneros-Zevallos, L. Screening methods to measure antioxidant activity of sorghum (sorghum bicolor) and sorghum products. J. Agric. Food. Chem. 2003, 51, 6657–6662. [Google Scholar]
- Hung, P.V.; Hatcher, D.W. Ultra-performance liquid chromatography (UPLC) quantification of carotenoids in durum wheat: Influence of genotype and environment in relation to the colour of yellow alkaline noodles (YAN). Food Chem. 2011, 125, 1510–1516. [Google Scholar] [CrossRef]
- Borrelli, G.M.; Troccoli, A.; Di Fonzo, N.; Fares, C. Durum wheat lipoxygenase activity and other quality parameters that affect pasta color. Cereal Chem. 1999, 76, 335–340. [Google Scholar] [CrossRef]
- Borrelli, G.M.; DeLeonardis, A.M.; Platani, C.; Troccoli, A. Distribution along durum wheat kernel of the components involved in semolina colour. J. Cereal Sci. 2008, 48, 494–502. [Google Scholar] [CrossRef]
- Panfili, G.; Fratianni, A.; Irano, M. Improved normal-phase high performance liquid chromatography procedure for the determination of carotenoids in cereals. J. Agric. Food Chem. 2004, 52, 6373–6377. [Google Scholar] [CrossRef] [PubMed]
- Beta, T.; Nam, S.; Dexter, J.E.; Sapirstein, H.D. Phenolic content and antioxidant activity of pearled wheat and roller-milled fractions. Cereal Chem. 2005, 82, 390–393. [Google Scholar] [CrossRef]
- Zhou, K.; Laux, J.J.; Yu, L. Comparison of Swiss red wheat grain and fractions for their antioxidant properties. J. Agric. Food Chem. 2004, 52, 1118–1123. [Google Scholar] [CrossRef] [PubMed]
- Fulcher, R.G.; O’Brien, T.P.; Lee, J.W. Studies on the aleurone layer I. Conventional and fluorescence microscopy of the cell wall with emphasis on phenol-carbohydrate complexes in wheat. Aust. J. Biol. Sci. 1972, 25, 23–34. [Google Scholar]
- Lavelli, V.; Hidalgo, A.; Pompei, C.; Brandolini, A. Radical scavenging activity of einkorn (Triticum monococcum L. subsp. monococcum) wholemeal flour and its relationship to soluble phenolic and lipophilic antioxidant content. J. Cereal Sci. 2009, 49, 319–321. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4303. [Google Scholar] [CrossRef] [PubMed]
- Adom, K.K.; Sorrells, M.E.; Liu, R.H. Phytochemical profiles and antioxidant activity of wheat varieties. J. Agric. Food Chem. 2003, 51, 7825–7834. [Google Scholar] [CrossRef] [PubMed]
- Robbins, R.J. Phenolic acids in foods: An overview of analytical methodology. J. Agric. Food Chem. 2003, 51, 2866–2887. [Google Scholar] [CrossRef] [PubMed]
- Mazzoncini, M.; Antichi, D.; Silvestri, N.; Giantelli, G.; Sgherri, C. Organically vs conventionally grown winter wheat: Effects on grain yield, technological quality, and on phenolic composition and antioxidant properties of bran and refined flour. Food Chem. 2015, 175, 445–451. [Google Scholar] [CrossRef] [PubMed]
| Fraction | TYP (μg/g) | TPC Methanol Extract (mg CE/100 g b) | TPC Yellow Pigment Extract (mg CE/100 g b) | |||
|---|---|---|---|---|---|---|
| Navigator | Strongfield | Navigator | Strongfield | Navigator | Strongfield | |
| Flour | 8.55 a ± 0.13 a | 7.83 d ± 0.01 | 33.8 c ± 5.0 b | 36.1 c ± 7.2 | 22.6 c ± 2.7 | 20.1 d ± 3.0 |
| Semolina | 8.65 a ± 0.09 | 7.91 cd ± 0.06 | 32.1 c ± 5.6 | 33.1 c ± 6.6 | 17.3 c ± 0.7 | 17.4 d ± 1.5 |
| Feed flour | 8.07 b ± 0.09 | 8.64 b ± 0.02 | 77.1 b ± 3.9 | 88.1 b ± 2.3 | 53.2 a ± 5.0 | 55.5 b ± 2.9 |
| Short bran | 7.40 c ± 0.00 | 8.94 a ± 0.09 | 101.2 a ± 10.4 | 116.1 a ± 3.2 | 67.3 a ± 6.8 | 73.3 a ± 2.8 |
| Bran | 7.42 c ± 0.15 | 8.90 a ± 0.02 | 92.8 ab ± 1.7 | 103.4 ab ± 6.3 | 62.1 a ± 4.9 | 68.4 a ± 6.8 |
| Wholewheat | 8.30 ab ± 0.18 | 8.04 c ± 0.05 | 45.7 c ± 2.1 | 46.8 c ± 1.9 | 35.5 b ± 3.5 | 35.3 c ± 1.9 |
| Fraction | Navigator | Strongfield | ||
|---|---|---|---|---|
| Lutein | Lutein Isomers | Lutein | Lutein Isomers | |
| Flour | 81.1 ± 1.7 | 15.4 ± 1.7 | 81.4 ± 1.5 | 16.6 ± 0.8 |
| Semolina | 80.9 ± 1.3 | 15.4 ± 0.8 | 82.6 ± 0.9 | 16.5 ± 0.6 |
| Feed flour | 79.4 ± 1.1 | 18.8 ± 1.6 | 76.5 ± 1.0 | 19.0 ± 0.8 |
| Short bran | 79.2 ±1.7 | 18.5 ± 1.6 | 75.4 ± 1.0 | 20.0 ± 1.3 |
| Bran | 80.2 ± 2.3 | 18.5 ± 1.6 | 76.5 ± 0.8 | 18.7 ± 0.9 |
| Wholewheat | 82.0 ± 1.7 | 16.0 ± 0.8 | 81.5 ± 2.1 | 15.3 ± 1.4 |
| Fraction | Navigator | Strongfield | ||||
|---|---|---|---|---|---|---|
| Unknown (x) | Lutein | Lutein + x | Unknown (x) | Lutein | Lutein + x | |
| Feed flour | 18.3 ± 3.7 | 59.5 ± 3.7 | 77.8 ± 2.8 | 19.5 ± 2.8 | 55.8 ± 3.3 | 75.3 ± 2.0 |
| Short bran | 23.3 ± 2.5 | 54.5 ± 4.4 | 77.8 ± 2.8 | 26.0 ± 1.2 | 50.3 ± 2.1 | 76.2 ± 1.2 |
| Bran | 23.2 ± 1.8 | 55.2 ± 2.7 | 78.4 ± 1.9 | 25.7 ± 1.8 | 51.2 ± 2.0 | 76.9 ± 1.1 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fu, B.X.; Chiremba, C.; Pozniak, C.J.; Wang, K.; Nam, S. Total Phenolic and Yellow Pigment Contents and Antioxidant Activities of Durum Wheat Milling Fractions. Antioxidants 2017, 6, 78. https://doi.org/10.3390/antiox6040078
Fu BX, Chiremba C, Pozniak CJ, Wang K, Nam S. Total Phenolic and Yellow Pigment Contents and Antioxidant Activities of Durum Wheat Milling Fractions. Antioxidants. 2017; 6(4):78. https://doi.org/10.3390/antiox6040078
Chicago/Turabian StyleFu, Bin Xiao, Constance Chiremba, Curtis J. Pozniak, Kun Wang, and Shin Nam. 2017. "Total Phenolic and Yellow Pigment Contents and Antioxidant Activities of Durum Wheat Milling Fractions" Antioxidants 6, no. 4: 78. https://doi.org/10.3390/antiox6040078




